Abstract
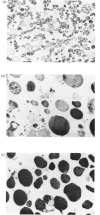
The present study was undertaken to isolate and investigate some physicochemical properties of renin granules from the rat kidney cortex. Two preparations of subcellular organelles were used: a primary-granule fraction, which allowed the properties of lysosomes to be compared simultaneously with those of renin granules, and a semi-purified preparation of the latter. The specific activity of renin in the primary-granule preparations was about 4-fold higher than in the original homogenate; that of the semi-purified renin-granule preparation was about 18-fold higher than in the homogenate, and consisted mainly of electron-dense granules but some mitochondria were also observed. Renin and acid phosphatase release from the primary-granule preparation was increased by lowering osmolality, by a low-molecular-weight solute (glucose) and by Triton X-100 or digitonin. Enzyme release was decreased by lowering the incubation temperature (4 degrees C) or the presence of CaCl2. Renin release from the partially purified granule preparation was not affected by cyclic AMP, cyclic GMP and ATP.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badenoch-Jones P., Baum H. Progesterone-induced lysis of rat kidney lysosomes as studied by changes in light-absorbance. Biochem J. 1974 Jul;142(1):1–6. doi: 10.1042/bj1420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Hellman B., Pihl E., Täljedal I. B. Physicochemical characteristics of insulin secretion granules. Biochem J. 1969 Jan;111(1):107–113. doi: 10.1042/bj1110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fynn M., Onomakpome N., Peart W. S. The effects of ionophores (A23187 and RO2-2985) on renin secretion and renal vasoconstriction. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):199–212. doi: 10.1098/rspb.1977.0135. [DOI] [PubMed] [Google Scholar]
- Giannattasio G., Zanini A., Meldolesi J. Molecular organization of rat prolactin granules. I. In vitro stability of intact and "membraneless" granules. J Cell Biol. 1975 Jan;64(1):246–251. doi: 10.1083/jcb.64.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B. The effects of calcium and divalent ionophores on the stability of isolated insulin secretory granules. FEBS Lett. 1975 Jul 1;54(3):343–346. doi: 10.1016/0014-5793(75)80936-7. [DOI] [PubMed] [Google Scholar]
- Herman L., Sato T., Hales C. N. The electron microscopic localization of cations to pancreatic islets of Langerhans and their possible tole in insulin secretion. J Ultrastruct Res. 1973 Feb;42(3):298–311. doi: 10.1016/s0022-5320(73)90058-0. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Poisner A. M. Properties of renin granules isolated from rat kidney. Mol Cell Endocrinol. 1976 Oct;5(5):331–337. doi: 10.1016/0303-7207(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Preservation of structural integrity of liver lysosomes and membrane-stabilizing action of anti-inflammatory drugs, catecholamines and cyclic adenosine monophosphate in isotonic salt media. Biochem Pharmacol. 1973 Jun 1;22(11):1269–1282. doi: 10.1016/0006-2952(73)90301-8. [DOI] [PubMed] [Google Scholar]
- Kirschbaum B. B. Effects of digitonin on mitochondrial and lysosomal fractions from renal cortex. Nephron. 1976;17(4):297–306. doi: 10.1159/000180734. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAUNSBACH A. B. CORRELATION OF ULTRASTRUCTURE, AUTOFLUORESCENCE AND ACID PHOSPHATASE ACTIVITY IN GRANULES ISOLATED FROM PROXIMAL TUBULES OF THE KIDNEY OF THE RAT. Nature. 1964 Jun 13;202:1131–1132. doi: 10.1038/2021131b0. [DOI] [PubMed] [Google Scholar]
- Mego J. L., Farb R. M., Barnes J. An adenosine triphosphate-dependent stabilization of proteolytic activity in heterolysosomes. Evidence for a proton pump. Biochem J. 1972 Jul;128(4):763–769. doi: 10.1042/bj1280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego J. L. The effect of pH on cathepsin activities in mouse liver heterolysosomes. Biochem J. 1971 May;122(4):445–452. doi: 10.1042/bj1220445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S., Yamamoto K., Ueda J. Isolation of renin granules from the dog kidney cortex. J Appl Physiol. 1972 Sep;33(3):306–311. doi: 10.1152/jappl.1972.33.3.306. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Johnston C. I. Isolation of renin granules from rat kidney cortex and evidence for an inactive form of renin (prorenin) in granules and plasma. Endocrinology. 1976 Jun;98(6):1466–1474. doi: 10.1210/endo-98-6-1466. [DOI] [PubMed] [Google Scholar]
- Nustad K., Rubin I. Subcellular localization of renin and kininogenase in the rat kidney. Br J Pharmacol. 1970 Oct;40(2):326–333. doi: 10.1111/j.1476-5381.1970.tb09925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoyama K., Hara M., Tanaka K., Omae T. Renin content in subcellular fractions of normal rat kidney extract. Jpn Heart J. 1973 Sep;14(5):440–444. doi: 10.1536/ihj.14.440. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart W. S., Quesada T., Tenyi I. The effects of EDTA and EGTA on renin secretion. Br J Pharmacol. 1977 Feb;59(2):247–252. doi: 10.1111/j.1476-5381.1977.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart W. S., Quesada T., Tenyi I. The effects of cyclic adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate and theophylline on renin secretion in the isolated perfused kidney of the rat. Br J Pharmacol. 1975 May;54(1):55–60. doi: 10.1111/j.1476-5381.1975.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L. The permeability of chromaffin granules to non-electrolytes. Biochem Pharmacol. 1976 May 1;25(9):1035–1038. doi: 10.1016/0006-2952(76)90492-5. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SHIBKO S., TAPPEL A. L. RAT-KIDNEY LYSOSOMES: ISOLATION AND PROPERTIES. Biochem J. 1965 Jun;95:731–741. doi: 10.1042/bj0950731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibko S., Pangborn J., Tappel A. L. Studies on the release of lysosomal enzymes from kidney lysosomes. J Cell Biol. 1965 Jun;25(3):479–483. doi: 10.1083/jcb.25.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner S. L. Improved assay methods for renin "concentration" and "activity" in human plasma. Methods using selective denaturation of renin substrate. Circ Res. 1967 Apr;20(4):391–402. doi: 10.1161/01.res.20.4.391. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Iwao H., Abe Y., Morimoto S. Effect of Ca on renin release in vitro and in vivo. Jpn Circ J. 1974 Dec;38(12):1127–1131. doi: 10.1253/jcj.38.1127. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Okahara T., Abe Y., Ueda J., Kishimoto T. Effects of cyclic AMP and dibutyryl cyclic AMP on renin release in vivo and in vitro. Jpn Circ J. 1973 Oct;37(10):1271–1276. doi: 10.1253/jcj.37.1271. [DOI] [PubMed] [Google Scholar]