Abstract
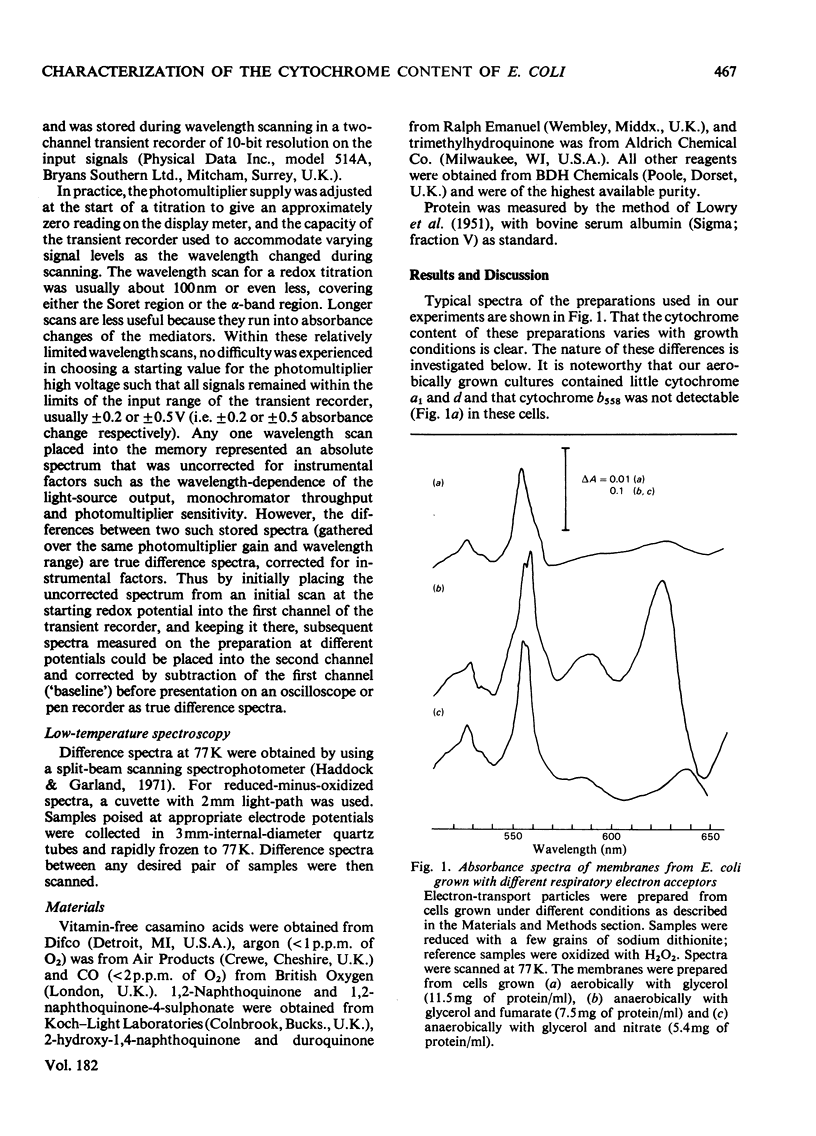
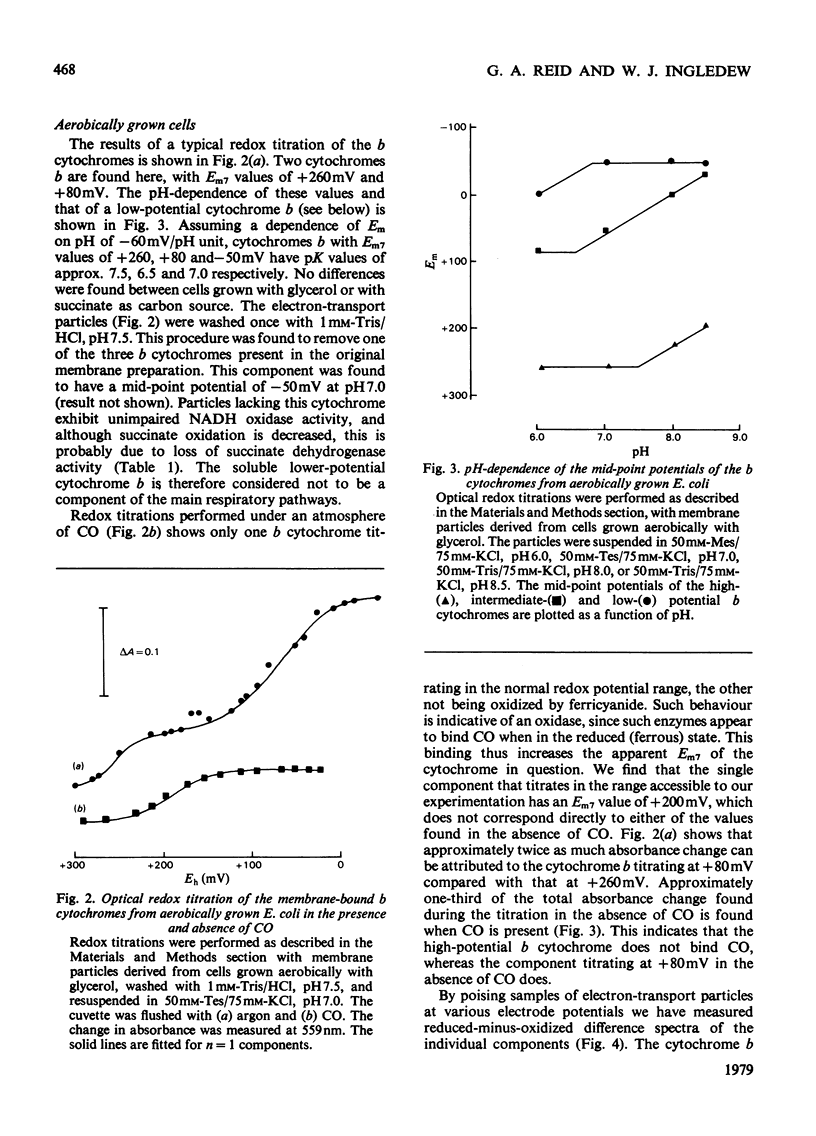
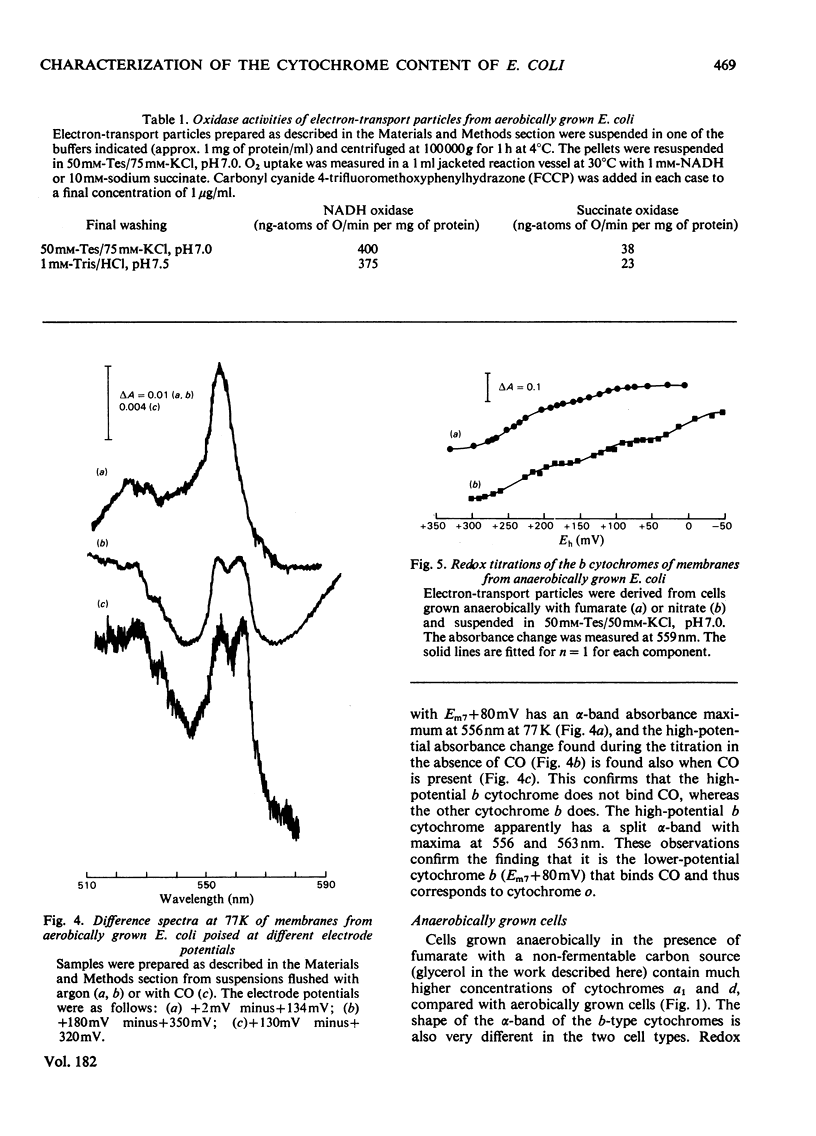
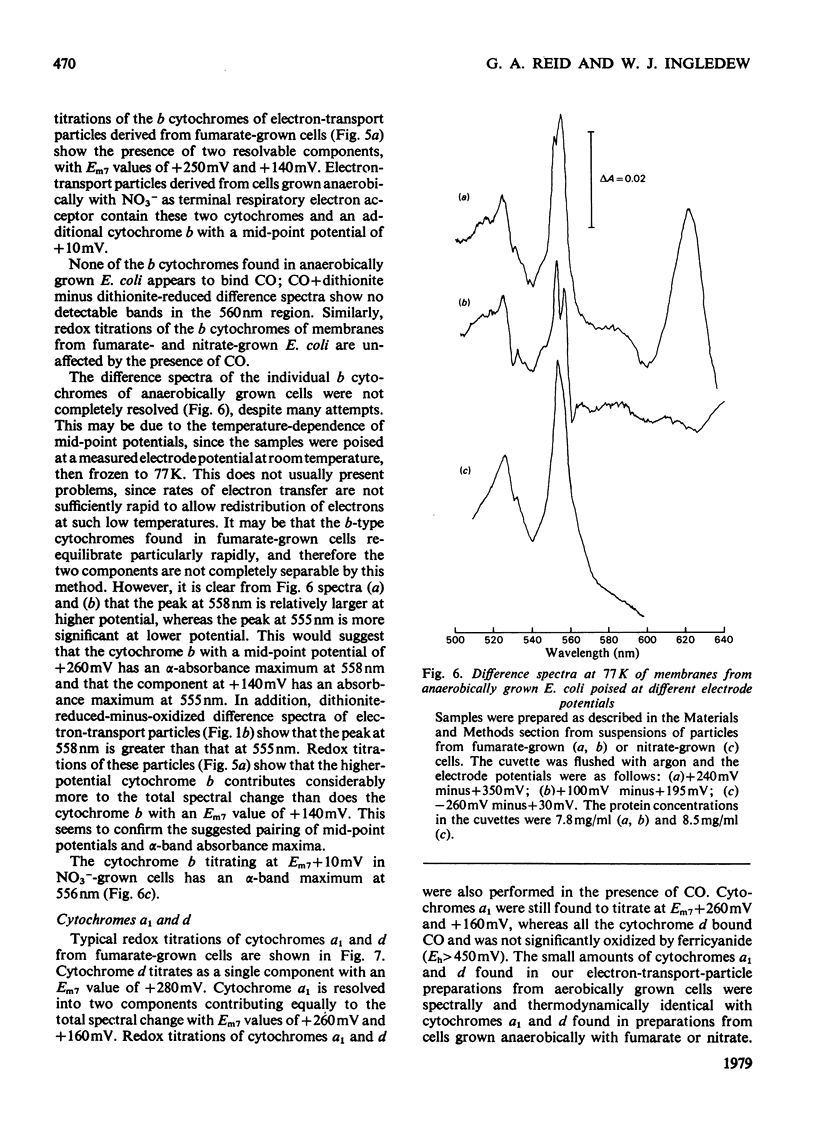
1. Electron-transport particles derived from Escherichia coli grown aerobically contain three b-type cytochromes with mid-point oxidation–reduction potentials at pH7 of +260mV, +80mV and −50mV, with n=1 for each. The variation of these values with pH was determined. 2. E. coli develops a different set of b-type cytochromes when grown anaerobically on glycerol with fumarate or nitrate as terminal electron acceptor. Electron-transport particles of fumarate-grown cells contain b-type cytochromes with mid-point potentials at pH7 of +140mV and +250mV (n=1). These two cytochromes are also present in cells grown with nitrate as terminal acceptor, where an additional cytochrome b with a mid-point potential of +10mV (n=1) is developed. 3. The wavelengths of the α-absorption-band maxima of the b-type cytochromes at 77K were: (a) for aerobically grown cells, cytochrome b (Em7 +260mV), 556nm and 563nm, cytochrome b (Em7 +80mV), 556nm and cytochrome b (Em7−50mV), 558nm; (b) for anaerobically grown cells, cytochrome b (Em7 +250mV), 558nm, cytochrome b (Em7 +40mV), 555nm and cytochrome b (Em7 +10mV), 556nm. 4. Cytochrome d was found to have a mid-point potential at pH7 of +280mV (n=1). 5. Cytochrome a1 was resolved as two components of equal magnitude with mid-point potentials of +260mV and +160mV (n=1). 6. Redox titrations performed in the presence of CO showed that one of the b-type cytochromes in the aerobically grown cultures was reduced, even at the upper limits of our range of electrode potentials (above +400mV). Cytochrome d was also not oxidizable in the presence of CO. Neither of the cytochromes a1 was affected by the presence of CO.
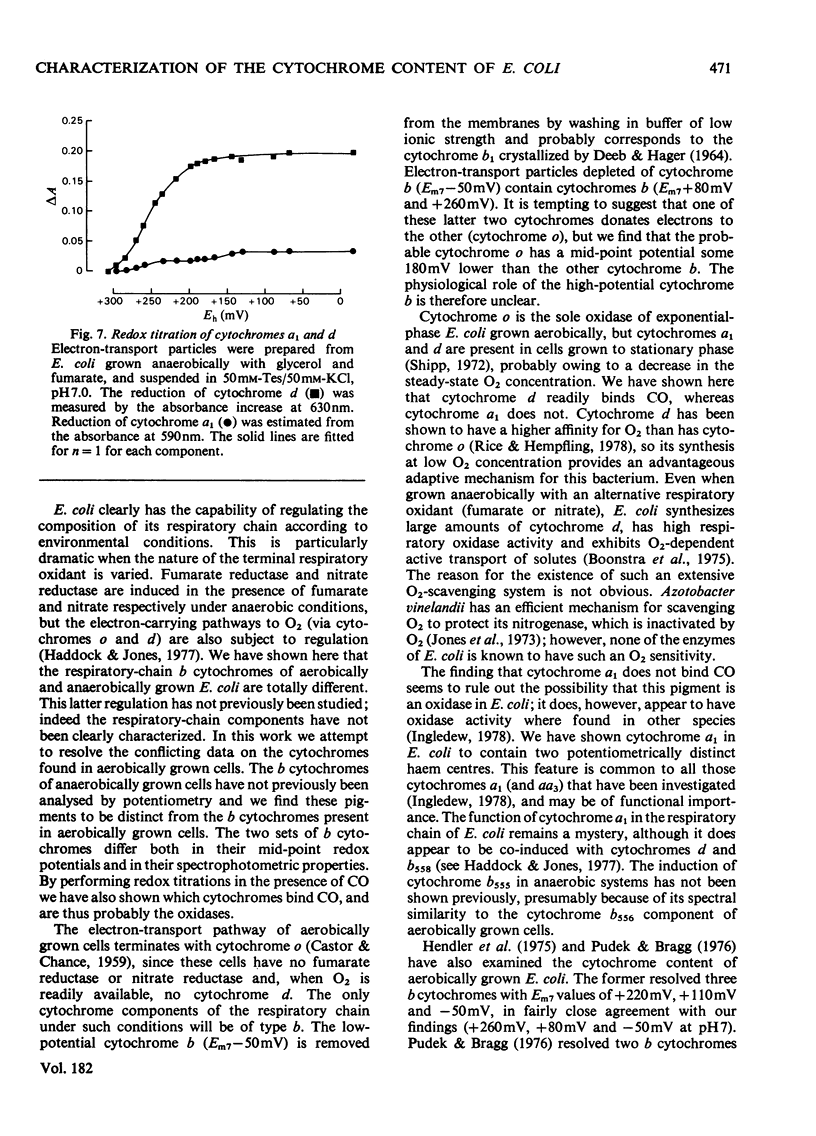
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boonstra J., Huttunen M. T., Konings W. N. Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem. 1975 Sep 10;250(17):6792–6798. [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- DEEB S. S., HAGER L. P. CRYSTALLINE CYTOCHROME B1 FROM ESCHERICHIA COLI. J Biol Chem. 1964 Apr;239:1024–1031. [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Fujita T. Studies on soluble cytochromes in Enterobacteriaceae. II. Cytochromes b-562 and c-550. J Biochem. 1966 Sep;60(3):329–334. doi: 10.1093/oxfordjournals.jbchem.a128440. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A., Garland P. B. Kinetic characterization of the membrane-bound cytochromes of Escherichia coli grown under a variety of conditions by using a stopped-flow dual-wavelength spectrophotometer. Biochem J. 1976 Feb 15;154(2):285–294. doi: 10.1042/bj1540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Towne D. W., Shrager R. I. Redox properties of beta-type cytochromes in Escherichia coli and rat liver mitochondria and techniques for their analysis. Biochim Biophys Acta. 1975 Jan 31;376(1):42–62. doi: 10.1016/0005-2728(75)90203-0. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Wright V., Ackrell B. A. Respiratory protection of nitrogenase in Azotobacter vinelandii. FEBS Lett. 1973 Jan 15;29(2):77–81. doi: 10.1016/0014-5793(73)80530-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Redox potentials of the cytochromes in the respiratory chain of aerobically grown Escherichia coli. Arch Biochem Biophys. 1976 Jun;174(2):546–552. doi: 10.1016/0003-9861(76)90382-9. [DOI] [PubMed] [Google Scholar]
- Revsin B., Brodie A. F. Carbon monoxide-binding pigments of Mycobacterium phlei and Escherichia coli. J Biol Chem. 1969 Jun 10;244(11):3101–3104. [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp W. S. Cytochromes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):459–472. doi: 10.1016/0003-9861(72)90063-x. [DOI] [PubMed] [Google Scholar]


