Abstract
Cellular effector function assays traditionally rely on bulk cell populations that mask complex heterogeneity and rare subpopulations. The Xdrop® droplet technology facilitates high-throughput compartmentalization of viable single cells or single-cell pairs in double-emulsion droplets, enabling the study of single cells or cell–cell interactions at an individual level. Effector cell molecule secretion and target cell killing can be evaluated independently or in combination. Compatibility with a wide range of commercial assay reagents allows for single-cell level readouts using common laboratory techniques such as flow cytometry or microscopy. Moreover, individual cells of interest can be viably isolated for further investigation or expansion. Here we demonstrate the application of the double-emulsion droplet technology with a range of cell types commonly utilized for adoptive cell therapy of cancer: natural killer cells, blood-derived T cells, tumor-infiltrating lymphocytes, and chimeric antigen receptor T cells. Single-cell compartmentalization offers unparalleled resolution, serving as a valuable tool for advancing the development and understanding of cellular therapy products.
Key words: Xdrop, cancer immunotherapy, single-cell analysis, cell therapy, microfluidics, cell characterization
Highlights
-
•
High-throughput single-cell microfluidics assays are routine with Xdrop® technology.
-
•
Single-cell Xdrop® assays are compatible with common effector cell types.
-
•
Viable cells with specific functions can be isolated from Xdrop® single-cell assays.
Background
Adoptive cell therapies (ACT) have demonstrated impressive curative effects against specific cancer types, as illustrated by the success of chimeric antigen receptor (CAR) T cells for hematological malignancies1,2 and expanded tumor-infiltrating lymphocytes (TILs) for advanced melanoma.3,4 Natural killer (NK) and γδ T cells are now also being extensively explored as potential immunotherapies.5,6 While both the utilization and impact of cellular therapies are expected to increase in the future, challenges persist in predicting their potency and improving treatment efficacy.
Our understanding of what drives clinical responses to ACT has evolved over time, in TILs progressing from initial studies highlighting increased numbers of CD8+ T cells in infusion products7 to more nuanced recent studies emphasizing the importance of neoantigen-specific8 and stem-like9 CD8+ T cells instead. Together with the incredible success of CAR-T cell clinical trials,10,11 where much fewer cells are administered, these findings suggest that small subpopulations of ACT products drive the antitumor effects.
Given that ACT products are comprised of effector cells considered to be primary mediators of direct tumor killing, a logical assumption is that highly potent cells will react strongly against their targets and could form an effective ACT product on their own. Such potential is often measured via functional responses to target cells e.g. cytokine secretion or cytotoxic ability. These efforts are dominated by bulk population assays which can often obscure these critical populations of interest. Single-cell RNA sequencing has alleviated this issue to some degree; however, its utility is limited by cost, frequent lack of RNA-to-protein-to-function translatability,12 and inability to select cells of interest for further assays or clinical use. Selection of viable effector cells for further applications is currently achievable via fluorescence and magnetic-activated cell sorting (FACS/MACS). These methodologies are limited to using surface molecules as surrogates of cellular activation and function or secretome-capture methods affected by cytokine crosstalk or requiring complex custom experimental equipment. Accurate identification and selection of rare, potent, and viable effector cells is therefore currently challenging, restricting their subsequent downstream study and potential generation of selectively expanded ACT products.
The Xdrop double-emulsion droplet technology highlighted here offers a solution by providing a standardized and user-friendly high-throughput platform for single-cell study of effector cell functions, such as direct tumor killing or cytokine secretion. The technology is compatible with downstream analysis and selective expansion of viable cells after assay.
The Xdrop technology
Few technologies can accommodate single-cell assessment of cytotoxicity or cytokine secretion,13, 14, 15, 16, 17 often requiring extensive microfluidics know-how and specialized equipment. The Xdrop instrument addresses these issues. The double-emulsion droplet technology facilitates high throughput compartmentalization of viable single cells or cell pairs in droplets, allowing the study of individual cells or cell–cell interactions over time (Figure 1). The technology uses the same droplet encapsulation technology previously employed for microbes and DNA,18,19 instead utilizing a larger 50 μm diameter double-emulsion droplet (DE50) to accommodate eukaryotic cells.
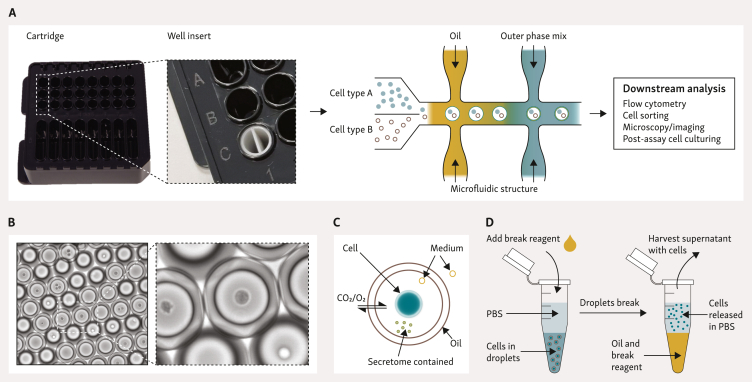
Figure 1.
Overview of DE50 droplet generation and characteristics. (A) Workflow for DE50 droplet generation and potential analyses. Microfluidic DE50 cartridges are loaded with oil, outer phase mix, and cells, covered with a gasket, and placed into the Xdrop instrument. Up to eight productions can be run in parallel per cartridge, with a run time of ∼8 min. When using two different cell types, the Xdrop Well Insert ensures separation of effector and target cells until DE50 droplet encapsulation. Additional assay reagents are added to the cell suspension according to the user’s experimental needs. DE50 droplets are harvested from the cartridge and incubated as necessary whilst the functional assay of choice occurs individually within each droplet. Downstream analysis can be conducted at various time points with a range of potential readouts. (B) Microscopy image of DE50 droplets containing cells. (C) Schematic diagram of DE50 droplet structure, including passive diffusion of smaller molecules to sustain viability and the retention of the secretome and cell/cells within the droplet. (D) Procedure for droplet breakage and recovery of cells. PBS, phosphate-buffered saline.
Before encapsulation, cells are stained with fluorescent cell-labelling dyes to facilitate deconvolution during analysis. During the production process, DE50 droplets can also be loaded with standard cell culture media, varied cell types, and supplementary reagents (e.g. viability stains and stimulatory molecules) tailored to specific readout requirements. The droplets are stable after production and can be transferred to common cell culturing plastic containers by pipetting. Droplets are kept in standard CO2 incubators to maintain viability, and we have observed as high as ∼80% viability 24 h after encapsulation. Decreases in viability over time are observable, although the extent of this is highly cell type-dependent. Assay optimization and monitoring of cell viability in samples is recommended to minimize background signals.
Analysis of droplets can be conducted at specific time points of interest using conventional cell analysis methods such as microscopy and flow cytometry. Continued culturing of cells after assay is possible by treating droplets with a break reagent (Figure 1D), similar to the Xdrop DE20 targeted enrichment procedure for recovery of DNA.18,19 The current droplet break procedure results in cell recovery of up to 60% with a viability >95%.
Detection and enrichment of potent cytokine secretors
Cytokine secretion upon stimulation is commonly detected within culture medium through singleplex [e.g. enzyme-linked immunosorbent assay (ELISA), enzyme-linked immunosorbent spot (ELISpot)] or elaborate multiplexed approaches (e.g. bead-based immunoassays). These assays, however, do not permit single-cell deconvolution. An alternative is intracellular cytokine staining which provides single-cell resolution, yet by nature measures intracellular accumulation of cytokines rather than actual secretion. Importantly, none of these approaches allow the viable isolation of specific cells.
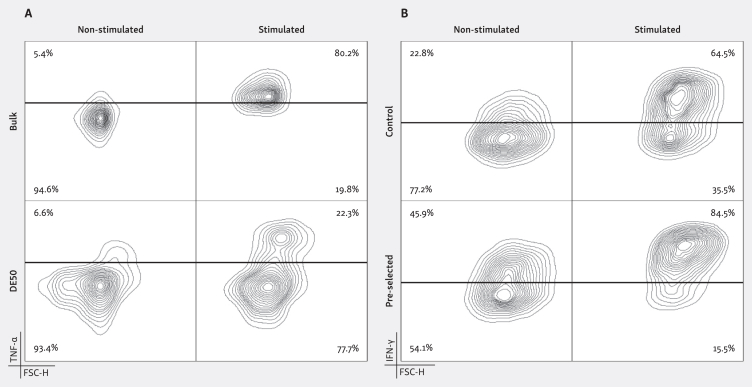
Contemporary detection technologies have evolved to capture secreted cytokines on the surface of the secreting cell without impacting viability, potentially providing a solution to the described challenges.20 Such approaches are vulnerable to cytokine crosstalk, however, where an excess of produced cytokine or premature saturation of capture reagents can cause false-positive and high background signals. It has previously been demonstrated that these pitfalls can be avoided by encapsulating assay reagents in microfluidic droplets,15,17 and cytokine secretion assays can similarly be carried out within DE50 droplets by loading detection reagents and single cells with capture antibody. For example, the increased resolution reveals a distinct high tumor necrosis factor-α (TNF-α)-producing subpopulation of T cells previously masked in a parallel bulk assay21 (Figure 2A), highlighting the importance of such single-cell resolution for identification of rare subpopulations.
Figure 2.
Detection and viable isolation of potent cytokine secreting effector cells using DE50 droplets. (A) T cells were labelled with TNF-α capture reagents and split into four groups: bulk samples (+/− PMA/ionomycin) and DE50 encapsulated samples (+/− PMA/ionomycin) and incubated for 4 h. For DE50 samples the TNF-α detection reagent was added during encapsulation whereas for bulk samples the detection reagent was added after the incubation. After incubation, cells from DE50 droplets were released using droplet break reagent and all samples were stained with α-CD3-PerCP and assessed by flow cytometry. The added resolution from the DE50 encapsulation reveals a high TNF-α-producing T-cell subpopulation not detectable when performing the assay in bulk21 (>3500 cells analyzed per plot). (B) NK cells were stimulated with IL-2 and labelled with IFN-γ capture reagent before being encapsulated in DE50 droplets together with IFN-γ detection reagent. After 4 h of incubation all droplets were broken as for (A) and high IFN-γ-secreting cells were enriched (FACS). Cells were then cultured for an additional 2 weeks, with control non-stimulated cells cultured in parallel. IFN-γ secretion following +/− IL-2 stimulation in the DE50 assay was then measured for both the non-enriched and enriched populations. The pre-selected population retained their enhanced IFN-γ-secreting abilities in comparison to the non-enriched control, confirming the successful isolation and subsequent expansion of effectors with an indicated increased potency22 (>14 000 cells analyzed per plot). FACS, fluorescence-activated cell sorter; FSC-H, forward scatter height; IFN, interferon; IL-2, interleukin 2; NK, natural killer; PMA, phorbol myristate acetate; TNF, tumor necrosis factor.
The translation of such increased resolution into downstream applications, such as deeper analysis or preferential expansion of the most potent secretors during ACT product generation, is often limited by technical constraints. The ability to break DE50 droplets and successfully recover viable cells helps bridge this gap, as demonstrated with cytokine-stimulated NK cells22 (Figure 2B). Following a DE50 interferon-γ (IFN-γ) assay, NK cells were released from droplets and a potent IFN-γ-secreting subpopulation of NK cells was sorted by fluorescence-activated cell sorter (FACS) and cultured in vitro for an additional 2 weeks. Subsequent analysis revealed that potent IFN-γ secretors retained their superior secretory abilities upon re-stimulation.22
Detection of effective target cell killers
Cytotoxicity assays are rational markers of effector cell potency that reflect the in vivo mode of action, a crucial feature for ACT product potency assays as noted in recent proposed Food and Drug Administration guidance documentation.23 Isotopic chromium release, bioluminescence, impedance, and flow cytometry-based assays are all commonly employed; however, the observed effect cannot be linked to specific individual cells. The observed cytotoxic abilities may therefore be due to confounding factors such as highly potent serially killing effectors16,24 masking a lack of cytotoxicity in the bulk of the population. Instead, DE50 droplets provide the single-cell resolution required to appropriately assess the cytotoxic potential of ACT products and potentially isolate this population.
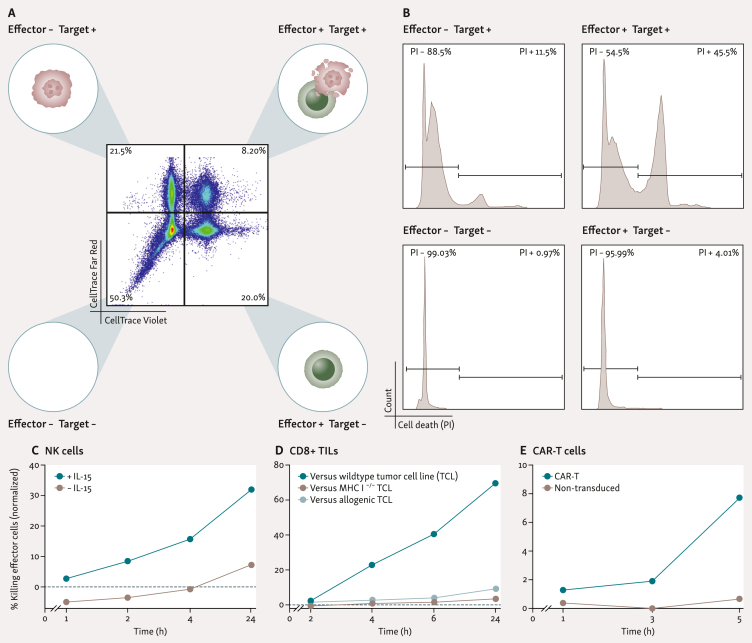
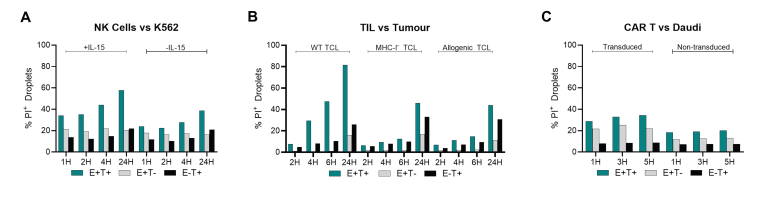
To quantify and identify effective killers, target and effector cells are labelled with distinct dyes and dead cell-staining propidium iodide (PI) is added immediately before encapsulation in DE50 droplets. The use of these dyes enables clear identification of droplet contents and the real-time labelling of newly dead cells throughout the incubation (Figure 3A and B). Calculation of true target cell killing utilizes the observed death in single-cell encapsulations (effector-only and target-only) to account for ‘background cell death’. This single-cell level cytotoxicity assay is compatible with a wide range of ACT-relevant cell types, including NK cells,25 TILs, and CAR-T cells (Figure 3C-E and Supplementary Figure S1, available at https://doi.org/10.1016/j.iotech.2024.100738).
Figure 3.
Cross-cell type compatibility of DE50 droplets with propidium-iodide-based cytotoxicity assays. (A) Distinct fluorescent labelling of effector and target cells before encapsulation in DE50 droplets allows deconvolution of droplet composition: effector− target−, effector− target+, effector+ target−, and target+ effector+. (B) Addition of propidium iodide (PI) to the encapsulation process allows detection of cell death as it occurs within droplets, which can be quantified within each unique droplet sub-population (those defined in A) during analysis to determine background cell death and true effector-induced target cell death. (C-E) Representative plots of effector cell-mediated killing of target cells in the described cytotoxicity assay, adjusted for non-cytotoxicity-related background cell death. (C) NK cells +/− IL-15 versus K562 target cells.25 (D) Expanded (rapid expansion process) CD8+ TILs versus autologous tumor cell line (TCL), allogenic TCL, or MHCI-deficient (B2M−) TCL (E) CD19-specific CAR-T31 (98% CAR+) or non-transduced T cells versus CD19+ Daudi target cells. All analyses conducted with flow cytometry. Cytotoxicity at each time point was calculated by normalizing the observed death in co-encapsulating droplets (minus background cell death) to all co-encapsulating droplets (minus background cell death): percentage of cytotoxic effector cells at time t(x) = [observed deatht(x) − background deatht(x)]/[100 − background deatht(x)]. Here background death is a measure for the death occurring inside co-encapsulated target and effector cells droplets not because of killing but rather resulting from other causes occurring within droplets or before encapsulation. The background death is estimated by measuring cell death in droplets with target cells only and effector cells only at all analysis time points. Background deatht(x) = [1 − (1 − death ratio in target-cell-only-droplets) × (1 − death ratio in effector-cell-only-droplets)] × 100 = [1 − (1 − %TCDt(x)/100) × (1 − %ECDt(x)/100)] × 100, where % TCD and ECD denote the % cell death measured at time x in target-cell-only droplets and effector-cell-only droplets, respectively. Original cell death values for each individual droplet population are plotted in Supplementary Figure S1, available at https://doi.org/10.1016/j.iotech.2024.100738. CAR-T cell, chimeric antigen receptor T-cell; IL-15, interleukin 15; MHCI, major histocompatibility complex class I; NK, natural killer; TILs, tumor-infiltrating lymphocytes.
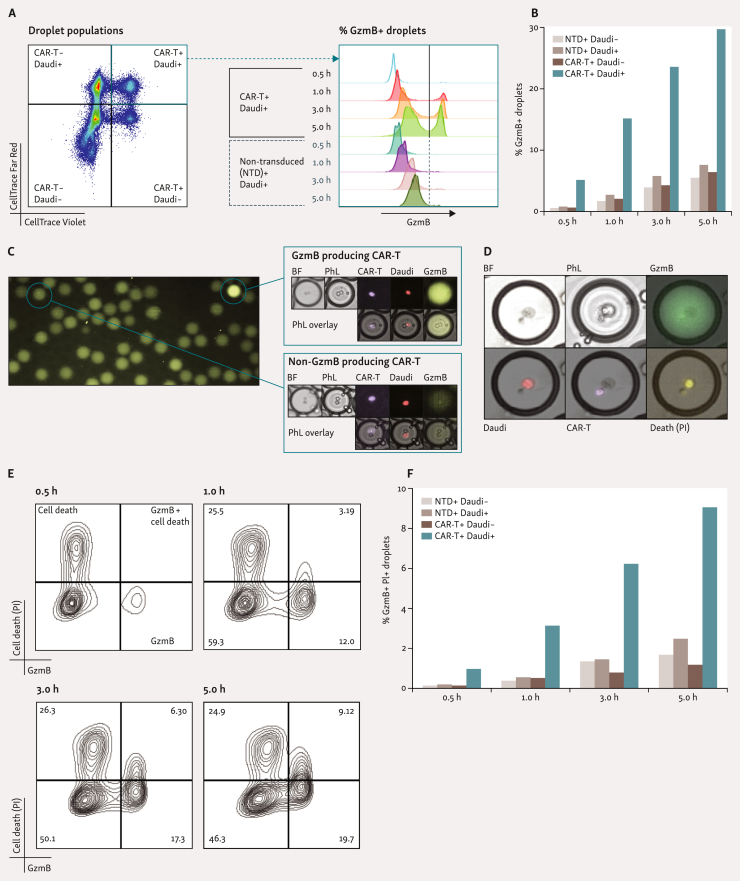
Alternative readouts of cytotoxicity can also be employed in the DE50 droplet setup. Cytotoxic T, CAR-T, and NK cells all utilize rapid release of pre-formed cytotoxic effector proteins (chiefly perforin and granzymes) as their primary target killing mechanism.26 In bulk cytotoxicity assays, target recognition-dependent secretion of granzyme B (GzmB) is utilized as a reliable measure of effector cell reactivity and as a surrogate of cytotoxic ability.27,28 The addition of a cleavage-triggered fluorescent GzmB substrate to the encapsulation process facilitates the rapid detection of its release,29 as exemplified here with CAR-T cells (Figure 4A-C). The granularity of this assay, and subsequent identification of highly potent effectors, can be achieved via combination with the PI-based cytotoxicity assay described above.29 Using CAR-T cells (>98% CAR+), we demonstrate the simultaneous detection of GzmB secretion and cell killing in DE50 droplets (Figure 4D-F). This combination reveals that not all GzmB+ encapsulations result in rapid target-cell killing, emphasizing the value of integrating multiple parameters for enhanced single-cell insights.
Figure 4.
Identification of potent effector cells via detection of granzyme B secretion. (A and B) The addition of a fluorescent-tagged granzyme B (GzmB) substrate, which fluoresces upon cleavage of the substrate, during the encapsulation process allows detection of GzmB secretion within DE50 droplets,29 as demonstrated with CD19-specific CAR-T31 (98% CAR+) and non-transduced (NTD) T cells versus CD19+ Daudi target cells. (C) Representative microscopy images of CAR-T+ Daudi+ DE50 droplets demonstrating the presence or lack of GzmB production. (D-F) Combination of the GzmB assay with the PI-based cytotoxicity assay29 described in Figure 3 provides additional functional information regarding the cell-killing ability of effector cells, as shown with CAR-T/Daudi pairs. (E) Highlights the likely cell killing mechanics at play. GzmB secretion occurs early (lower right quadrant) and precedes death resulting from cell killing (upper right quadrant). The change in the number of cells dead before encapsulation or dying from other causes than GzmB-induced killing throughput the assay is negligible and remains constant (upper left quadrant). A, B, E, and F analyzed via flow cytometry. More than 6.1 × 103 droplets were analyzed per plot. (F) Quantitation of NTD+ Daudi−, NTD+ Daudi+, CAR-T+ Daudi− and CAR-T+ Daudi+ co-encapsulating droplets with both GzmB and cell death (GzmB+ PI+). BF, bright-field; CAR-T, chimeric antigen receptor T-cell; PhL, phase contrast (Nikon Eclipse Ts2R); PI, propidium iodide.
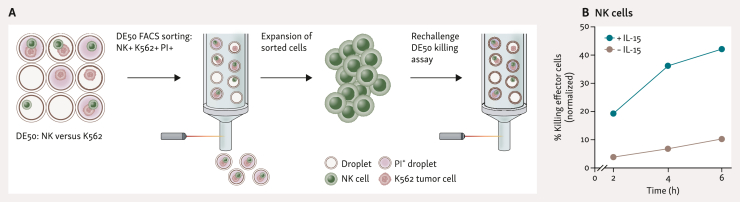
Isolation and expansion of cells with indicated killing ability is of great interest for improving ACT products. Our preliminary studies with DE50 NK/K562 cell co-encapsulations demonstrate the potential feasibility of applying the Xdrop technology to this context (Figure 5). Intact PI+ droplets were FACS sorted and the recovered NK cells cultured in vitro. Cytotoxic ability was then retested in another DE50 droplet cytotoxicity assay where the sorted NK cells clearly demonstrated their retained cell-killing ability. The experiment applied adjusted sorting conditions which typically yield sorting efficiencies of 40%-60% and purity >95%; however, sorting can be further optimized to obtain 80%-90% efficiency.
Figure 5.
Isolation and expansion of functionally active cytotoxic NK cells. (A) NK cells were co-encapsulated with K562 cells using the same procedure as in Figure 3. After 5 h the PI+ fraction of NK+ K562+ droplets was FACS sorted and the recovered cells were expanded for >3 weeks. CD56+ cells were purified (MACS-based separation), expanded for an additional 10 days, and then restimulated with K562 cells and +/− IL-15 in a co-encapsulation assay. (B) Cytotoxicity measurement of sorted and expanded NK cells, carried out as in (A), demonstrating cytotoxic ability of sorted cells. FACS, fluorescence-activated cell sorter; IL-15, interleukin 15; MACS, magnetic activated cell sorting; NK, natural killer; PI, propidium iodide.
Conclusion and future perspectives
The double-emulsion droplet technology presented here is a robust single-cell level platform that overcomes many current limitations in detecting highly potent effector cells within ACT products and demonstrates significant potential for their isolation and continued expansion.
The highly customizable nature of the assay promotes flexibility across many cell types and potency parameters, and the ability to isolate viable truly potent effectors allows a wide range of downstream in-depth characterizations concerning general phenotype, proliferative ability, response to repeated stimulation, functional profiles, and other such attributes. Findings resulting from these kinds of studies are likely to inform the future development of improved ACT by guiding the production of highly potent infusion products. Revealing the true functional profiles of individual cells in ACT products may also produce advances in linking clinical responses to these traits, and in the future, there is substantial potential for direct downstream therapeutic ACT applications. In particular, the technology presents an exciting prospect for the generation of selective ACT products.
Although promising, the described technology has certain limitations. Droplets with more than two cells can occur and confound analyses, but this can be mitigated by optimizing loading ratios and concentrations as cell encapsulation follows a Poisson distribution.30 Genuine cell-pair interaction assays form a minority of the total droplet population, but this still represents a substantial number due to the high number of generated droplets (750 000 per sample). Importantly, the singlet encapsulating droplets facilitated by the Poisson distribution function are valuable assay controls avoiding the need for multiple parallel assay control productions.
Background cell death does occur throughout the assay, but this can be accounted for when calculating target cell killing and can be significantly reduced by optimizing pre-assay cell handling procedures for each cell type. The cytotoxicity assays presented here cannot definitively confirm which cell of the pair within the droplet has died when analyzed using flow cytometry; however, complementary imaging analyses can confirm the observed/expected trends, as demonstrated in Figure 4D. Alternatively, target cell lines engineered to express specific fluorescent markers (e.g. GFP/YFP/RFP) upon death are likely an effective strategy for clarifying any ambiguity. Continued expansion after assay may be affected by potential cytotoxic effects of the droplet break reagent, although suitable recovery is still possible as demonstrated. This issue may be especially problematic in the case of extremely rare populations, although effector cell expansion protocols often result in significant fold expansions from even minimal starting material. While the data presented here highlight the feasibility of direct sorting of droplets of interest, we believe the effectiveness may be improved through additional optimization.
In conclusion, the double-emulsion droplet technology has the potential to play a sizable role in properly understanding and harnessing highly potent effector cells in an ACT context.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT3.5 and Grammarly v1.2 in order to improve the readability of the manuscript. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Acknowledgements
The authors thank all patients who donated the clinical material used in this study. Written informed consent was provided by all patients before sample collection. Samples were collected from patients enrolled in clinical protocols at the National Center for Cancer Immune Therapy (CCIT-DK), Department of Oncology, Copenhagen University Hospital, Herlev, Denmark. We thank the healthcare personnel involved in these studies, in particular Eva Ellebaek, Rikke Andersen, and Troels Holz Borch. All procedures were approved by the Ethics Committee of the Capital Region of Denmark and national regulations for biomedical research (Ethical approval reference: H-20070020; Data Protection approval P-2021-303). The CD19-specific CAR T cell construct31 was generously provided by the Holt group at the BC Cancer Research Institute (Vancouver, Canada). GraphPad Prism v10 (GraphPad Software, Boston, Massachusetts, USA), and FlowJo™ v10.0 Software (BD Life Sciences, Ashland, Oregon, USA) were used for data analysis and figure generation. Samplix® and Xdrop® are registered trademarks of Samplix ApS.
Funding
This work was supported by the European Innovation Council [Project 190144395] to Samplix ApS. Salary for ACKR was supported by the Innovation Fund Denmark (3194-00037B), salary for MD was supported by Independent Research Fund Denmark (2034-00406B), and salary for CAC was supported by Lundbeck Foundation (R307-2018-3636).
Disclosure
ACKR, DLP, MJJ, MM, and PM are employees of Samplix ApS. MD has received access to research data from Bristol Myers Squibb and Genentech, and is an advisor for Achilles Therapeutics (past 2 years). All other authors have declared no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.iotech.2024.100738.
Contributor Information
C.A. Chamberlain, Email: christopher.aled.chamberlain@regionh.dk.
P. Mouritzen, Email: pmo@samplix.com.
Supplementary data
Supplementary Figure S1.
Supplementary Figure 1. Cross-cell type compatibility of DE50 droplets with propidium-iodide based cytotoxicity assays. (A-C) Individual viability values for each co-encapsulation condition from Figure 3C-E. These data demonstrate cell type-specific differences in cell death and the stability of non-killing associated viability throughout the assay timepoints.
References
- 1.Westin J.R., Oluwole O.O., Kersten M.J., et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389:148–157. doi: 10.1056/NEJMoa2301665. [DOI] [PubMed] [Google Scholar]
- 2.Maude S.L., Laetsch T.W., Buechner J., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohaan M.W., Borch T.H., van den Berg J.H., et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med. 2022;387:2113–2125. doi: 10.1056/NEJMoa2210233. [DOI] [PubMed] [Google Scholar]
- 4.Dafni U., Michielin O., Lluesma S.M., et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol. 2019;30:1902–1913. doi: 10.1093/annonc/mdz398. [DOI] [PubMed] [Google Scholar]
- 5.Laskowski T.J., Biederstadt A., Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22:557–575. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensurado S., Blanco-Dominguez R., Silva-Santos B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023;20:178–191. doi: 10.1038/s41571-022-00722-1. [DOI] [PubMed] [Google Scholar]
- 7.Radvanyi L.G., Bernatchez C., Zhang M., et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristensen N.P., Heeke C., Tvingsholm S.A., et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma. J Clin Invest. 2022;132 doi: 10.1172/JCI150535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna S., Lowery F.J., Copeland A.R., et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science. 2020;370:1328–1334. doi: 10.1126/science.abb9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappell K.M., Kochenderfer J.N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359–371. doi: 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J.H., Geyer M.B., Brentjens R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimegard J., Tarbier M., Danielsson M., et al. A combined approach for single-cell mRNA and intracellular protein expression analysis. Commun Biol. 2021;4:624. doi: 10.1038/s42003-021-02142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le K., Tan C., Gupta S., et al. A novel mammalian cell line development platform utilizing nanofluidics and optoelectro positioning technology. Biotechnol Prog. 2018;34:1438–1446. doi: 10.1002/btpr.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephides D., Davoli S., Whitley W., et al. Cyto-Mine: an integrated, picodroplet system for high-throughput single-cell analysis, sorting, dispensing, and monoclonality assurance. SLAS Technol. 2020;25:177–189. doi: 10.1177/2472630319892571. [DOI] [PubMed] [Google Scholar]
- 15.Antona S., Abele T., Jahnke K., et al. Droplet-based combinatorial assay for cell cytotoxicity and cytokine release evaluation. Adv Funct Mater. 2020;30 [Google Scholar]
- 16.Antona S., Platzman I., Spatz J.P. Droplet-based cytotoxicity assay: implementation of time-efficient screening of antitumor activity of natural killer cells. ACS Omega. 2020;5:24674–24683. doi: 10.1021/acsomega.0c03264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y., Brouchon J., Calvo-Calle J.M., et al. Droplet encapsulation improves accuracy of immune cell cytokine capture assays. Lab Chip. 2020;20:1513–1520. doi: 10.1039/c9lc01261c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blondal T., Gamba C., Møller Jagd L., et al. Verification of CRISPR editing and finding transgenic inserts by Xdrop indirect sequence capture followed by short- and long-read sequencing. Methods. 2021;191:68–77. doi: 10.1016/j.ymeth.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Madsen E.B., Hoijer I., Kvist T., Ameur A., Mikkelsen M.J. Xdrop: targeted sequencing of long DNA molecules from low input samples using droplet sorting. Hum Mutat. 2020;41:1671–1679. doi: 10.1002/humu.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng N., Mosmann T.R. Optimization of the cytokine secretion assay for human IL-2 in single and combination assays. Cytometry A. 2015;87:777–783. doi: 10.1002/cyto.a.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen MJ, Hussing C, Højmark RS, Frøsig TM, Mouritzen P, Mikkelsen MJ. Application Note: Identifying highly potent TNF-α-secreting T cells using the Xdrop® single-cell format. Samplix ApS; 2022. https://samplix.com/files/samplix/downloads/AppNotes/Identify%20TNFa_ver.01_SX-000699-AN.pdf [Google Scholar]
- 22.Jacobsen MJ, Amasia M, Hussing C, et al. Application Note: Revealing and retrieving highly potent IFN-γ secretors using an Xdrop single-cell format workflow based on double-emulsion droplets. Samplix ApS; 2023. https://samplix.com/files/samplix/downloads/AppNotes/IFN-y%20secretion_ver.01_SX-000701-AN.pdf [Google Scholar]
- 23.Food and Drug Administration; 2023. FDA guidance for industry: potency assurance for cellular and gene therapy products.https://www.fda.gov/media/175132/download [Google Scholar]
- 24.Davenport A.J., Jenkins M.R., Ritchie D.S., et al. CAR-T cells are serial killers. Oncoimmunology. 2015;4 doi: 10.1080/2162402X.2015.1053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen MJ, Peterson DL, Amasia M., et al. Application Note: Rapidly identifying active natural killer cells. Samplix ApS; 2023. https://samplix.com/files/samplix/downloads/AppNotes/Agilent_Samplix_AppNote_Cell_killing_assays_Nov2023.pdf [Google Scholar]
- 26.Voskoboinik I., Whisstock J.C., Trapani J.A. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 27.Packard B.Z., Telford W.G., Komoriya A., Henkart P.A. Granzyme B activity in target cells detects attack by cytotoxic lymphocytes. J Immunol. 2007;179:3812–3820. doi: 10.4049/jimmunol.179.6.3812. [DOI] [PubMed] [Google Scholar]
- 28.Glienke W., Dragon A.C., Zimmermann K., et al. GMP-compliant manufacturing of TRUCKs: CAR T cells targeting GD(2) and releasing inducible IL-18. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.839783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen DP, Jacobsen MJ, Schlicht B, Mikkelsen MJ, Mouritzen P. Application Note: Revealing granzyme B secretion and cell killing dynamics in a single-cell format. Samplix ApS; 2024. https://samplix.com/files/samplix/downloads/AppNotes/GranzymeB-ver.01_SSX-000649-AN.pdf [Google Scholar]
- 30.Cell distribution calculator. Samplix ApS; 2024. https://samplix.com/products/digital-tools [Google Scholar]
- 31.Kekre N., Hay K.A., Webb J.R., et al. CLIC-01: Manufacture and distribution of non-cryopreserved CAR-T cells for patients with CD19 positive hematologic malignancies. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.