Abstract
All members of the herpesvirus family persist in their host throughout life. In doing so, herpesviruses exploit a surprising number of different strategies to evade the immune system. Human herpesvirus 7 (HHV-7) is a relatively recently discovered member of the herpesvirus family, and little is known about how it escapes immune detection. Here we show that HHV-7 infection results in premature degradation of major histocompatibility complex class I molecules. We identify and characterize a protein from HHV-7, U21, that binds to and diverts properly folded class I molecules to a lysosomal compartment. Thus, U21 is likely to function in the normal course of HHV-7 infection to downregulate surface class I molecules and prevent recognition of infected cells by cytotoxic T lymphocytes.
Human herpesvirus 7 (HHV-7) is a betaherpesvirus most closely related to HHV-6. HHV-7 and HHV-6 have colinear genomes and share many biological properties. Both viruses display CD4+ T lymphotropism, both cause xanthem subitum (roseola) (5, 29, 32), and more than 90% of adults are seropositive for both HHV-6 and HHV-7 (38). HHV-6 and -7-infected cells exhibit cytomegaly and are prone to syncytium formation, features reminiscent of those seen in human cytomegalovirus (HCMV) infection. The sequences of HHV-6 and -7 genomes confirm their relationship to HCMV (25).
Little is known about the immunobiology of HHV-6 or -7. Like all other herpesviruses, HHV-6 and -7 remain latent or establish persistent infections. To do so, they must avoid detection and elimination by the immune system. Viral immune evasion strategies include restriction of viral gene expression, infection at immunoprivileged sites, obstruction of antiviral cytokine function, and interference with antigen presentation (34). Notably, all of the herpesviruses thus far examined employ the latter strategy of interfering with viral antigen presentation to cytotoxic T lymphocytes (CTLs).
To present antigen at the cell surface, major histocompatibility complex (MHC) class I heavy chains must form a complex with the light chain, β2-microglobulin (β2m), and peptide to acquire a stable conformation. MHC class I-associated peptides are generated in the cytoplasm mainly by the proteasome and are transported into the endoplasmic reticulum (ER) by the transporter TAP (transporter associated with antigen processing). Once in the ER, the peptides are loaded onto MHC class I molecules. Stable class I-β2m-peptide complexes then traverse the Golgi en route to the cell surface, where they can interact with CD8+ CTLs.
Herpesvirus gene products influence MHC class I antigen presentation in numerous and diverse ways. Some herpesvirus proteins interfere with proteolysis of antigens (13) or peptide transport into the ER (3, 15, 33, 40). Others retain or destroy class I molecules (2, 19, 37, 41), enhance the internalization of class I molecules (12, 17, 31), or divert class I molecules to lysosomes for degradation (30; for reviews, see references 4 and 34). Judging from the number and molecular diversity of these strategies, the removal of MHC class I-peptide complexes from the cell surface must be evolutionarily advantageous to these viruses as a means of escaping immune detection.
Most of the immunoevasins encoded by herpesviruses are unique, yet all herpesviruses analyzed manage to interfere with class I antigen presentation in one way or another. No obvious homologies are apparent between any HHV-6 or -7 genes and the genes that encode immunoevasins in other herpesviruses; nonetheless it seemed likely that HHV-6 and -7 also encode immunoevasins. Here we describe a gene product from HHV-7 that binds tightly to properly folded MHC class I molecules and diverts them to lysosomes.
MATERIALS AND METHODS
Cell lines.
SupT1 cells were cultured in RPMI–5% fetal bovine serum and were infected by coculturing with HHV-7 (strain SB)-infected SupT1 cells (P. Pellett, Centers for Disease Control and Prevention, Atlanta, Ga.) (1). Infected cells were used for experiments 2 to 6 days postinfection. U373 astrocytoma cells were cultured in Dulbecco's modified Eagle medium (DMEM)–10% fetal bovine serum. U373 astrocytoma cells were infected with a retrovirus encoding U21 or U21 with an influenza virus hemagglutinin (HA) epitope tag.
Antibodies.
W6/32 is a monoclonal antibody (MAb) that recognizes assembled, β2m-associated HLA-A, -B, or -C molecules (6). For some immunofluorescence studies, W6/32 was covalently coupled to Alexa Fluor 488 dye (Molecular Probes, Eugene, Oreg.). 12CA5 is a MAb that recognizes the influenza virus HA epitope tag. Antibody (Ab) 2441 is a rabbit polyclonal Ab that was raised against glutathione S-transferase (GST) fused in frame to the C terminus of U21. The FITC-conjugated HLA-A-B-C Ab was purchased from PharMingen. The MAb α-EEA1 was obtained from Becton Dickinson, Lexington, Ky., anti-γ-adaptin MAb 100/3 was purchased from Sigma (St. Louis, Mo.). α-lamp1 and -lamp2 MAbs were the generous gift of T. August (Johns Hopkins Medical School), and the polyclonal CI-M6PR Ab recognizes the cation-independent mannose 6-phosphate receptor (14). The 66Ig10 MAb recognizes the transferrin receptor (35). Alexa- or FITC-conjugated secondary antibodies were purchased from Molecular Probes or Jackson Immunologicals (West Grove, Pa.). Alexa 488 was covalently coupled to the W6/32 MAb according to the manufacturer's instructions.
Pulse-chase experiments.
Cells were detached with trypsin and incubated with methionine- and cysteine-free DMEM for 30 min at 37°C. Cells were labeled with 500 or 1,000 μCi of [35S]methionine-cysteine (1,200 Ci/mmol; NEN-Dupont, Boston, Mass.) per milliliter at 37°C for the indicated times and chased with complete DMEM supplemented with nonradiolabeled methionine and cysteine to a final concentration of 1 mM at 37°C for the indicated times. Cells were lysed in digitonin lysis buffer (1% digitonin, 25 mM HEPES [pH 7.7], 150 mM potassium acetate) or Nonidet P-40 lysis buffer (50 mM Tris-HCl [pH 7.4], 0.5% NP-40, 5 mM MgCl2). Lysates were centrifuged for 5 to 10 min at 14,000 rpm at 4°C in an Eppendorf model 5415C centrifuge to pellet nuclei and debris, and precleared with protein A-agarose (BRL Life Sciences, Grand Island, N.Y.) and 3 μl of normal mouse serum, followed by immunoprecipitation with specific antiserum and protein A-agarose. Immunoprecipitations were normalized to equal trichloroacetic acid-precipitable 35S-labeled protein. The immunoprecipitates were washed five times with 1 ml of digitonin wash buffer (0.2% digitonin, 25 mM HEPES [pH 7.7], 150 mM potassium acetate) or NP-40 wash buffer (50 mM Tris-HCl [pH 7.4], 0.5% NP-40, 5 mM EDTA, 150 mM NaCl) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or one-dimensional isoelectric focusing (IEF). Leupeptin (Sigma), in dimethyl sulfoxide, was added to the cells 45 min prior to the pulse at a final concentration of 200 μM. Concanamycin B (ConB) (Ajinimoto Co., Kanagawa, Japan) (39) was added to the cells 45 min prior to the pulse at 20 nM final concentration. Brefeldin A (BFA) (Sigma), was added to the pulse and chase medium at a final concentration of 10 μg/ml 5 min prior to the start of the chase period. Endoglycosidase Hf (New England Biolabs, Beverly, Mass.) was added to the immunoprecipitates according to the vendor's instructions.
Cloning of U20 and U21.
PCR amplification of U20 and U21 was performed from cDNA made from infected SupT1 cells, which yielded DNA fragments of the predicted sizes. The resulting fragments were cloned into the PCR-Blunt II TOPO-expression vector (Invitrogen), and the sequences were confirmed. PCR was performed from cDNA made from HHV-7-infected cells using the following primers corresponding to the 5′ and 3′ ends of the U21 and U20 open reading frames (nucleotides 31242 to 32431 (U20) and 32422 to 33714 (U21) from HHV-7, strain RK): U20, 5′-gccaccATGTTTGTGAAAAAAAC-3′ and 5′-ccTTAATGACACATGAAATCT-3′; U21, 5′-gccaccATGTGGACTATCTTGCTGTTTT-3′ and 5′-TAATCACAAACATTGCTGT-3′. The U20 and U21 cDNAs were then subcloned into the retroviral vector LNCX (Clontech, Palo Alto, Calif.). HA-tagged U20 and U21 were amplified from primers containing the additional HA sequence: GCCTACCCATACGACGTACCAGACTACGCA.
Flow cytometry.
Cells were removed from the plates with phosphate-buffered saline (PBS)–1 mM EDTA, washed with ice-cold PBS, and incubated with FITC-conjugated anti-HLA-A-B-C Ab or with the 66IG10 monoclonal anti-transferrin receptor Ab for 30 min on ice, washed thoroughly, and either resuspended in ice-cold PBS or incubated with FITC-conjugated goat anti-mouse secondary Ab and washed again before evaluating the surface levels of fluorescence with a Beckman FACScalibur flow cytometer.
Immunofluorescence microscopy.
Cells on coverslips were washed with PBS and fixed with 3% paraformaldehyde for 10 min; permeabilized with 0.5% saponin or NP-40 in PBS and 3% BSA or 2% fish skin gelatin; incubated with primary antibodies or Alexa 488-conjugated W6/32 for 30 min; washed; incubated with FITC-, Alexa 488-, or Alexa 598-conjugated secondary antibodies for 30 min; washed; and mounted on microscope slides.
RESULTS
HHV-7 infection in vitro is limited to primary CD4+ T cells and the SupT1 thymocyte-derived T-cell line (1, 7). One of the limitations to working with HHV-7 is that attempts at preparation of a high-titer cell-free virus have been unsuccessful (for a review, see reference 9). As is the case for varicella-zoster virus and HCMV, HHV-7 infection is best spread via cell-cell contact (1); thus, infection of cultured SupT1 cells is accomplished by mixing SupT1-infected with noninfected cells at a ratio of 1:5. An average of 30% of these cells become positive for an HHV-7 nuclear antigen (8), a source of some variability in the outcome of biochemical analysis. Cytomegaly and syncytium formation provide a more rapid indication of infection efficiency.
A 60-kDa glycoprotein associates tightly with properly folded class I molecules in HHV-7 infected cells.
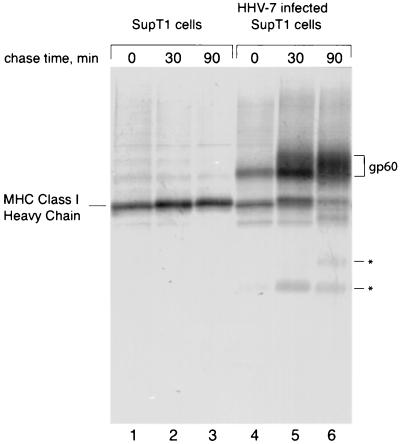
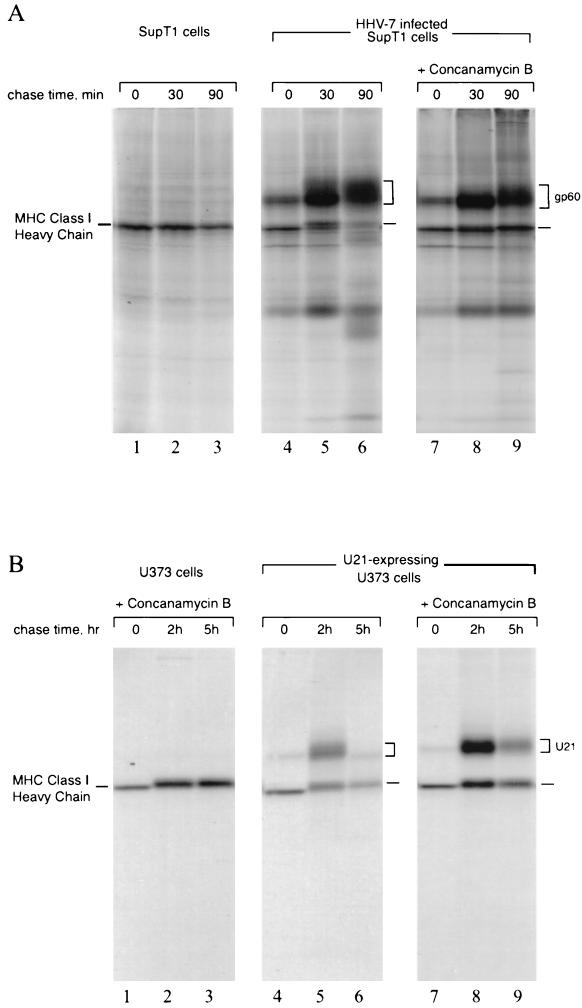
To determine whether infection with HHV-7 affected class I maturation, we immunoprecipitated MHC class I molecules from [35S]methionine-labeled HHV-7-infected SupT1 T cells using the conformation-specific class I Ab W6/32, which recognizes only properly folded class I-β2m complexes. In HHV-7-infected cells, where approximately 50 to 60% of the cells exhibited cytomegaly, we observed a coimmunoprecipitating protein of approximately 60 kDa (Fig. 1, lanes 4 to 6), which was absent from noninfected SupT1 cells (Fig. 1, lanes 1 to 3). This coprecipitating protein, designated gp60, associated with class I molecules soon after synthesis, since this association was already apparent after a 10-min pulse-label period (Fig. 1, lane 4). The class I-associated polypeptide became more diffuse at later chase times, suggesting heterogeneous glycosylation upon traversal of the secretory pathway (Fig. 1, lanes 5 and 6). In infected cells, the amount of immunoprecipitated class I heavy chain molecules decreased in the course of the chase period, compared to noninfected cells (lanes 6 and 3), suggesting destabilization of class I molecules. After 90 min of chase, the amount of W6/32-reactive class I heavy chain was reduced, but the amount of gp60 recovered by W6/32 coimmunoprecipitation increased (lanes 4 to 6). A pool of free radiolabeled gp60, synthesized during the pulse and capable of interacting with unlabeled class I molecules synthesized during the chase, would explain this observation. The polypeptides of low molecular weight in lanes 5 and 6 may represent degradation products of the class I heavy chain that retain the W6/32 epitope. Alternatively, they could be fragments of gp60 that continue to associate with class I molecules. We have not pursued the identification of these polypeptides further.
FIG. 1.
A 60-kDa protein associates tightly with MHC class I complexes in HHV-7-infected cells. Noninfected or HHV-7-infected SupT1 cells (72 h postinfection) were pulse-labeled for 10 min and then chased for the indicated times. The cells were washed, lysed, and immunoprecipitated with W6/32. The class I heavy chain and gp60 are indicated. Asterisks denote degradation products.
The association between the 60-kDa protein and MHC class I is strong, as it persisted through four washes with lysis buffer containing 0.01% SDS (data not shown). gp60 did not coimmunoprecipitate with normal mouse serum and thus is not an Fc receptor, nor did it coprecipitate with anti-transferrin receptor antibodies. Finally, antisera directed against free class I heavy chains failed to recover gp60 from HHV-7-infected cells (data not shown), suggesting that gp60 binds exclusively to properly folded β2m-bound class I molecules.
gp60 contains multiple N-linked glycans and is further modified in the Golgi.
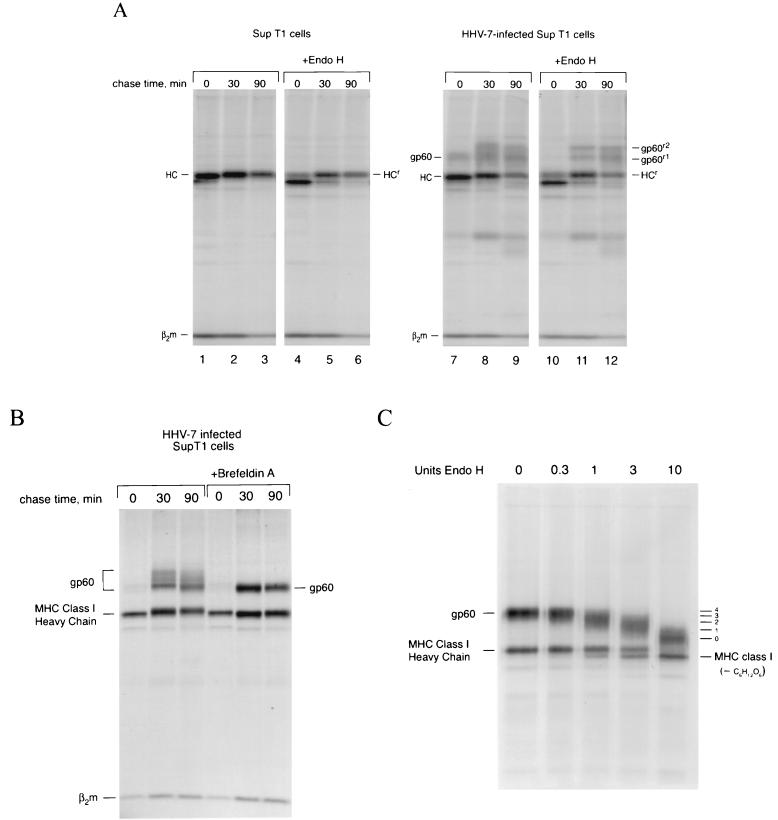
Endoglycosidase H (Endo-H) cleaves high-mannose N-linked glycans found on proteins in the ER and early Golgi. Transport of glycoproteins through the medial Golgi usually results in resistance to Endo-H, the consequence of complex-type glycan modifications. To determine whether gp60 contained N-linked oligosaccharide modifications and whether gp60 affected the trafficking of class I molecules through the Golgi, we performed a pulse-chase experiment identical to that described in Fig. 1 and treated the immunoprecipitated samples with Endo-H (Fig. 2A). Most newly synthesized glycoproteins remain in the ER and are Endo-H sensitive after 10 min of pulse-labeling with [35S]methionine. When W6/32 immunoprecipitates were treated with Endo-H, the single N-linked glycan on the class I heavy chain was removed (Fig. 2A, compare lanes 1 and 4). Some Endo-H-resistant heavy chain molecules were present in this experiment after only 10 min of labeling (lane 4, HCr). gp60 is also Endo-H sensitive; the 60-kDa polypeptide appears to collapse to a molecular mass coincident with Endo-H-resistant class I heavy chains (Fig. 2A, compare lanes 10 and 7). After a 30-min chase period, a greater percentage of MHC class I heavy chain molecules are Endo-H resistant (lane 5), and we begin to see the appearance of Endo-H-resistant gp60 molecules (lane 11). gp60 appears to have two Endo-H-resistant forms, consistent with incomplete conversion of at least one of its glycans. In contrast to the experiments depicted in Fig. 1, little gp60 was visible in association with class I molecules after the pulse-label (compare Fig. 2A, lane 7, with Fig. 1, lane 4). We attribute this to poor infection efficiency of the cells used in Fig. 2A, as judged by the low percentage (20 to 30%) of cells exhibiting the characteristic HHV-7-associated cytomegaly.
FIG. 2.
The MHC class I-associated protein is a glycoprotein. (A) HHV-7-infected SupT1 cells (72 h postinfection) were pulse-labeled for 10 min and then chased for the indicated times. W6/32 immune complexes were digested with or without Endo-H. Endo-H-resistant forms of MHC class I heavy chain (HCr) and gp60 (gp60r1 and gp60r2) are indicated. (B) HHV-7-infected SupT1 cells were pulse-labeled for 10 min and then chased for the indicated times in the absence or presence of BFA and immunoprecipitated with W6/32. (C) HHV-7-infected SupT1 cells were pulse-labeled for 10 min and then chased for 40 min in the presence of BFA. W6/32 immune complexes were treated with increasing concentrations of Endo-H, as indicated. The estimated number of glycans added to gp60 are indicated on the right.
Its diffuse nature on SDS-polyacrylamide gels suggested that gp60 contains complex-type N-linked glycans. When HHV-7-infected cells were treated with BFA, which inhibits ER-to-Golgi traffic and results in the redistribution of Golgi membranes and resident enzymes into the ER (22), gp60 presented as a more discrete polypeptide, consistent with the normal occurrence of complex-type oligosaccharide modifications on gp60 (Fig. 2B). We conclude that gp60 and the class I products with which it associates form a complex early in biosynthesis and travel together through the secretory pathway. In this experiment, the percentage of HHV-7-infected cells was similar to that shown in Fig. 2A.
Glycoproteins remain mostly Endo-H sensitive in BFA-treated cells; thus, BFA treatment should also increase the fraction of Endo-H-sensitive glycoproteins. To determine the number of N-linked glycans added to gp60, class I-gp60 complexes were recovered from HHV-7-infected cells treated with BFA to prevent addition of complex-type N-linked glycans. The immune complexes were then treated with a range of concentrations of Endo-H. At the highest Endo-H concentration, a fully deglycosylated polypeptide of approximately 45 kDa became apparent (Fig. 2C). Combined with the estimated loss of ∼15 kDa for the fully deglycosylated species, we infer the presence of three or four N-linked glycans, although the exact number could not be verified experimentally.
Identification of the candidate gene that specifies gp60.
The DNA sequence of HHV-7 is known, and homologies between HHV-7 genes and other herpesvirus genes have been assigned (24, 25). The HHV-7 genome does not contain genes that are homologous to any of the HCMV genes whose products bind to MHC class I molecules. In HCMV, these genes are located in the unique short region of the genome, a region without an equivalent in the smaller HHV-7 genome. We therefore surmised that an HHV-7 gene encoding an MHC class I binding protein might be unique to HHV-7.
Based on the predicted molecular mass of the endo H-treated 45-kDa polypeptide, there were eight candidate open reading frames for the MHC class I-associated protein in the HHV-7 genome (Table 1). Of these, four fulfilled the criterion of possessing at least three consensus N-linked glycosylation sites. Two of the four candidate genes, U45 and U60, were homologs of genes conserved among all herpesviruses and predicted to be involved in DNA packaging or nucleotide catabolism. These were not further considered. The remaining two open reading frames, U20 and U21, had no obvious counterparts in HCMV or other herpesviruses, and thus were attractive candidates for the gene encoding gp60.
TABLE 1.
Candidate open reading frames for gp60 from HHV-7a
| Name | Length (amino acids) | No. of NXS/T | HCMV homologs | Notes |
|---|---|---|---|---|
| U2 | 359 | 2 | UL23 | US22 gene family |
| U3 | 384 | 1 | UL24 | US22 gene family |
| U8 | 362 | 2 | UL29 | US22 gene family |
| U20 | 391 | 7 | Glycoprotein | |
| U21 | 430 | 5 | Glycoprotein | |
| U27 | 364 | 1 | UL44, all herpesviruses | Subunit of DNA pol |
| U45 | 379 | 6 | UL72, all herpesviruses | dUTPase |
| U60 | 394 | 6 | UL89, all herpesviruses | Late spliced gene; DNA packaging |
The number of PROSITE-predicted potential N-linked glycosylation sites is listed, as well as the names of the HCMV homologs, if any, and putative function of the homolog, if known. Modified from data reported in reference (25).
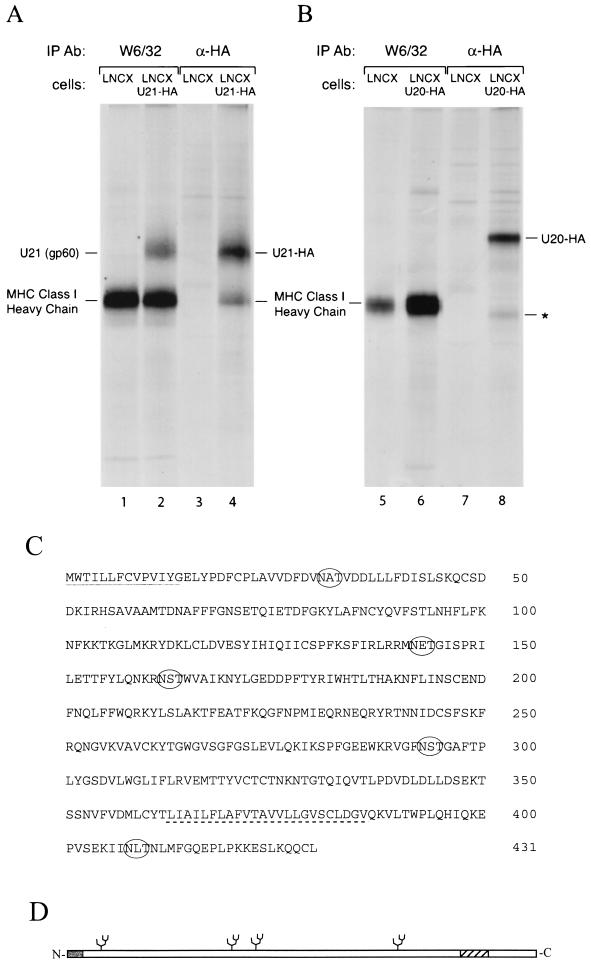
PCR amplification of U20 and U21 was performed from cDNA isolated from infected SupT1 cells, yielding DNA fragments of the predicted sizes. HA-tagged and nontagged U20 and U21 retroviral expression vectors were generated and used to stably transduce human U373 astrocytoma cells, which express high levels of MHC class I products. Immunoprecipitation of class I MHC products from U21-expressing cells yielded a protein of an apparent molecular weight identical to that of gp60 recovered from HHV-7 infected cells (Fig. 3A). Moreover, immunoprecipitation using an anti-HA Ab yielded the same 60-kDa polypeptide, together with material of a molecular mass corresponding to that of MHC class I heavy chain. Expression of U20 did not result in coimmunoprecipitation of the HA-tagged U20 protein with MHC class I heavy chain when precipitated with the W6/32 class I Ab, nor did the HA-tagged U20 comigrate at the same molecular weight as gp60 when it was precipitated with the anti-HA antiserum (Fig. 3B). The coprecipitating polypeptide in the HA-U20 lane is not the MHC class I heavy chain, as this polypeptide migrates slightly faster than MHC class I molecules (lane 8). It could be a degradation product of HA-U20.
FIG. 3.
Coimmunoprecipitation of U21, but not U20, with class I molecules from astrocytoma cells. (A and B) Cells expressing C-terminally HA epitope-tagged U20 (A), U21-HA (B), or retroviral vector alone (LNCX) were labeled for 1 h with [35S]methionine, lysed, and immunoprecipitated with either W6/32 or an anti-HA Ab. The asterisk denotes a probable degradation product of U20 that is smaller than the class I heavy chain. (C) Amino acid sequence of U21. The predicted signal sequence (continuous line) and transmembrane region (dashed line) are underlined. Predicted N-glycosylation sites are circled. (D) Schematic representation of sequence in panel C, with the predicted signal sequence shaded and the transmembrane region hatched.
The relative amount of U21 that coimmunoprecipitated with class I from retrovirally infected U21 astrocytoma cells is comparable to that seen in HHV-7-infected SupT1 cells. The retrovirally transduced U21 levels could therefore be in the approximate range of physiological U21 expression levels seen in HHV-7-infected cells. No homologies were apparent between U21 and any other protein, except between that of its counterpart in the colinear genome of HHV-6. Four of the five potential N-linked glycosylation sites in the U21 sequence are N-terminal to the predicted transmembrane domain (Fig. 3C). Since U21 is glycosylated at least three times (Fig. 2B), we infer it to be a type I membrane protein with a predicted cleavable signal sequence (26), four potential N-linked glycosylation sites, and a short cytoplasmic tail (Fig. 3D).
Ab directed against U21.
We raised a rabbit antiserum directed against the cytoplasmic tail of U21 fused at its N terminus to GST. This antiserum immunoprecipitates a protein with mobility identical to that of the U21 recovered in a complex with class I molecules from U21 retrovirus-transduced U373 cells (Fig. 4A, lane 4). The protein recovered by the antiserum to U21 was indistinguishable in size from that isolated from HHV-7-infected SupT1 cells. However, the U21 product in astrocytoma cells appeared less diffuse than in HHV-7-infected SupT1 T cells (compare Fig. 1, lane 5, with U21 in Fig. 3B or 4A). This discrepancy may be due to inherent differences in complex-type glycan modifications that occur in the two cell lines. These data confirm that U21 is indeed the MHC class I-associated protein and further demonstrate that U21 can associate with MHC class I complexes in the absence of other HHV-7-encoded proteins.
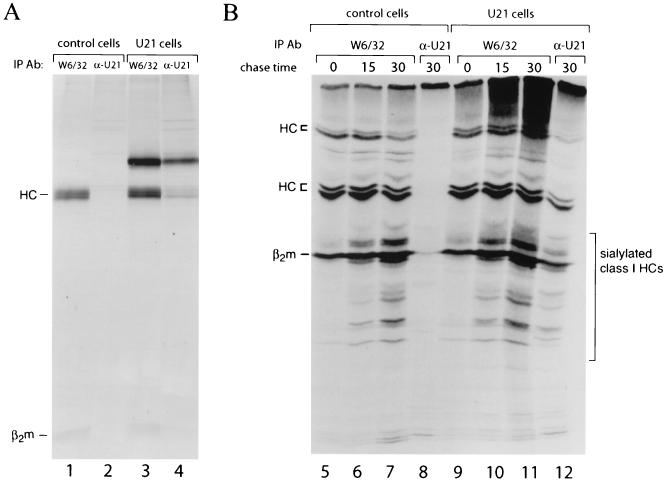
FIG. 4.
U21 does not discriminate against the products of different class I loci. (A) Class I complexes from control and U21-expressing cells were precipitated with either W6/32 or a polyclonal anti-U21 Ab and separated by SDS-PAGE. (B) IEF gel of W6/32 and α-U21 immunoprecipitations shown in panel A. Lanes 3, 4, 7, and 8 are from the same samples as those in lanes 1 to 4 from the SDS-PAGE gel in panel A. Heavy chains of class I (HC), β2m, and sialylated class I heavy chain molecules are indicated.
U21 associates with multiple alleles of MHC class I.
The human MHC encodes three class I heavy chain gene products, termed HLA-A, -B, and -C. These genes are highly polymorphic; thus, most human cell lines would be expected to possess two different alleles of each heavy chain gene. The generation of anti-U21 serum allowed us to ask whether all MHC class I products associate equally well with U21. To resolve the MHC class I allelic products immunoprecipitated by W6/32 and U21, we employed one-dimensional IEF gels. Immunoprecipitations were carried out from 35S-labeled control and U21-expressing cells using the W6/32 and U21 antibodies. The same immunoprecipitate shown in Fig. 4, lane 1, was resolved on an IEF gel, where the class I heavy chains resolve as multiple bands, reflecting the presence of multiple distinct class I products in the U373 cell line. As MHC class I heavy chains acquire sialic acids in the course of their maturation, polypeptides that focus at more acidic isoelectric point are visible at later time points. The immunoprecipitation from control cells using the U21-specific Ab is predictably devoid of bands in both SDS-PAGE and IEF gels (lanes 2 and 8). The pattern of bands precipitated with W6/32 (lanes 3 and 11) from U21-expressing cells is essentially indistinguishable from that in control cells (lanes 1 and 7), with the exception of some heterodisperse material at the top of the lanes from U21 cells. This is likely to be U21 itself, since the predicted pI of U21 is 7.5, outside of the resolvable pH range of the gel system used. IEF of the U21 immunoprecipitates from U21 cells (lanes 4 and 12) shows an almost identical pattern of heavy chains to that of W6/32 immunoprecipitates from control cells (lane 7), indicating that U21 associates with the same allelic class I products that are immunoprecipitated with W6/32. We conclude that U21 interacts with all of the major class I products in SupT1 cells. The allelic class I products expressed by SupT1 cells have not been identified. The acquisition of sialic acids on class I heavy chains is not impeded by the presence of U21. Likewise, U21-associated heavy chains are sialylated. Thus, the presence of U21 does not interfere with passage of class I heavy chains through the Golgi.
ConB prevents degradation of U21 and class I molecules.
One of the viral gene products from mouse cytomegalovirus (mCMV) that downregulates MHC class I, designated m06, diverts MHC class I molecules to the lysosomes for degradation (30). HHV-7 infection results in the loss of recovery of pulse-labeled MHC class I molecules, possibly due to degradation (Fig. 1). Degradation must occur after the complex has received complex-type oligosaccharide modifications in the Golgi, since the acquisition of Endo-H resistance for both class I and U21 (Fig. 2A) and the presence of sialic acids on U21-associated class I products (Fig. 4B) is inconsistent with retention of this complex in the ER. We therefore examined whether MHC class I complexes in HHV-7-infected cells could be stabilized in the presence of ConB, an inhibitor of the vacuolar H+-ATPase. Lysosomal protease function is compromised at more neutral pH, and thus stabilization of a protein in the presence of ConB would suggest involvement of lysosomal proteases in the turnover of that particular protein. MHC class I molecules in HHV-7-infected cells were stabilized in the presence of ConB (Fig. 5A, compare lanes 5 and 6 with lanes 8 and 9). Thus, elevation of intraorganellar pH stabilizes MHC class I molecules in HHV-7-infected cells.
FIG. 5.
ConB prevents degradation of U21 and class I molecules. (A) HHV-7-infected SupT1 cells (72 h postinfection) were pulse-labeled for 10 min and then chased for the indicated times in the presence of ConB, lanes 7 to 9. gp60 and class I heavy chains are indicated. (B) U21-expressing U373 astrocytoma cells were pulse-labeled for 10 min and then chased for the indicated times in the presence of ConB, lanes 1 to 3 and 7 to 9.
In astrocytoma cells, U21 expression alone resulted in a reduction in the levels of class I products (Fig. 5B, lanes 5 and 6), and both class I and U21 molecules became stabilized with ConB treatment (Fig. 5B, compare lanes 5 and 6 with lanes 8 and 9). These observations suggest that U21 may escort MHC class I molecules to an acidic compartment, where they are destroyed.
U21 expression results in redistribution of MHC class I molecules to lysosomes.
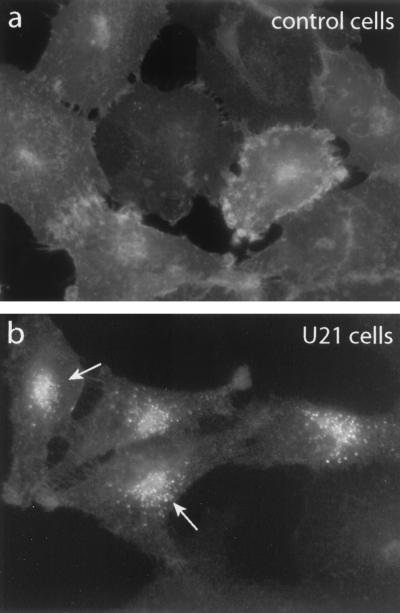
A lysosomal targeting function for U21 should manifest itself in a redistribution of class I molecules complexed with U21. The HHV-7-permissive SupT1 T cells are not conducive to satisfactory morphological analysis, as they possess only a thin shell of cytoplasm around the nucleus. We therefore focused on the U21-expressing adherent U373 astrocytoma cell line for immunocytochemistry. Immunofluorescence microscopy was performed on control and U21-expressing U373 astrocytoma cells using the W6/32 Ab. Class I immunolabeling of control astrocytoma cells shows a typical plasma membrane labeling pattern (Fig. 6a). Weakly labeled intracellular structures are likely to be the ER and Golgi apparatus. In contrast, U21-expressing cells exhibit intense perinuclear fluorescent labeling when analyzed with the W6/32 Ab (Fig. 6b), and plasma membrane labeling is diminished. Strong perinuclear localization was readily apparent in HHV-7-infected SupT1 cells as well (data not shown).
FIG. 6.
Immunofluorescence of MHC class I in U373 astrocytoma cells. Control U373 cells (a) or U21-expressing U373 cells (b) were labeled with the W6/32 Ab, directed against properly folded MHC class I molecules. Arrows indicate perinuclear W6/32 labeling that is absent from control cells.
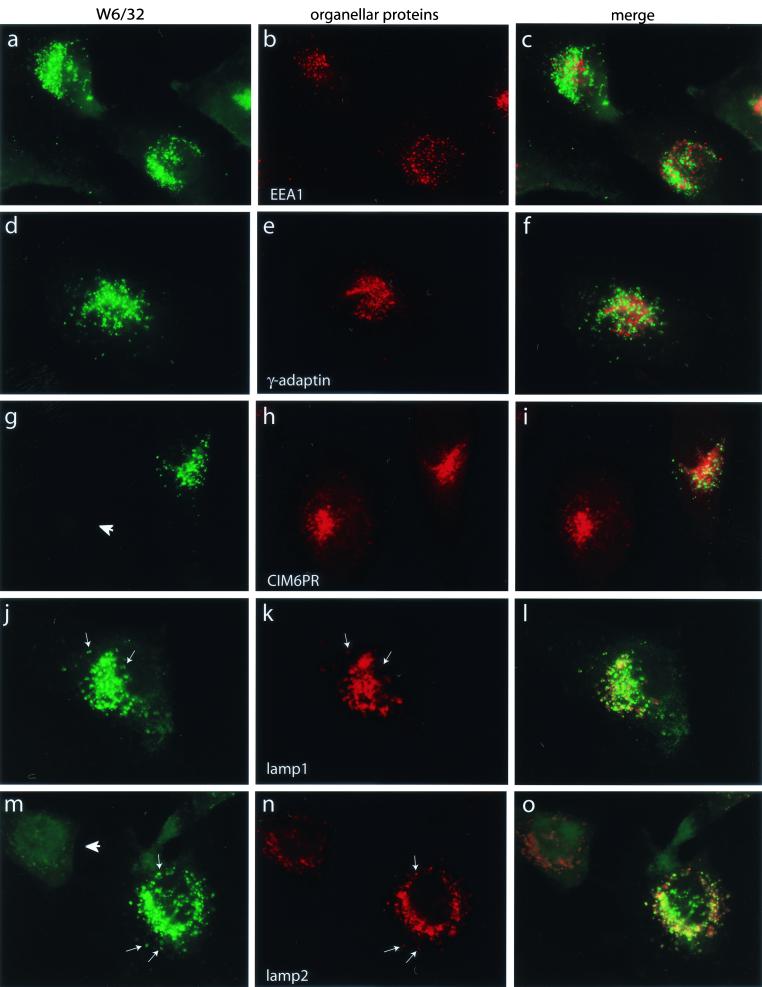
In HHV-7 infected cells, MHC class I molecules are diverted to a lysosomal compartment where they are most likely degraded (Fig. 5). Since U21 binds tightly to class I molecules and we observed relocalization of W6/32 fluorescence in U21-expressing cells, it follows that U21 might be responsible for the rerouting class I molecules to lysosomes for degradation. To identify the perinuclear organelle to which class I molecules were relocated in U21 cells, more extensive immunofluorescent colocalization experiments were carried out with W6/32 and antisera directed against EEA1 as a marker for early endosomes, γ-adaptin for the trans-Golgi network, cation-independent mannose-6-phosphate receptor (CI-M6PR) for late endosomes, and lamp1 and lamp2 for lysosomes (Fig. 7). The perinuclearly localized W6/32-reactive class I molecules are entirely coincident with the lysosomal markers lamp1 and lamp2, demonstrating that U21 expression results in the sequestration of MHC class I molecules in a lysosomal compartment. Not all of the U21 cells appear to sequester class I molecules (Fig. 7g and m). This variation is likely due to variability in U21 expression levels in the retrovirally transduced pools of cells. The U21-specific antiserum (Fig. 4) did not yield signals in immunofluorescence labeling experiments, so localization of U21 itself could not be directly verified.
FIG. 7.
Colocalization of MHC class I and lysosomal marker proteins. U21-expressing U373 cells were labeled with antisera directed against W6/32 (left panels) and the marker proteins EEA1, γ-adaptin, CI-M6PR, and lamp-1 and -2 (middle panels). The left column shows W6/32 immunolabeling. The right column shows merged images. Arrows indicate specific points of colocalization between the lamp-1 and lamp-2 Ab labeling and W6/32 labeling. Arrowheads denote cells that are assumed not to express U21.
U21 expression results in reduced class I on the cell surface.
With most of the class I molecules sequestered in a lysosomal compartment in the U373-U21 cell line, the number of class I molecules at the plasma membrane of U21-expressing cells should be reduced. Fluorescence-activated cell sorter analysis of neomycin-resistant pools of retrovirally transduced U21 astrocytoma cells shows that this is indeed the case (Fig. 8). In U21-expressing cells, some cells exhibit greatly reduced surface labeling with W6/32, while others display intermediate levels (Fig. 8A). This variation in cell surface class I expression is likely attributable to differences in U21 expression levels, since subcloning of neomycin-resistant cells resulted in the generation of some clonal cell lines in which the entire population of cells exhibited a more homogeneous reduction in surface expression of class I products (Fig. 8B). Even this clonal population of cells does not appear entirely homogeneous. Although the retrovirally transduced cell lines were maintained under selective pressure, for unknown reasons, surface expression of class I molecules in this clonal population increased with time in culture and later altogether discontinued expression of U21. U21 appears specific for MHC class I molecules, since the level of surface transferrin receptors in U21-expressing cells was indistinguishable from that in control cells (Fig. 8C).
FIG. 8.
MHC class I surface expression is downregulated in U21 cells. (A) Control U373 cells (red) or U21-expressing astrocytoma cells (blue) were incubated with a FITC-conjugated anti-HLA-A-B-C Ab and analyzed by flow cytometry. Fluorescence-activated cell sorter profiles of cells incubated without FITC-conjugated Ab are in black. (B) A clonal population of U21-expressing cells is indicated by the green trace. (C) Surface expression of transferrin receptor is unaffected by U21 expression.
DISCUSSION
Here we demonstrated degradation of MHC class I molecules in cells infected with HHV-7. We identified the HHV-7 U21 gene product as the protein responsible for this degradation. The U21-encoded protein is a transmembrane glycoprotein that associates tightly with properly folded MHC class I molecules shortly after synthesis in the ER. Expression of U21 results in the sequestration of class I molecules to a lysosomal compartment and presumably reduces the number of class I molecules available to present antigen to CTLs at the cell surface.
The identification of the U21 gene as the MHC class I binding protein was based on its molecular weight and the number of predicted N-linked glycans in its primary sequence, both of which were consistent with the properties of gp60 recovered in a complex with class I molecules. Infection of astrocytoma cells with a retrovirus specifying the production of U21 resulted in expression of a protein product indistinguishable from that recovered in HHV-7-infected cells, including its tight association with properly folded class I molecules. Antisera directed against a GST fusion protein with the predicted C terminus of U21 confirmed the identification of U21 as the correct gene.
There are no obvious predicted sequence homologies between U21 and any other protein, other than that of the U21 encoded by the colinear genome of HHV-6. Although HHV-6 U21 is approximately 50% conserved with HHV-7 U21 at the amino acid level, immunoprecipitations of MHC class I products have so far failed to yield coprecipitation of U21 from HHV-6-infected cells.
The m06 gene product from murine cytomegalovirus (MCMV) has been shown to bind to and redirect MHC class I molecules to lysosomes (30). HHV-7 U21 appears functionally similar to the mCMV m06 gene product, although the two proteins share no homology. The hydropathy profile of m06 suggests that it, too, is a type I membrane protein. Both the m06 and U21 gene products possess multiple N-glycosylation sites. U21 binds to a similar array of class I gene products as the HLA-A-B-C–reactive W6/32 Ab, and expression of U21 in astrocytoma cells results in degradation of all of them. Presumably MCMV m06 also displays no MHC allele specificity in its binding, since m06 expression results in surface downregulation of multiple class I alleles in NIH 3T3 cells (30).
MCMV m06 possesses within its C-terminal tail a dileucine sorting signal that is responsible for targeting the m06 class I complex to lysosomes (30). Most lysosomal targeting signals contain a tyrosine, a dileucine sequence, or clusters of acidic amino acids (10, 16, 21, 27, 28, 36; for reviews, see references 20 and 23) (Table 2. ). The C terminus of U21 possesses none of these sequences. A dilysine sequence, usually located at the −3 and −4 positions from the extreme carboxyl terminus of a protein, is responsible for ER retention of some ER resident proteins (18). U21 does possess a pair of lysines in its C terminus, but these are not at positions −3 and −4. The metabotropic glutamate receptor contains a dilysine ER retention sequence that is not located at the extreme C terminus of its cytoplasmic tail (11), but this retention sequence also requires the presence of two proximal arginine residues (RRKK). The two lysines in the cytoplasmic tail of U21 are not adjacent to arginine residues. Thus, unlike MCMV m06, U21 does not seem to possess an obvious predicted sorting signal in its cytoplasmic tail.
TABLE 2.
Examples of known cytoplasmic sorting signalsa
| Signal | Protein | Sequenceb | Localizationc |
|---|---|---|---|
| Tyrosine based (NPXY) | LDL receptor | TM-12 aa-FDNPVY-32 aa | Internalization |
| Tyrosine based (YXXΦ) | Tfn receptor | 17 aa-LSYTRF-45 aa-TM | Internalization |
| TGN38 | TM-24 aa-SDYQRLNLKL-COOH | TGN | |
| Lamp-1 | TM-RKRSHAGYQTI-COOH | Lysosomes | |
| Lamp-2 | TM-KSIRSGYEVM-COOH | Lysosomes | |
| HLA-DMβ | TM-WRRAGHSSYTPLP-COOH | MIIC | |
| PIgR | TM-79 aa-LAYSAF-141 aa | Basolateral PM | |
| CD-MPR | TM-24 aa-YKYSKV-135 aa | Late endosomes | |
| CI-MPR | TM-42 aa-AAYRGV-19 aa | Late endosomes | |
| Dileucine based | CI-MPR tail | TM-24 aa-LVSFHDDSDEDLLHI-COOH | Late endosomes |
| CD-MPR tail | TM-42 aa-LGEESEERDDHLLPM-COOH | ||
| Ii | NH-MDDQRDLISNNEQLPMLGRR-COOH | MIIC | |
| Acidic clusters | Furin | TM-28 aa-EAWQEECPSDSEEDE-15 aa | TGN |
| Dilysine (KKFF) | ERGIC-53 | -RSQQEAAAKKFF-COOH | Intermediate compartment |
| Adenovirus E3/19K | -KYKSRRSFIDEKKMP-COOH | ER | |
| mGLUR-1 | -FLNIFRRKKPGAGNAKKRQ-16 aa | ER |
The HHV-7 U21 C-terminal sequence is TM-SCLDGVQKVLTWPLQHIQKEPVSEKIINLTNLMFGQEPLPKKESLKQQCL-COOH. Modified from tables in Kirchhausen et al. (20) and Marks et al. (23).
aa, amino acids; TM, transmembrane region; COOH, C terminus.
TGN, trans-Golgi network; PM, plasma membrane; MIIC, MHC class II compartment.
U21 acquires endo H resistance after a 30-min chase period (Fig. 2A), and U21-associated class I molecules acquire sialic acids within the same time period (Fig. 4B), indicating that U21 proceeds in complex with class I molecules at least as far as the trans-Golgi. Its tight association with lysosome-destined MHC class I molecules suggests that U21 accompanies class I to lysosomes. Furthermore, in U21-expressing astrocytoma cells treated with ConB, the U21-class I complex is stabilized, suggesting that both U21 and MHC class I are degraded together in an acidic compartment (Fig. 5B). The possibility that U21 might shuttle between the trans-Golgi network and an acidic compartment to accomplish delivery of class I molecules to lysosomes deserves to be examined further.
We suggest that the HHV-7 gene product U21 functions in the course of the HHV-7 life cycle to divert MHC class I molecules to the lysosomes for degradation and in so doing allows the HHV-7-infected cell to bypass surveillance by CTLs. The specific mechanism by which U21 redirects class I molecules remains to be elucidated. It is not clear whether U21 accompanies class I molecules to the cell surface or whether it diverts class I molecules directly to the lysosome from the Golgi, though the absence of class I molecules from the plasma membrane of U21-expressing cells at steady state suggests that the diversion is less likely to involve trafficking via the plasma membrane. U21 may contain a novel lysosomal targeting sequence, or, equally intriguing, it may cause a conformational change in the class I molecule that prevents its interaction with a cellular trafficking protein, or act as an adapter to connect MHC class I molecules to a cellular protein that directs it to lysosomes.
ACKNOWLEDGMENTS
We thank Phil Pellett (Centers for Disease Control and Prevention) and Jodi Black (National Institutes of Health) for the HHV-7-infected cells, HHV-7-specific antisera, and helpful discussions and Domenic Tortorella, Susanne Wells, and Patricio Meneses for critical reading of the manuscript and helpful discussions.
A.W.H. is supported by a fellowship from the Irvington Institute for Immunological Research (New York, N.Y.). This work was supported by NIH grant PO1-AI-42257 (to P.M.H. and H.L.P.).
REFERENCES
- 1.Ablashi D V, Handy M, Bernbaum J, Chatlynne L G, Lapps W, Kramarsky B, Berneman Z N, Komaroff A L, Whitman J E. Propagation and characterization of human herpesvirus-7 (HHV-7) isolates in a continuous T-lymphoblastoid cell line (SupT1) J Virol Methods. 1998;73:123–140. doi: 10.1016/s0166-0934(98)00037-8. [DOI] [PubMed] [Google Scholar]
- 2.Ahn K, Angulo A, Ghazal P, Peterson P A, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 4.Alcami A, Koszinowski U H. Viral mechanisms of immune evasion. Trends Microbiol. 2000;8:410–418. doi: 10.1016/S0966-842X(00)01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano Y, Suga S, Yoshikawa T, Yazaki T, Uchikawa T. Clinical features and viral excretion in an infant with primary human herpesvirus 7 infection. Pediatrics. 1995;95:187–190. [PubMed] [Google Scholar]
- 6.Barnstable C J, Bodmer W F, Brown G, Galfre G, Milstein C, Williams A F, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens: new tools for genetic analysis. Cell. 1978;14:9. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 7.Berneman Z N, Ablashi D V, Li G, Eger-Fletcher M, Reitz M S, Jr, Hung C L, Brus I, Komaroff A L, Gallo R C. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci USA. 1992;89:10552–10556. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black J B, Burns D A, Goldsmith C S, Feorino P M, Kite-Powell K, Schinazi R F, Krug P W, Pellett P E. Biologic properties of human herpesvirus 7 strain SB. Virus Res. 1997;52:25–41. doi: 10.1016/s0168-1702(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 9.Black J B, Pellett P E. Human herpesvirus 7. Rev Med Virol. 1999;9:245–262. doi: 10.1002/(sici)1099-1654(199910/12)9:4<245::aid-rmv253>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Canfield W M, Johnson K F, Ye R D, Gregory W, Kornfeld S. Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24–29 of the cytoplasmic tail. J Biol Chem. 1991;266:5682–5688. [PubMed] [Google Scholar]
- 11.Chan W Y, Soloviev M M, Ciruela F, McIlhinney R A. Molecular determinants of metabotropic glutamate receptor 1B trafficking. Mol Cell Neurosci. 2001;17:577–588. doi: 10.1006/mcne.2001.0965. [DOI] [PubMed] [Google Scholar]
- 12.Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci USA. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert M J, Riddell S R, Plachter B, Greenberg P D. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 15.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 16.Honing S, Griffith J, Geuze H J, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- 17.Ishido S, Wang C, Lee B S, Cohen G B, Jung J U. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson M R, Nilsson T, Peterson P A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones T R, Wiertz E J, Sun L, Fish K N, Nelson J A, Ploegh H L. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhausen T, Bonifacino J S, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 21.Letourneur F, Klausner R D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 22.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner R D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 23.Marks M S, Ohno H, Kirchhausen T, Bonifacino J S. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 24.Megaw A G, Rapaport D, Avidor B, Frenkel N, Davison A J. The DNA sequence of the RK strain of human herpesvirus 7. Virology. 1998;244:119–132. doi: 10.1006/viro.1998.9105. [DOI] [PubMed] [Google Scholar]
- 25.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Ohno H, Stewart J, Fournier M C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 28.Peters C, Braun M, Weber B, Wendland M, Schmidt B, Pohlmann R, Waheed A, von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990;9:3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portolani M, Cermelli C, Mirandola P, Di Luca D. Isolation of human herpesvirus 7 from an infant with febrile syndrome. J Med Virol. 1995;45:282–283. doi: 10.1002/jmv.1890450307. [DOI] [PubMed] [Google Scholar]
- 30.Reusch U, Muranyi W, Lucin P, Burgert H G, Hengel H, Koszinowski U H. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18:1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson P G, Efstathiou S, Doherty P C, Lehner P J. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc Natl Acad Sci USA. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka K, Kondo T, Torigoe S, Okada S, Mukai T, Yamanishi K. Human herpesvirus 7: another causal agent for roseola (exanthem subitum) J Pediatr. 1994;125:1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- 33.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 34.Tortorella D, Gewurz B E, Furman M H, Schust D J, Ploegh H L. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 35.van M. de Rijn, Geurts van Kessel A H, Kroezen V, van Agthoven A J, Verstijnen K, Terhorst C, Hilgers J. Localization of a gene controlling the expression of the human transferrin receptor to the region q12 leads to qter of chromosome 3. Cytogenet Cell Genet. 1983;36:525–531. doi: 10.1159/000131967. [DOI] [PubMed] [Google Scholar]
- 36.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiertz E J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 38.Wyatt L S, Rodriguez W J, Balachandran N, Frenkel N. Human herpesvirus 7: antigenic properties and prevalence in children and adults. J Virol. 1991;65:6260–6265. doi: 10.1128/jvi.65.11.6260-6265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilla M, Tan A, Ito K, Miwa K, Ploegh H L. Involvement of the vacuolar H+-ATPases in the secretory pathway of HepG2 cells. J Biol Chem. 1993;268:19092–19100. [PubMed] [Google Scholar]
- 40.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski U H. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]