ABSTRACT
Fibroids are the most common benign tumors of the female reproductive system. Most patients with fibroids are asymptomatic, but the presence of fibroids can still cause some abnormal clinical symptoms, such as increased menstrual volume, abnormal uterine bleeding, pelvic pain, urinary tract and gastrointestinal tract compression symptoms, etc. The impact of fibroids on pregnancy is worth discussing. At present, it is believed that submucosal myoma and intramural myoma affecting uterine cavity shape affect the pregnancy outcome of patients, while the impact of type III intramural myoma on pregnancy is still controversial. A number of studies have found that in addition to direct contact with the endometrial compression, uterine myoma also affects the endometrial flexibility through other ways. In this review, we summarized the effects of fibroids on endometrial receptivity and discussed in depth the mechanisms of such effects, including secretion of cytokines, changes in endometrial blood flow and angiogenesis, effects on endometrial peristalsis and mechanical stress conduction, changes in uterine microecological environment, and abnormal signal transduction pathways. Understanding the mechanism of endometrial receptivity affected by fibroids is significant for exploring the treatment of fibroids, improving the pregnancy outcome of patients with fibroids and increasing the clinical pregnancy rate.
Keywords: decidualization, endometrial receptivity, endometrium, fibroids, pregnancy
1. Introduction
Fibroids are the most common gynecological benign tumors in the reproductive system of women of reproductive age, with an incidence of about 70%, of which about 30% of patients can show clinical symptoms, including increased menstrual volume, anemia, pelvic compression symptoms and pain, dyspareunia, infertility, early abortion, and recurrent abortion [1]. Submucous myoma (SMM) reduces pregnancy rate by directly pressing the endometrium and altering uterine cavity morphology. In recent years, studies have found that even IMM (intramural myoma) does not affect uterine morphology, it may still have adverse effects on the clinical pregnancy rate and live birth rate of patients [2, 3, 4]. Fibroids may affect endometrial receptivity through several mechanisms, such as affecting endometrial decidualization process, interfering with endometrial peristalsis, affecting uterine blood flow and endometrial vascularization, immune factors, changes in signal transduction pathways, and changes in reproductive tract microflora. The purpose of this review is to systematically review the mechanisms related to the effect of fibroids on endometrial receptivity, to clarify the specific mechanisms by which fibroids may reduce the pregnancy rate.
2. Methods
We used a variety of strategies to search the literature related to fibroids and endometrial receptivity, focusing on mechanisms affecting endometrial receptivity, infertility, and so forth. When searching literature, we did not limit the publication time of literature, PubMed was used for the literature search, and “fibroids, uterine leiomyoma,” “endometrial receptivity” and “infertility, subfertility” were used for combined or separate search. In addition, we made reference to the relevant references in the literature we read, and comprehensively read the literature results. We summarized the mechanisms by which related fibroids affect endometrial receptivity and then affect embryo implantation, leading to infertility and adverse pregnancy sac outcomes in this review.
3. Results
3.1. Origin of Fibroids
In the past decade, the drivers of genetic mutations that cause fibroids are becoming increasingly clear. Whole‐genome sequencing of fibroids found that the same variation was observed in some of the individual tumor nodules, suggesting that these nodules share a common origin [5]. The majority of fibroids exhibit highly specific mutations in the MED12 gene, which occurs in 40%–90% of fibroids. MED12 acts as a bridge between transcription factors and basic RNA polymerase II transcription mechanisms. The types of mutations include heterozygous missense mononucleotide variants, heterozygous in‐frame deletions/insertion–deletions, mononucleotide variants that affect splicing, heterozygous deletions/insertion–deletions that cross intron–exon 2 boundaries, affecting splicing receptor sites, and so forth. Among them, nearly 70% of fibroids have tumorigenic mutations in MED12 exon 2 [6]. Recently, it was found that mediator kinase subunits are enriched in myometrial stem cells (MMSC), and in addition, chemical inhibition of CDK8/19 in MMSC leads to reduced phosphorylation of stem cell‐enriched transcription factors and altered expression of myogenic genes. MED12 exon 2 mutation may provide a selective advantage to myometrium stem cells by disrupting mediator kinase activity and by altering the growth or differentiation trajectory of myometrium stem cells to form myoma stem cells, which in turn seed and sustain monoclonal tumor growth [7, 8, 9]. Mutations in fumarate hydratase (FH) on chromosome 1 in band q42 were found in fibroids [10, 11], the mechanism of tumorigenesis induced by FH mutations remains unclear [12]. The most widely studied hypothesis is activation of the hypoxia pathway. FH biallelic deletion leads to intracellular accumulation of fumaric acid. High levels of fumarate modify cysteine residues of KEAP1 (Kelch‐like epoxy chloropropane‐associated protein 1), leading to the accumulation and activation of NRF2 [13, 14]. Nrf2 has been shown to contribute to the malignant phenotypes of cancer, including aggressive proliferation [15]. Studies have found that NRF2 signaling pathway is the single most significant dysregulated pathway in FH subtype leiomyoma [16].
For leiomyomas lacking MED12 mutations and FH mutations, whole‐genome sequencing revealed complex chromosomal rearrangements (CCRs) in these subgroups of leiomyomas. These CCRs are similar to chromothripsis, in which one or more chromosomes break into pieces in a single event and are randomly spliced together [5]. Fibroids may be formed through those CCR events that create tumor‐promoting genetic changes, which can impair control of cell‐cycle checkpoints and repair of DNA double‐strand breaks, such as translocations of the HMGA2 and RAD51B loci [5]. It was found that the expression of HMGA2 in chromosomal rearrangements of 12q15 fibroids was significantly higher than that in karyotypically normal uterine leiomyomas or in myometrium [16, 17]. The proto‐oncogene pleomorphic adenoma gene 1 (PLAG1) as one of the most uniquely up‐regulated genes in leiomyomas with HMGA2 or HMGA1 aberrations. PLAG1 encodes for a transcription factor whose ectopic expression can trigger the development of several benign mesenchymal tumors, HMGA2 and HMGA1 promote tumorigenesis through the activation of PLAG1 [18]. COL4A5/COL4A6 deletion is a rare subtype that accounts for about 2% of fibroids. Alterations in the COL4A5–COL4A6 locus recurred in fibroids and also arose through chromothripsis [8].
3.2. Receptivity of Endometrium
The endometrium consists of two regions: functionalis and basalis. In the functionalis region, functional glands extend vertically to the uterine cavity, where the functional cavity cell layer is arranged on the surface of the endometrium. The functional region responds to hormones and undergoes dynamic remodeling of cell morphology and function during the menstrual cycle. The basal glands contain epithelial stem/progenitor cells that are required to regenerate functional glands after menstruation [19]. Stromal cells, fibroblasts, perivascular (PV), and endothelial cells provide support and structural integrity, including a rich vascular system within the tissue [20]. Successful embryo implantation requires the endometrium of the acceptable embryo to be synchronized and functionally coordinated with embryonic development, and the endometrium receptive window period usually occurs 6–10 days after ovulation [21]. After ovulation, the luteum forms and the endometrium begins the process of decidualization. During the secretory phase, decidualized stromal cells specific to the secretory phase (dStromal cells) are described as different cellular states in the early, middle, and late secretory phases and function through different mechanisms [20]. The receptive endometrium is characterized by the appearance of microvilli on the apical surface of luminal epithelial cells, called pinopodes [22, 23]. It was found that there was a close association between the number of microvilli and the endometrial receptivity to the embryo [23, 24]. All of these molecules play an essential role in the communication between the endometrium and the blastocyst.
HOXA is a transcription factor, which is expressed in the endometrium of adult mice and humans, and its expression can be seen in the endometrium proliferation stage, and increases in the secretion stage, reaching its peak in the implantation window period [25, 26]. Studies have found that the endometrial receptivity of thin endometrial mice can be improved by upregulation of HOXA10 expression [27], and the experiments related to endometriosis, low implantation rate, and impaired endometrial receptivity showed decreased HOXA10/HOXA11 expression [26, 28, 29], those results showed that HOXA10/HOXA11 expression was closely related to endometrial receptivity.
Embryo implantation is a complex and precise process, in which a variety of cytokines, growth factors, adhesion factors, transcription factors, and so forth are involved and regulated [19, 30, 31]. Immune cells are essential for endometrial receptivity in embryo implantation and early placental development [32, 33]. In addition, endometrial peristalsis abnormalities, endometrial microbial flora disorders, endometrial blood flow changes, and so forth may lead to endometrial receptivity impairment.
3.3. Effect of Fibroids on Endometrial Receptivity
According to the available research, fertilities are decreased in females with submucosal fibroids [34], and myomectomy prior to in vitro fertilization is recommended to improve reproductive outcomes. Subserosal fibroids do not affect fertility outcomes, and removal does not confer benefits. As for IMM, its effect on patient reproductive outcomes remains controversial. Extensive researches have reported that IMMs even non‐cavity distorting IMMs effect the reproductive outcomes [35, 36, 37]. A multitude of mechanisms that may influence reproductive outcomes were proposed, for example, effects on implantation, synchronicity disruption, sperm transport, uterine contractility, and endometrial changes (Figure 1). Here, we elucidate the mechanism by which fibroids affect endometrial receptivity.
FIGURE 1.
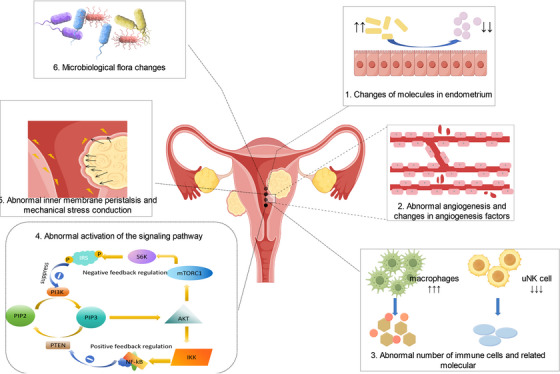
Overview of the mechanism by which fibroids affect endometrial receptivity.
3.3.1. Changes of Molecules in Endometrium
There is a consensus that SMM can reduce the implantation rate and clinical pregnancy rate. SMM may interfere with embryo implantation by changing the uterine cavity morphology and endometrial peristalsis, and large fibroids may even hinder sperm passage through the cervix and Ostium fallopian tube. However, impaired fertility can also be observed in women with small submucosal fibroids and IMMs that do not change the uterine morphology, thus considering that leiomyoma paracrine signaling may lead to myoma‐related infertility by affecting endometrial receptivity. Key regulators of implantation, such as homeobox‐A10 (HOXA10), HOXA11, and leukemia inhibitory factor (LIF), are known to have defective endometrial receptivity leading to infertility [38]. In patients with SMM infertility, HOXA10 and HOXA11 were reduced, and the results of a study by Rackow et al. showed that global endometrial HOXA10 expression was reduced in SMM and not confined to the endometrial portion covering the fibroids, suggesting that implantation defective can be mediated by signal diffusion [39]. Bone morphogenetic protein‐2 (BMP‐2) is an important multifunctional growth factor associated with embryo implantation and regulates the expression of HOXA and LIF [40]. BMP‐2 usually binds to BMPR in endometrial stromal cells (ESCs) to promote endometrial decidualization. The disappearance of BMP‐2 in mouse endometrium will lead to decidualization and embryo implantation failure. Studies show that the transforming growth factor‐β 3 (TGF‐β 3) has a weak affinity for BMPR. In the presence of leiomyomas, excess TGF‐β 3 binds to BMPR, resulting in inhibition of BMPR 1B and BMPR 2 in ESC, resulting in a lack of response to BMP. Neutralization of TGF‐β prevented the reduction of BMPR expression and restored endometrial BMP‐responsive [38] (Figure 2). In addition, endometrial TGF‐β expression is influenced by estrogen and progesterone. A previous study reported that TGF‐β expression is stimulated by progesterone, but recent studies confirmed that progesterone directly inhibited the endometrial expression of TGF‐β 2/3 [41]. These findings suggest that TGF‐β is a paracrine mediator of leiomyoma‐associated infertility and pregnancy loss and that pharmacologic therapies designed to reduce TGF‐β levels in the endometrium or to inhibit TGF‐β signaling may be a strategy to improve endometrial receptivity and increase pregnancy rate in patients.
FIGURE 2.
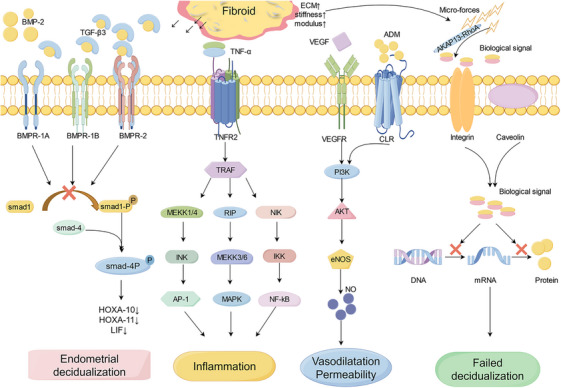
Transforming growth factor‐beta3 (TGFβ3) produced by fibroid reduces bone morphogenetic protein receptor (BMPR) expression and interfere with bone morphogenetic protein‐2 (BMP‐2) binding to receptors results in decreased expression of homeobox‐A10 、11 and leukemia inhibitory factor (HOXA10, HOXA11, LIF), thus affecting endometrial decidualization and endometrial receptivity. Tumor necrosis factor‐alpha (TNF‐α) responds to the immune response in three ways, abnormal expression of TNF‐α may lead to implantation failure and infertility. Vascular endothelial cell growth factor (VEGF) and adrenomedullin (ADM) are both angiogenic factors that cause abnormal angiogenesis through the PI3K‐AKT signaling pathway. The mechanical stress generated by fibroids is transformed into biological signals that interfere with gene expression, resulting in abnormal endometrial decidualization.
3.3.2. Endometrial Blood Flow and Angiogenic Factors
It is believed that increased endometrial blood flow is beneficial to embryo implantation and in‐vitro fertilization (IVF) outcomes [42, 43]. However, studies have found that with the change of menstrual cycle sex hormone levels, there is a specific vascular balance environment in the endometrium, and any increase or decrease in blood flow will lead to endometrial dysfunctional [44]. Fibroids may lead to abnormal intimal blood flow by causing abnormal endometrial angiogenesis and vascular maturation. A study included 182 patients with IMMs, endometrial volume, and blood flow index measured with 3D Doppler ultrasound, the results found that endometrial vessels in uterine fibroid (>4 cm) group increased significantly [45]. Moon et al. found that the resistance index (RI) and pulsatile index (PI) of the lower endometrial artery in patients with affected uterine cavity fibroids were significantly higher than that of patients with normal uterine cavity, and the clinical pregnancy rate and embryo implantation rate in this group were significantly reduced [46]. Compared with patients without fibroids, patients with in fibroid group had significantly increased endometrial venous pool, indicating increased vascular diameter, indicating impaired vascular maturity, and increased vascular fragility and permeability [47].
The presence of fibroids upregulated the endometrial angiogenic factors expression, for example, adrenomedullin (ADM) and vascular endothelial cell growth factor (VEGF). VEGF is the major initiator of angiogenesis, ADM could combine with calcitonin receptor‐like receptor (CLR), regulating the creation of nitric oxide (NO), and then stimulating the angiogenic [48]. This indicates abnormal endometrial angiogenesis in patients, and may involve disturbances in vascular maturation, leading to an increased vascular fragility [49]. Endometrial nitric oxide synthase (eNOS) is the major isomer of human endometrial NO and an important angiogenic factor involved in vasodilation and endothelial cell permeability which is induced by VEGF. Although eNOS significantly affects sustained pregnancy and maintaining uterine quiescence during implantation, endometrial eNOS levels were significantly increased in patients with fibroids [50]. The overexpression of eNOS can induce apoptosis or impair the endometrium and its function, supporting the hypothesis that fibroids can impair implantation or increase the risk of abortion [49, 51] (Figure 2). In addition, the Extracellular matrix (ECM) of fibroids contains a lot of basic fibroblast growth factor (bFGF), an angiogenic growth factor that is highly mitogenic to capillary endothelial cells in vitro and can induce angiogenesis in vivo. It has been proposed that fibroids are a reservoir of bFGF, which may affect the endometrial vasculature through paracrine and local endocrine effects [52]. In patients with fibroid and abnormal uterine bleeding, ESC bFGF‐R1 expression is disturbed in the early luteal phase, consistent with embryonic attachment and implantation time, so dysregulation of the bFGF receptor/ligand system is implicated in female infertility with leiomyoma [53]. In conclusion, the findings suggest that fibroids may affect intima tolerance by affecting the generation of angiogenic factors and endometrial blood flow, but many factors are only confirmed in a single study, and more studies are needed to verify these conclusions.
3.3.3. Immune Factors
Endometrial immune cells show significant periodic fluctuation following the menstrual cycle. Abnormal changes in immune cells may affect endometrial decidualization and affect angiogenesis and embryo implantation. Two uterine macrophage (uM) populations (uM1 and uM2) previously identified in the endometrium during pregnancy [20], uM1 expresses pro‐inflammatory genes such as IL 1β and EREG, while uM2 expresses anti‐inflammatory genes such as HMOX1. At different stages of the menstrual cycle, macrophages in the endometrial specifically expressed role‐specific markers. During the proliferative phase, macrophages expressed adhesion and activation markers (CD71, CD69, CD54), suggesting a potential role in the regeneration and proliferation of the endometrial functional layer [54, 55]. In addition, uMs up‐regulate TNF (uM1) as well as growth factors such as IGF 1 (uM2) and EREG (uM1) which could stimulate the proliferation and survival of eStromal MMP by binding to their corresponding receptors. Both uM also express immunomodulatory genes (IL 10, LGALS 9, TREM2) that enhance the anti‐inflammatory response of the proliferating endometrium. Additionally, macrophages are also involved in the angiogenesis process, with uMs expressing multiple growth factor members of the pro‐angiogenesis VEGF family. For example, uM1 express VEGFA and uM2 express VEGFB, TNF61 is expressed in uM1 and OSM 62 and CXCL 8 expressed in both uM types, all of them are vascular remodeling factors [20].
Macrophages begin to increase before menstruation and peak during menstruation, accounting for about 15% of the total number of endometrial leukocytes, in particular, macrophages increased significantly at the implantation site [56]. A significant increase in macrophage number can be observed in the second half of the menstrual cycle, thus considered to play an important role in fertility regulation. In addition, macrophages can secrete LIF which is an important cytokine during embryo implantation [54]. LIF is also considered an effective chemoattractant of macrophages. One study showed that a mouse model with LIF knocked out showed a 50% reduction in the number of macrophages, which led to the failure of embryo implantation [57]. It is therefore concluded that macrophages play a crucial role in fertility and inducing the production of pro‐inflammatory cytokines [56]. The study found that the density of macrophages in the endometrial tissue close to the fibroid was significantly higher than that of the endometrial tissue away from the fibroid [58], and the infiltration in patients with muscle layer and endometrium in SMM and IMM was higher than that in patients with subserous fibroid, and the muscle layer and its corresponding macrophages in the endometrium did not depend on the size of the fibroids. Monocyte chemotaxis protein‐1 (MCP‐1) showed a significant positive correlation with tissue infiltration of macrophages in the endometrium of IMM patients [59]. Therefore, when fibroids exist, the inflammatory reaction change is not only limited to the fibroids itself, but also affect the patients in the endometrial tissue. Direct compression of the endometrial by SMM leads to changes in endometrial ischemia or hypoxia, increased endometrial angiogenesis, increased endometrial vascular activity, and subsequently increased recruitment of inflammatory cells. The disturbance of endometrial inflammatory environment may further affect the abnormal formation of endometrial pro‐inflammatory environment, resulting in the disturbance of membrane environment during embryo implantation window, thus affecting the successful implantation of embryos. TNF‐α is a type II transmembrane protein which produced by macrophages. TNF‐α is involved in tissue homeostasis and systemic inflammation, and is one of the cytokines that cause acute phase reaction [60]. The expression level of TNF‐α in fibroid is higher than that in the adjacent myometrium. Moreover, serum TNF‐α level is significantly increased in patients with clinically symptomatic fibroids. Studies have shown that compared with normal endometrium, endometrium expression of TNF‐α was significantly increased in patients with IMMs [61], and TNF‐α was involved in preimplantation development of embryos, loss of immune pregnancy, and regulation of trophoblast invasion. Elevated serum TNF‐α levels have been associated with serious pregnancy complications, such as recurrent abortion, premature rupture of membranes, preeclampsia, and intrauterine growth restriction [62]. Abnormal expression of TNF‐α may lead to implantation failure and infertility (Figure 2).
In the planting window, uterine natural killer cells (uNK) cells are the main key immune cells to regulate immune tolerance, trophoblast migration and invasion, and spiral artery transformation [63], endometrial uNK cells increase rapidly after ovulation, and further increas in the first trimester. Studies found that the uNK cell is related to the process of decidualization of endometrial, the number of uNK cells began to increase about 3 days after LH peak, so uNK cells increased before endometrial decidualization, especially around the arteries and glands. In the late secretion (about 11–13 days after LH peak), a large number of uNK cells are densely distributed in the inner membrane matrix [64]. Mouse studies found that loss of uNK cells led to normal development and maintenance failure of the decidua and maternal arteries [65]. A significant increase of uNK cell levels was observed in the endometrium of patients with recurrent abortion and repeated implant failure, indicating that potential disturbances of the immune environment may eventually lead to implantation or placenta formation failure [66]. However, insufficient activation of uNK cells may also lead to recurrent abortion, so moderate and controlled activation of uNK cells is conducive to the pregnancy [32]. A study compared the patients with fibroids and no fibroids different location of the number of endometrial uNK cells and found that away from the location of the endometrium of the uNK cells were significantly less than the control samples, and the number of uNK cells in the endometrial near fibroids were less than away from the location of the endometrium in the proliferative period and after the secretion period [67]. Low levels of IL‐11 in fibroid patients may be associated with reduced uNK cell numbers, IL‐11 involved in the regulation of trophoblast invasion, and reduced IL‐11 may contribute to implantation failure in these women; however, further studies are needed to evaluate this association [58].
3.3.4. Aberrant Activation of the Cell Signaling Pathway
During the process of endometrial decidualization, several cellular pathways are altered, including: WNT/β‐catenin pathway, cAMP/PKA pathway, Notch signaling pathway, ERK1/2 pathway, TGF β signaling pathway, BMP2‐WNT4 signaling cascade, phosphatidylinositol 3‐kinase (PI3K)/AKT pathway, and so on [68, 69]. The alteration of abnormal signaling pathways may affect endometrial decidualization and thus affect the successful implantation of embryos. Since the endometrial epithelium is the first site of contact between maternal and embryonic tissue, significant changes in the adhesion properties of the uterine epithelium are critical to the process of implantation. The study suggests that Wnt‐/β‐catenin signaling pathway may play an important role in the implantation process of human embryos [70]. Downregulation or deletion of E‐cadherin and β‐catenin (important intracellular mediators of the Wnt signaling pathway) protein expression during the implantation window may be necessary to enable epithelial cell segregation and blastocyst invasion [71]. Matsuzaki et al. studied the expression of E‐cadherin and β‐catenin in the endometrium of patients with endometriosis, uterine fibroids, and infertility, and found that in healthy control groups, the expression of E‐cadherin in glandular and coelomic epithelium at the proliferative stage, early and middle secretory stage was significantly lower than that in the later secretory stage. However, in patients with fibroid infertility, no matter the coelomic epithelium or glandular epithelium, the expression of E‐cadherin was significantly lower than that in the later secretory stage. There were no significant periodic differences in the expression of E‐cadherin [72].
Relevant studies have found that IMM may affect endometrial decidualization by affecting PI3K‐AKT pathway. Although the signaling mechanisms and pathways of decidual degeneration are still unknown, several lines of evidence point to the PI3K/AKT pathway as the main regulator of decidual cell proliferation and survival [73, 74, 75]. Activated Akt (p‐Akt) phosphorylates and activates a variety of downstream proteins, and is involved in various cellular functions, including growth, proliferation, differentiation, survival, apoptosis, and migration. After the addition of PI3K/Akt signaling pathway inhibitor, the number of embryo implantation was significantly reduced [76, 77]. The expression levels and distribution characteristics of genes related to the PI3K/Akt signaling pathway at implantation sites and interimplantation sites in endometrial suggest that the PI3K/Akt signaling pathway is involved in the early embryo implantation process, especially during the embryo implantation window period, and may promote stromal cell migration [77]. Akt expression in stromal cells was detected only in the functional layer, where tissue remodeling occurs during menstruation or implantation. Therefore, Akt activation may be involved in human endometrial and decidual cell survival and extracellular matrix remodeling [73, 78]. Makker et al. took patients without IMM as the control group to study the expression, cell distribution, and activation status of key signaling components of PI3K/PTEN/Akt pathway in endometrium during the mesocrine phase in IMM infertility patients [79]. It was found that nuclear PTEN staining in endometrial mesenchyme in the experimental group was significantly lower than that in fertile women. The expression of p‐PTEN and the ratio of p‐PTEN to PTEN increased in the nucleus, suggesting the loss of PTEN biological activity. Although there is no information on pPTEN/PTEN expression in the endometrium, a study of women with tubal infertility showed that pregnancy success was associated with the highest expression levels of PTEN mRNA, suggesting that PTEN is essential for endometrial receptivity and successful implantation [80]. Laguee et al. found that PTEN regulates decidual degeneration and trophoblast invasion, and indicated that PI3K/AKT signaling activity must be suppressed in decidual cells at the maternal/fetal interface to allow these processes on the rails [81]. Loss of PTEN may lead to changes in the function of the uterine glands, resulting in sterility or loss of pregnancy in these mice. In addition, Akt1 mRNA levels and nuclear Akt1 expression were significantly up‐regulated in the endometrial stroma of IMM infertile women. Elevated Akt1 expression is associated with reduced fertility in endometriosis patients [82]. In the fibroid group, the expression of Akt2 in the endometrial stroma was significantly up‐regulated. Akt2 is a key factor in the PI3K/Akt signaling pathway and is involved in the maintenance of glucose homeostasis. Abnormalities in this process may be represented by implantation failure and poor pregnancy outcomes. It has been reported that alterations in glycolytic metabolism lead to endometrial dysfunction in patients with PCOS [83, 84]. PCOS, endometriosis, and fibroids are estrogen‐dependent disorders which associated with infertility [85]. The presence of fibroids leads to changes in the expression of PI3K/PTEN/Akt pathway signaling components in endometrium. The decrease of nuclear PTEN is accompanied by the increase of nuclear phosphorylated PTEN and the subsequent activation of nuclear Akt in the stroma, which may affect endometrial decidualization through regulating endometrial proliferation and apoptosis during implantation, and participate in the infertility mechanism of patients with fibroids (Figure 3).
FIGURE 3.
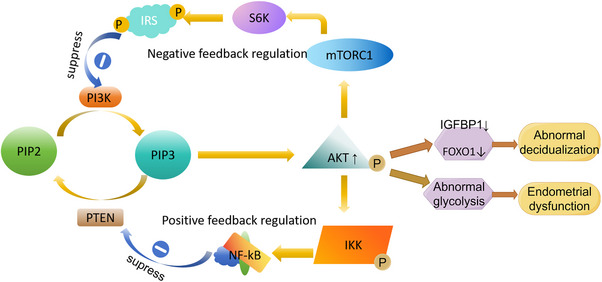
PI3K‐AKT signal path: PI3K phosphorylates phosphatidylinositol‐4, 5‐diphosphate (PIP2) to phosphatidylinositol‐3,4, 5‐triphosphate (PIP3), and PIP3 activates AKT. Increased phosphorylated AKT reduces the expression of deciduation‐specific gene insulin‐like growth factor binding protein‐1 (IGFBP1) and decreases the level of nuclear forkhead transcription factors of the O class (FOXO1). Causes abnormal decidualization of endometrial stromal cells in women with endometriosis. It can also lead to endometrial dysfunction by causing changes in glycolytic metabolism.
3.3.5. Abnormal Inner Membrane Peristalsis and Mechanical Stress Conduction
The direction of peristalsis of the uterus changed significantly with different stages of the menstrual cycle. During menstruation, the uterus peristalsis moved from the base of the uterus to the cervix, while the direction of ovulation was the cervix to the base of the uterus. The frequency of uterine peristalsis decreased significantly after ovulation, and this peristalsis pattern was conducive to activities such as menstrual blood discharge, sperm transport, and embryo implantation [86]. However, the presence of fibroids may affect embryo implantation by affecting the direction, frequency, and even amplitude of peristalsis of the endometrium muscularis junction [87]. Fanchin et al. examined the uterine peristalsis of infertility patients without uterine abnormalities with ultrasound, and the study proved that the frequency of uterine peristalsis on the day of embryo transfer was negatively correlated with pregnancy outcome [88]. Abnormal uterine peristalsis may expel the embryo from the uterine cavity [89]. A previous study of endometrial peristalsis using Cine magnetic resonance imaging (Cine MRI) found abnormal menstrual and midluteal peristalsis in three out of five patients with fibroids [90]. In a study of 51 IMM patients, it was found that 22 patients with high‐frequency endometrial peristalsis had no pregnancy, while 34% of patients with low‐frequency peristalsis had pregnancy, so a higher midluteal uterine peristalsis frequency may be one of the reasons for infertility in IMM patients [86]. However, the relationship between uterine peristalsis and fibroids is unclear. Since estrogen can induce peristalsis by increasing endometrial oxytocin receptor density, expression of aromatase in fibroids may lead to increased tissue estrogen concentration, which in turn leads to increased endometrial peristalsis [86, 91]. The study found that hysteromyectomy reduced the frequency of abnormal peristalsis in all patients, and 14 of 15 patients with IMM who underwent surgery returned the frequency of peristalsis to the normal range (0 or 1/3 min). In addition, 6 of the 14 patients had a successful pregnancy after hysteromyectomy [92]. Therefore, the presence of fibroids may lead to increased abnormal endometrial peristalsis leading to infertility, and myomectomy may increase the pregnancy rate of patients by restoring the frequency of endometrial peristalsis [93].
The ECM, composed of glycosaminoglycan and interstitial collagen, is the determining factor of the hardness of fibroids, which are 2–4 times harder at different locations than adjacent muscle layers [94, 95]. The hardness produced by each biological cell or the response to mechanical signals is different. Fibroid stem cells are more likely to proliferate and generate mechanical signals at higher hardness. The increased hardness not only makes the mechanical signals stronger but also makes the signal transmission more efficient and faster. The transmission of mechanical signals from myoma to adjacent myometrium and then to endometrium is a solid conduction process, and the transformation of mechanical signals into biochemical signals requires a series of pathways. Rho‐GTPases are key enzymes in this pathway. The ECM composition and Rho‐GTPases of fibroids are the main factors that determine the efficiency of this pathway. Mechanical signals from fibroids are converted into biochemical signals and transmitted to fibroids, myometrium, and endometrium by transmembrane receptor integrins, cadherins, or caveolins [95]. Signals directly entering fibroids may stimulate fibroid growth and those directly entering the endometrium may impair the receptibility of endometrium [93, 94, 95] (Figure 2). Under physiological conditions, micromechanical forces caused by subendometrial muscle contraction reach the endometrium and activate epithelial sodium channel (ENaC). ENaC activation has a positive effect on receptivity by stimulating the activation of prostaglandin E2. If the mechanical signal binds to the lysophatidic acid (LPA) receptor in the individual cell, the Rho GTPase is activated, and then the actin–myosin complex comes into play, and this whole process produces a positive progression in decidualization [95]. Due to the low ECM content in the early stage of muscle nucleus formation, small strength and small force may have a positive effect on decidualization. However, the continuity of mechanical signals and the effect on decidualization are impaired due to increased ECM and hardness. Because of the different hardness and modulus of fibroids, the influence of different location and size of fibroids on endometrial receptivity is different. The myometrium has an elastic modulus due to its biological properties, and fibroids have a chondroid modulus due to their collagen and glycosaminoglycan content. The chondroid modulus makes the fibroid five times harder than the muscle layer, and the different modulus of the fibroid and muscle layer convert the mechanical signals into biological signals and transmit them to the adjacent muscle layer and endometrium [94, 96]. Therefore, fibroids in the early stages of development, if the fibroids are not hard enough, may not be enough to produce biological signals. The main factor determining fibroid hardness is not size but matrix composition, and if a fibroid is small and dense, it may produce stronger mechanical stimulation than a larger fibroid with less ECM. In this way, the mechanical signals of small and hard fibroids that do not directly touch the endometrial membrane can also reach the endometrial membrane and affect the endometrial decidualization process.
3.3.6. Microbiological Flora Changes
The microbiota of the human body plays an important role in affecting the health status of the body. In the past, the uterine cavity was thought to be a sterile environment, but now it is found that there is a unique microbiome in the female reproductive tract, which accounts for about 9% of the total bacteria in the female body [97]. Researchers have found differences between endometrial and vaginal microbial communities in terms of microflora classification and relative abundance, and further study found that lactobacillus is the most representative bacterial genus in endometrial samples [98, 99]. A large‐scale study by Chen et al. evaluated the microbiome composition characteristics of 110 women at six sites in the female reproductive tract. The research demonstrated that lactobacillus is the main taxa of the vaginal flora. However, this gradually disappears as the sample enters the endometrium and upper reproductive tract. The degree of correlation between lower and upper reproductive tract microbiota varies among different patient populations, possibly reflecting health and disease phenotypes [100, 101, 102]. The results of a number of studies on infertility in people treated with ART suggest the possibility that lactobacillus is the dominant microflora in the endometrial [103, 104, 105]. However, subsequent studies did not confirm the lactobacillus advantage originally assumed. Higher diversity appears to be associated with better outcomes after IVF surgery [106, 107]. The results of the current study suggest that the composition of uterine microflora is unclear and needs to be further investigated. It is worth noting that contamination during sampling and environmental contamination received (including kit reagents, surgery, and laboratory environment) should be excluded during the study [108].
The endometrial microbiome may predict reproductive outcomes, some evidence linking the endometrial microbiota to early miscarriage. However, whether there is a causal relationship between the need's further clarification [101]. The endometrial microbiome may have a symbiotic relationship with the endometrium and its local immune mediators, promoting implantation and regulating immune tolerance [109]. The mother's immune system is crucial to the success of early pregnancy, immune‐mediated trophoblast invasion and spiral artery remodeling are the keys to success. Liu et al. compared the microbiota characteristics of the endometrial lavage fluid in patients with recurrent miscarriages (RM) with healthy patients and found that β‐diversity was significantly higher in the endometrial lavage fluid samples, but β‐diversity was not found in tissue biopsies from patients who experienced RM [110]. In addition, concomitant inflammatory changes were observed in these patients, although implantation is an inflammatory event, excessive inflammation outside the implantation window is associated with reproductive dysfunction. In a study of patients with endometritis, it was found that the relative abundance of lactobacillus was significantly reduced in patients with endometritis [111]. If the content of lactobacillus in endometrial is less than 90%, it is not conducive to reproductive outcomes and can be determined as an ecological imbalance, >90% lactobacillus content was significantly associated with better implantation results and sustained pregnancy [112]. Relevant studies have also shown that CE is associated with a significant decrease in α diversity within the endometrial microbiota and an increase in the expression of pro‐inflammatory genes associated with apoptotic pathways, including IFN‐α and tumor necrosis factor‐α [113, 114]. Endometriosis, ovarian or endometrial cancer, and polycystic ovary syndrome have been shown to affect the microbiota within the female reproductive tract [115]. In addition, there may be cross‐regulation between the microbiota and the uterine immune system. Emerging ideas suggest that the host immune system and the microbiota maintain a symbiotic relationship, and the microbiota can affect the host immune system function. However, the nature of the interaction between the microbiota and immune cells in the uterus is unclear. The symbiotic relationship between the uterine microbiota and the innate and adaptive immune systems may play an important role in maintaining a balanced inflammatory environment, as this mild bacterial stimulation can induce the formation of a microenvironment conducive to embryo implantation. On the other hand, the regulatory stimulation of the immune system by the microbiome helps build tolerance to semen [56]. The presence of fibroids may affect the vagino‐uterine microbiome because fibroids may cause inflammation and changes in local nutrients, such as increased menstrual bleeding and prolonged periods, which can affect the vagino‐uterine microbiome. Winters et al. found different distribution of microflora in vaginal and endometrial samples of patients with fibroids. The endometrial samples were dominated by acinetobacter, pseudomonas, syncytiaceae, and pipiobacterium, rather than lactobacillus [116], Mao et al. found that the cervical and vaginal microbiome of patients with fibroids increased in firmicutes. In the difference analysis of relative abundance, the abundance of Clostridium erysipelosus, myxospira, and fingerolderia increased, while the abundance of erysipelothrix and spolactobacter decreased. The presence of fibroids may lead to the imbalance of vaginal and cervical microecology [117]. However, there are few relevant studies on patients with fibroids, and further studies are needed to prove the relationship between changes in intrauterine microecology and pregnancy outcome, as well as the impact of fibroids on intrauterine microecology.
4. Conclusion
In summary, the most direct reason why fibroids affect endometrial function is that direct compression on endometrium and changes uterine cavity shape, thus affecting endometrial peristalsis, resulting in endometrial angiogenesis and abnormal blood flow. In addition, myoma without uterine compression can also change endometrial receptivity by producing excessive growth factors and cytokines [38, 40]. In addition, fibroids interfere with endometrial receptivity by affecting endometrial angiogenesis, causing inflammatory environment, abnormal mechanical stress conduction, abnormal protein kinase expression during signal transduction pathways, and abnormal endometrial microbial environment. However, it is far enough to simply understand the mechanism of the effect of fibroids on endometrium. More researches are needed to discover and demonstrate the effects of fibroids on the endometrium and other possible molecular mechanisms. Our review provides a reference for clinicians to understand the effects of fibroids on endometrial receptivity, and provides a basis for future solutions to problems such as fibroids affecting embryo implantation and causing abortion.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The figures were created by Figdraw.com.
Data Availability Statement
The data that support the findings of this study are openly available in figshare at https://pubmed.ncbi.nlm.nih.gov/.
References
- 1. Navarro A., Bariani M. V., Yang Q., et al., “Understanding the Impact of Uterine Fibroids on Human Endometrium Function,” Frontiers in Cell and Developmental Biology 9 (2021): 633180, 10.3389/fcell.2021.633180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rikhraj K., Tan J., Taskin O., et al., “The Impact of Noncavity‐Distorting Intramural Fibroids on Live Birth Rate in In Vitro Fertilization Cycles: A Systematic Review and Meta‐Analysis,” Journal of Womens Health (2002) 29 (2020): 210–219, 10.1089/jwh.2019.7813. [DOI] [PubMed] [Google Scholar]
- 3. Favilli A., Etrusco A., Chiantera V., et al., “Impact of FIGO Type 3 Uterine Fibroids on In Vitro Fertilization Outcomes: A Systematic Review and Meta‐Analysis,” International Journal of Gynaecology and Obstetrics 163 (2023): 528–539, 10.1002/ijgo.14838. [DOI] [PubMed] [Google Scholar]
- 4. Erden M., Uyanik E., Polat M., et al., “The Effect of ≤6 cm Sized Noncavity‐Distorting Intramural Fibroids on In Vitro Fertilization Outcomes: A Systematic Review and Meta‐Analysis,” Fertility and Sterility 119 (2023): 996–1007, 10.1016/j.fertnstert.2023.02.018. [DOI] [PubMed] [Google Scholar]
- 5. Mehine M., Kaasinen E., Mäkinen N., et al., “Characterization of Uterine Leiomyomas by Whole‐Genome Sequencing,” New England Journal of Medicine 369 (2013): 43–53, 10.1056/NEJMoa1302736. [DOI] [PubMed] [Google Scholar]
- 6. Croce S. and Chibon F., “MED12 and Uterine Smooth Muscle Oncogenesis: State of the Art and Perspectives,” European Journal of Cancer 51 (2015): 1603–1610, 10.1016/j.ejca.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 7. Mehine M., Mäkinen N., Heinonen H. R., et al., “Genomics of Uterine Leiomyomas: Insights From High‐Throughput Sequencing,” Fertility and Sterility 102 (2014): 621–629, 10.1016/j.fertnstert.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 8. Yang Q., Ciebiera M., Bariani M. V., et al., “Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment,” Endocrine Reviews 43 (2022): 678–719, 10.1210/endrev/bnab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turunen M., Spaeth J. M., Keskitalo S., et al., “Uterine Leiomyoma‐Linked MED12 Mutations Disrupt Mediator‐Associated CDK Activity,” Cell Reports 7 (2014): 654–660, 10.1016/j.celrep.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badeloe S., Bladergroen R. S., Jonkman M. F., et al., “Hereditary Multiple Cutaneous Leiomyoma Resulting From Novel Mutations in the Fumarate Hydratase Gene,” Journal of Dermatological Science 51 (2008): 139–143, 10.1016/j.jdermsci.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 11. Stewart L., Glenn G. M., Stratton P., et al., “Association of Germline Mutations in the Fumarate Hydratase Gene and Uterine Fibroids in Women with Hereditary Leiomyomatosis and Renal Cell Cancer,” Archives of Dermatology 144 (2008): 1584–1592, 10.1001/archdermatol.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lehtonen H. J., “Hereditary Leiomyomatosis and Renal Cell Cancer: Update on Clinical and Molecular Characteristics,” Familial Cancer 10 (2011): 397–411, 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 13. Adam J., Hatipoglu E., O'Flaherty L., et al., “Renal Cyst Formation in Fh1‐Deficient Mice is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling,” Cancer Cell 20 (2011): 524–537, 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kinch L., Grishin N. V., and Brugarolas J., “Succination of Keap1 and Activation of Nrf2‐Dependent Antioxidant Pathways in FH‐Deficient Papillary Renal Cell Carcinoma Type 2,” Cancer Cell 20 (2011): 418–420, 10.1016/j.ccr.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitsuishi Y., Taguchi K., Kawatani Y., et al., “Nrf2 Redirects Glucose and Glutamine Into Anabolic Pathways in Metabolic Reprogramming,” Cancer Cell 22 (2012): 66–79, 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 16. Mehine M., Kaasinen E., Heinonen H. R., et al., “Integrated Data Analysis Reveals Uterine Leiomyoma Subtypes With Distinct Driver Pathways and Biomarkers,” Proceedings of the National Academy of Sciences 113 (2016): 1315–1320, 10.1073/pnas.1518752113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gross K. L., Neskey D. M., Manchanda N., et al., “HMGA2 Expression in Uterine Leiomyomata and Myometrium: Quantitative Analysis and Tissue Culture Studies,” Genes, Chromosomes & Cancer 38 (2003): 68–79, 10.1002/gcc.10240. [DOI] [PubMed] [Google Scholar]
- 18. Jokinen V., Mehine M., Reinikka S., et al., “3'RNA and Whole‐Genome Sequencing of Archival Uterine Leiomyomas Reveal a Tumor Subtype With Chromosomal Rearrangements Affecting Either HMGA2, HMGA1, or PLAG1,” Genes, Chromosomes & Cancer 62 (2023): 27–38, 10.1002/gcc.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ochoa‐Bernal M. A. and Fazleabas A. T., “Physiologic Events of Embryo Implantation and Decidualization in Human and Non‐Human Primates,” International Journal of Molecular Sciences 21 (2020): 1973, 10.3390/ijms21061973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marečková M., Garcia‐Alonso L., Moullet M., et al., “An Integrated Single‐Cell Reference Atlas of the Human Endometrium,” Nature Genetics 56 (2024): 1925–1937, 10.1038/s41588-024-01873-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Achache H. and Revel A., “Endometrial Receptivity Markers, the Journey to Successful Embryo Implantation,” Human Reproduction Update 12 (2006): 731–746, 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 22. Nikas G. and Makrigiannakis A., “Endometrial Pinopodes and Uterine Receptivity,” Annals of the New York Academy of Sciences 997 (2003): 120–123, 10.1196/annals.1290.042. [DOI] [PubMed] [Google Scholar]
- 23. Rarani F. Z., Borhani F., and Rashidi B., “Endometrial Pinopode Biomarkers: Molecules and microRNAs,” Journal of Cellular Physiology 233 (2018): 9145–9158, 10.1002/jcp.26852. [DOI] [PubMed] [Google Scholar]
- 24. Stavreus‐Evers A., Nikas G., Sahlin L., et al., “Formation of Pinopodes in Human Endometrium is Associated With the Concentrations of Progesterone and Progesterone Receptors,” Fertility and Sterility 76 (2001): 782–791, 10.1016/s0015-0282(01)01993-8. [DOI] [PubMed] [Google Scholar]
- 25. Du H. and Taylor H. S., “The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility,” Cold Spring Harbor Perspectives in Medicine 6 (2015): a023002, 10.1101/cshperspect.a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao Y., Chen X., Liu X., et al., “Exposure of Mice to Benzo(a)Pyrene Impairs Endometrial Receptivity and Reduces the Number of Implantation Sites During Early Pregnancy,” Food and Chemical Toxicology 69 (2014): 244–251, 10.1016/j.fct.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 27. Wei W., Wang N., Zhu Y., et al., “GM‐CSF Improves Endometrial Receptivity in a Thin Endometrium Rat Model by Upregulating HOXA10,” Molecular Human Reproduction 30 (2023): gaad042, 10.1093/molehr/gaad042. [DOI] [PubMed] [Google Scholar]
- 28. Celik O., Unlu C., Otlu B., et al., “Laparoscopic Endometrioma Resection Increases Peri‐Implantation Endometrial HOXA‐10 and HOXA‐11 mRNA Expression,” Fertility and Sterility 104 (2015): 356–365, 10.1016/j.fertnstert.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 29. Fambrini M., Sorbi F., Bussani C., et al., “Hypermethylation of HOXA10 Gene in Mid‐Luteal Endometrium From Women With Ovarian Endometriomas,” Acta Obstetricia et Gynecologica Scandinavica 92 (2013): 1331–1334, 10.1111/aogs.12236. [DOI] [PubMed] [Google Scholar]
- 30. Chen J. R., Cheng J. G., Shatzer T., et al., “Leukemia Inhibitory Factor Can Substitute for Nidatory Estrogen and Is Essential to Inducing a Receptive Uterus for Implantation But Is Not Essential for Subsequent Embryogenesis,” Endocrinology 141 (2000): 4365–4372, 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- 31. Cullinan E. B., Abbondanzo S. J., Anderson P. S., et al., “Leukemia Inhibitory Factor (LIF) and LIF Receptor Expression in Human Endometrium Suggests a Potential Autocrine/Paracrine Function in Regulating Embryo Implantation,” Proceedings of the National Academy of Sciences 93 (1996): 3115–3120, 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robertson S. A., Moldenhauer L. M., Green E. S., et al., “Immune Determinants of Endometrial Receptivity: A Biological Perspective,” Fertility and Sterility 117 (2022): 1107–1120, 10.1016/j.fertnstert.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 33. Tirado‐González I., Barrientos G., Freitag N., et al., “Uterine NK Cells Are Critical in Shaping DC Immunogenic Functions Compatible With Pregnancy Progression,” PLoS ONE 7 (2012): e46755, 10.1371/journal.pone.0046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pritts E. A., Parker W. H., and Olive D. L., “Fibroids and Infertility: An Updated Systematic Review of the Evidence,” Fertility and Sterility 91 (2009): 1215–1223, 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 35. Bai X., Lin Y., Chen Y., et al., “The Impact of FIGO Type 3 Fibroids on In‐Vitro Fertilization Outcomes: A Nested Retrospective Case‐Control Study,” European Journal of Obstetrics, Gynecology, and Reproductive Biology 247 (2020): 176–180, 10.1016/j.ejogrb.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 36. Wang X., Chen L., Wang H., et al., “The Impact of Noncavity‐Distorting Intramural Fibroids on the Efficacy of In Vitro Fertilization‐Embryo Transfer: An Updated Meta‐Analysis,” BioMed Research International 2018 (2018): 8924703, 10.1155/2018/8924703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sunkara S. K., Khairy M., El‐Toukhy T., et al., “The Effect of Intramural Fibroids Without Uterine Cavity Involvement on the Outcome of IVF Treatment: A Systematic Review and Meta‐Analysis,” Human Reproduction 25 (2010): 418–429, 10.1093/humrep/dep396. [DOI] [PubMed] [Google Scholar]
- 38. Sinclair D. C., Mastroyannis A., and Taylor H. S., “Leiomyoma Simultaneously Impair Endometrial BMP‐2‐Mediated Decidualization and Anticoagulant Expression Through Secretion of TGF‐β3,” Journal of Clinical Endocrinology and Metabolism 96 (2011): 412–421, 10.1210/jc.2010-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rackow B. W. and Taylor H. S., “Submucosal Uterine Leiomyomas Have a Global Effect on Molecular Determinants of Endometrial Receptivity,” Fertility and Sterility 93 (2010): 2027–2034, 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doherty L. F. and Taylor H. S., “Leiomyoma‐Derived Transforming Growth Factor‐β Impairs Bone Morphogenetic Protein‐2‐Mediated Endometrial Receptivity,” Fertility and Sterility 103 (2015): 845–852, 10.1016/j.fertnstert.2014.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gaide Chevronnay H. P., Cornet P. B., Delvaux D., et al., “Opposite Regulation of Transforming Growth Factors‐beta2 and ‐beta3 Expression in the Human Endometrium,” Endocrinology 149 (2008): 1015–1025, 10.1210/en.2007-0849. [DOI] [PubMed] [Google Scholar]
- 42. Mercé L. T., Barco M. J., Bau S., et al., “Are Endometrial Parameters by Three‐Dimensional Ultrasound and Power Doppler Angiography Related to In Vitro Fertilization/Embryo Transfer Outcome?,” Fertility and Sterility 89 (2008): 111–117, 10.1016/j.fertnstert.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 43. Wang L., Qiao J., Li R., et al., “Role of Endometrial Blood Flow Assessment With Color Doppler Energy in Predicting Pregnancy Outcome of IVF‐ET Cycles,” Reproductive Biology and Endocrinology 8 (2010): 122, 10.1186/1477-7827-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raine‐Fenning N. J., Campbell B. K., Kendall N. R., et al., “Quantifying the Changes in Endometrial Vascularity Throughout the Normal Menstrual Cycle With Three‐Dimensional Power Doppler Angiography,” Human Reproduction 19 (2004): 330–338, 10.1093/humrep/deh056. [DOI] [PubMed] [Google Scholar]
- 45. Kamel A., El‐Mazny A., Ramadan W., et al., “Effect of Intramural Fibroid on Uterine and Endometrial Vascularity in Infertile Women Scheduled for In‐Vitro Fertilization,” Archives of Gynecology and Obstetrics 297 (2018): 539–545, 10.1007/s00404-017-4607-2. [DOI] [PubMed] [Google Scholar]
- 46. Gianaroli L., Gordts S., D'Angelo A., et al., “Effect of Inner Myometrium Fibroid on Reproductive Outcome After IVF,” Reproductive Biomedicine Online 10 (2005): 473–477, 10.1016/s1472-6483(10)60823-1. [DOI] [PubMed] [Google Scholar]
- 47. Bereza T., Tomaszewski K. A., Lis G. J., et al., “Venous Lakes'—A Corrosion Cast Scanning Electron Microscopy Study of Regular and Myomatous Human Uterine Blood Vessels,” Folia Morphologiica 73 (2014): 164–168, 10.5603/fm.2014.0024. [DOI] [PubMed] [Google Scholar]
- 48. Middelkoop M. A., Don E. E., Hehenkamp W. J. K., et al., “Angiogenesis in Abnormal Uterine Bleeding: A Narrative Review,” Human Reproduction Update 29 (2023): 457–485, 10.1093/humupd/dmad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Don E. E., Mijatovic V., and Huirne J. A. F., “Infertility in Patients With Uterine Fibroids: A Debate About the Hypothetical Mechanisms,” Human Reproduction (2023): 2045–2054, 10.1093/humrep/dead194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hague S., Zhang L., Oehler M. K., et al., “Expression of the Hypoxically Regulated Angiogenic Factor Adrenomedullin Correlates With Uterine Leiomyoma Vascular Density,” Clinical Cancer Research 6 (2000): 2808–2814. [PubMed] [Google Scholar]
- 51. Najafi T., Ghaffari Novin M., Pakravesh J., et al., “Immunohistochemical Localization of Endothelial Nitric Oxide Synthase in Endometrial Tissue of Women With Unexplained Infertility,” Iranian Journal of Reproductive Medicine 10 (2012): 121–126. [PMC free article] [PubMed] [Google Scholar]
- 52. Wu X., Blanck A., Olovsson M., et al., “Expression of Basic Fibroblast Growth Factor (bFGF), FGF Receptor 1 and FGF Receptor 2 in Uterine Leiomyomas and Myometrium During the Menstrual Cycle, After Menopause and GnRHa Treatment,” Acta Obstetricia et Gynecologica Scandinavica 80 (2001): 497–504. [PubMed] [Google Scholar]
- 53. Anania C. A., Stewart E. A., Quade B. J., et al., “Expression of the Fibroblast Growth Factor Receptor in Women With Leiomyomas and Abnormal Uterine Bleeding,” Molecular Human Reproduction 3 (1997): 685–691, 10.1093/molehr/3.8.685. [DOI] [PubMed] [Google Scholar]
- 54. Thiruchelvam U., Dransfield I., Saunders P. T., et al., “The Importance of the Macrophage Within the Human Endometrium,” Journal of Leukocyte Biology 93 (2013): 217–225, 10.1189/jlb.0712327. [DOI] [PubMed] [Google Scholar]
- 55. Eidukaite A. and Tamosiunas V., “Endometrial and Peritoneal Macrophages: Expression of Activation and Adhesion Molecules,” American Journal of Reproductive Immunology 52 (2004): 113–117, 10.1111/j.1600-0897.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 56. Agostinis C., Mangogna A., Bossi F., et al., “Uterine Immunity and Microbiota: A Shifting Paradigm,” Frontiers in Immunology 10 (2019): 2387, 10.3389/fimmu.2019.02387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schofield G. and Kimber S. J., “Leukocyte Subpopulations in the Uteri of Leukemia Inhibitory Factor Knockout Mice During Early Pregnancy,” Biology of Reproduction 72 (2005): 872–878, 10.1095/biolreprod.104.034876. [DOI] [PubMed] [Google Scholar]
- 58. Ikhena D. E. and Bulun S. E., “Literature Review on the Role of Uterine Fibroids in Endometrial Function,” Reproductive Sciences 25 (2018): 635–643, 10.1177/1933719117725827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miura S., Khan K. N., Kitajima M., et al., “Differential Infiltration of Macrophages and Prostaglandin Production by Different Uterine Leiomyomas,” Human Reproduction 21 (2006): 2545–2554, 10.1093/humrep/del205. [DOI] [PubMed] [Google Scholar]
- 60. Ciebiera M., Włodarczyk M., Zgliczyńska M., et al., “The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms,” International Journal of Molecular Sciences 19 (2018), 10.3390/ijms19123869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luddi A., Marrocco C., Governini L., et al., “Increased Expression of Neurogenic Factors in Uterine Fibroids,” Human Reproduction 34 (2019): 2153–2162, 10.1093/humrep/dez182. [DOI] [PubMed] [Google Scholar]
- 62. Azizieh F. Y. and Raghupathy R. G., “Tumor Necrosis Factor‐α and Pregnancy Complications: A Prospective Study,” Medical Principles and Practice 24 (2015): 165–170, 10.1159/000369363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang F., Qualls A. E., Marques‐Fernandez L., et al., “Biology and Pathology of the Uterine Microenvironment and Its Natural Killer Cells,” Cellular and Molecular Immunology 18 (2021): 2101–2113, 10.1038/s41423-021-00739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. King A., “Uterine Leukocytes and Decidualization,” Human Reproduction Update 6 (2000): 28–36, 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 65. Guimond M. J., Wang B., and Croy B. A., “Engraftment of Bone Marrow From Severe Combined Immunodeficient (SCID) Mice Reverses the Reproductive Deficits in Natural Killer Cell‐Deficient Tg Epsilon 26 Mice,” Journal of Experimental Medicine 187 (1998): 217–223, 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Von Woon E., Greer O., Shah N., et al., “Number and Function of Uterine Natural Killer Cells in Recurrent Miscarriage and Implantation Failure: A Systematic Review and Meta‐Analysis,” Human Reproduction Update 28 (2022): 548–582, 10.1093/humupd/dmac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kitaya K. and Yasuo T., “Leukocyte Density and Composition in Human Cycling Endometrium With Uterine Fibroids,” Human Immunology 71 (2010): 158–163, 10.1016/j.humimm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 68. Da Silva I. D., Wuidar V., Zielonka M., et al., “Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview,” Cells 13 (2024): 1236, 10.3390/cells13151236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ticconi C., Di Simone N., Campagnolo L., et al., “Clinical Consequences of Defective Decidualization,” Tissue & Cell 72 (2021): 101586, 10.1016/j.tice.2021.101586. [DOI] [PubMed] [Google Scholar]
- 70. Tulac S., Overgaard M. T., Hamilton A. E., et al., “Dickkopf‐1, An Inhibitor of Wnt Signaling, Is Regulated by Progesterone in Human Endometrial Stromal Cells,” Journal of Clinical Endocrinology and Metabolism 91 (2006): 1453–1461, 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- 71. Li J., Zhang J. V., Cao Y. J., et al., “Inhibition of the Beta‐Catenin Signaling Pathway in Blastocyst and Uterus During the Window of Implantation in Mice,” Biology of Reproduction 72 (2005): 700–706, 10.1095/biolreprod.104.033837. [DOI] [PubMed] [Google Scholar]
- 72. Matsuzaki S., Darcha C., Maleysson E., et al., “Impaired Down‐Regulation of E‐Cadherin and Beta‐Catenin Protein Expression in Endometrial Epithelial Cells in the Mid‐Secretory Endometrium of Infertile Patients With Endometriosis,” Journal of Clinical Endocrinology and Metabolism 95 (2010): 3437–3445, 10.1210/jc.2009-2713. [DOI] [PubMed] [Google Scholar]
- 73. Toyofuku A., Hara T., Taguchi T., et al., “Cyclic and Characteristic Expression of Phosphorylated Akt in Human Endometrium and Decidual Cells In Vivo and In Vitro,” Human Reproduction 21 (2006): 1122–1128, 10.1093/humrep/dei454. [DOI] [PubMed] [Google Scholar]
- 74. Shooner C., Caron P. L., Fréchette‐Frigon G., et al., “TGF‐Beta Expression During Rat Pregnancy and Activity on Decidual Cell Survival,” Reproductive Biology and Endocrinology 3 (2005): 20, 10.1186/1477-7827-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tessier C., Prigent‐Tessier A., Ferguson‐Gottschall S., et al., “PRL Antiapoptotic Effect in the Rat Decidua Involves the PI3K/Protein Kinase B‐Mediated Inhibition of Caspase‐3 Activity,” Endocrinology 142 (2001): 4086–4094, 10.1210/endo.142.9.8381. [DOI] [PubMed] [Google Scholar]
- 76. Veillette A., Grenier K., Brasseur K., et al., “Regulation of the PI3‐K/Akt Survival Pathway in the Rat Endometrium,” Biology of Reproduction 88 (2013): 79, 10.1095/biolreprod.112.107136. [DOI] [PubMed] [Google Scholar]
- 77. Liu L., Wang Y., and Yu Q., “The PI3K/Akt Signaling Pathway Exerts Effects on the Implantation of Mouse Embryos by Regulating the Expression of RhoA,” International Journal of Molecular Medicine 33 (2014): 1089–1096, 10.3892/ijmm.2014.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Herington J. L. and Bany B. M., “Do Molecular Signals From the Conceptus Influence Endometrium Decidualization in Rodents?,” Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 312 (2009): 797–816, 10.1002/jez.b.21308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Makker A., Goel M. M., Nigam D., et al., “Aberrant Akt Activation During Implantation Window in Infertile Women With Intramural Uterine Fibroids,” Reproductive Sciences 25 (2018): 1243–1253, 10.1177/1933719117737844. [DOI] [PubMed] [Google Scholar]
- 80. Antsiferova Y. S., Sotnikova N. Y., Bogatova I. K., et al., “Changes of Apoptosis Regulation in the Endometrium of Infertile Women With Tubal Factor and Endometriosis Undergoing In Vitro Fertilization Treatment,” JBRA Assisted Reproduction 18 (2014): 2–6, 10.5935/1518-0557.20140084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laguë M. N., Detmar J., Paquet M., et al., “Decidual PTEN Expression Is Required for Trophoblast Invasion in the Mouse,” American Journal of Physiology Endocrinology and Metabolism 299 (2010): E936–E946, 10.1152/ajpendo.00255.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Laudanski P., Szamatowicz J., Kowalczuk O., et al., “Expression of Selected Tumor Suppressor and Oncogenes in Endometrium of Women With Endometriosis,” Human Reproduction 24 (2009): 1880–1890, 10.1093/humrep/dep175. [DOI] [PubMed] [Google Scholar]
- 83. Ujvari D., Hulchiy M., Calaby A., et al., “Lifestyle Intervention Up‐Regulates Gene and Protein Levels of Molecules Involved in Insulin Signaling in the Endometrium of Overweight/Obese Women With Polycystic Ovary Syndrome,” Human Reproduction 29 (2014): 1526–1535, 10.1093/humrep/deu114. [DOI] [PubMed] [Google Scholar]
- 84. Zhang Y., Sun X., Sun X., et al., “Molecular Characterization of Insulin Resistance and Glycolytic Metabolism in the Rat Uterus,” Scientific Reports 6 (2016): 30679, 10.1038/srep30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Makker A., Goel M. M., Das V., et al., “PI3K‐Akt‐mTOR and MAPK Signaling Pathways in Polycystic Ovarian Syndrome, Uterine Leiomyomas and Endometriosis: An Update,” Gynecological Endocrinology 28 (2012): 175–181, 10.3109/09513590.2011.583955. [DOI] [PubMed] [Google Scholar]
- 86. Yoshino O., Hayashi T., Osuga Y., et al., “Decreased Pregnancy Rate Is Linked to Abnormal Uterine Peristalsis Caused by Intramural Fibroids,” Human Reproduction 25 (2010): 2475–2479, 10.1093/humrep/deq222. [DOI] [PubMed] [Google Scholar]
- 87. Tanos V., Lingwood L., and Balami S., “Junctional Zone Endometrium Morphological Characteristics and Functionality: Review of the Literature,” Gynecologic and Obstetric Investigation 85 (2020): 107–117, 10.1159/000505650. [DOI] [PubMed] [Google Scholar]
- 88. Fanchin R., Righini C., Olivennes F., et al., “Uterine Contractions at the Time of Embryo Transfer Alter Pregnancy Rates After In‐Vitro Fertilization,” Human Reproduction 13 (1998): 1968–1974, 10.1093/humrep/13.7.1968. [DOI] [PubMed] [Google Scholar]
- 89. Bulletti C. and de Ziegler D., “Uterine Contractility and Embryo Implantation,” Current Opinion in Obstetrics & Gynecology 18 (2006): 473–484, 10.1097/01.gco.0000233947.97543.c4. [DOI] [PubMed] [Google Scholar]
- 90. Orisaka M., Kurokawa T., Shukunami K., et al., “A Comparison of Uterine Peristalsis in Women With Normal Uteri and Uterine Leiomyoma by Cine Magnetic Resonance Imaging,” European Journal of Obstetrics, Gynecology, and Reproductive Biology 135 (2007): 111–115, 10.1016/j.ejogrb.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 91. Mueller A., Siemer J., Schreiner S., et al., “Role of Estrogen and Progesterone in the Regulation of Uterine Peristalsis: Results From Perfused Non‐Pregnant Swine Uteri,” Human Reproduction 21 (2006): 1863–1868, 10.1093/humrep/del056. [DOI] [PubMed] [Google Scholar]
- 92. Yoshino O., Nishii O., Osuga Y., et al., “Myomectomy Decreases Abnormal Uterine Peristalsis and Increases Pregnancy Rate,” Journal of Minimally Invasive Gynecology 19 (2012): 63–67, 10.1016/j.jmig.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 93. Norian J. M., Owen C. M., Taboas J., et al., “Characterization of Tissue Biomechanics and Mechanical Signaling in Uterine Leiomyoma,” Matrix Biology 31 (2012): 57–65, 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rogers R., Norian J., Malik M., et al., “Mechanical Homeostasis Is Altered in Uterine Leiomyoma,” American Journal of Obstetrics and Gynecology 198 (2008): 474, 10.1016/j.ajog.2007.11.057. e1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Celik O., Celik N., Gungor N. D., et al., “Biomechanical Forces Determine Fibroid Stem Cell Transformation and the Receptivity Status of the Endometrium: A Critical Appraisal,” International Journal of Molecular Sciences 23 (2022): 14201, 10.3390/ijms232214201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wettschureck N. and Offermanns S., “Rho/Rho‐Kinase Mediated Signaling in Physiology and Pathophysiology,” Journal of Molecular Medicine (Berlin) 80 (2002): 629–638, 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 97. Moreno I. and Simon C., “Deciphering the Effect of Reproductive Tract Microbiota on Human Reproduction,” Reproductive Medicine and Biology 18 (2019): 40–50, 10.1002/rmb2.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Moreno I. and Simon C., “Relevance of Assessing the Uterine Microbiota in Infertility,” Fertility and Sterility 110 (2018): 337–343, 10.1016/j.fertnstert.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 99. Franasiak J. M., Werner M. D., Juneau C. R., et al., “Endometrial Microbiome at the Time of Embryo Transfer: Next‐Generation Sequencing of the 16S Ribosomal Subunit,” Journal of Assisted Reproduction and Genetics 33 (2016): 129–136, 10.1007/s10815-015-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen C., Song X., Wei W., et al., “The Microbiota Continuum Along the Female Reproductive Tract and Its Relation to Uterine‐Related Diseases,” Nature Communications 8 (2017): 875, 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Odendaal J., Black N., Bennett P. R., et al., “The Endometrial Microbiota and Early Pregnancy Loss,” Human Reproduction 39 (2024): 638–646, 10.1093/humrep/dead274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sirota I., Zarek S. M., and Segars J. H., “Potential Influence of the Microbiome on Infertility and Assisted Reproductive Technology,” Seminars in Reproductive Medicine 32 (2014): 35–42, 10.1055/s-0033-1361821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tao X., Franasiak J. M., Zhan Y., et al., “Characterizing the Endometrial Microbiome by Analyzing the Ultra‐Low Bacteria From Embryo Transfer Catheter Tips in IVF Cycles: Next Generation Sequencing (NGS) Analysis of the 16S Ribosomal Gene,” Human Microbiome Journal 3 (2017): 15–21, 10.1016/j.humic.2017.01.004. [DOI] [Google Scholar]
- 104. Kyono K., Hashimoto T., Nagai Y., et al., “Analysis of Endometrial Microbiota by 16S Ribosomal RNA Gene Sequencing Among Infertile Patients: A Single‐Center Pilot Study,” Reproductive Medicine and Biology 17 (2018): 297–306, 10.1002/rmb2.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Carosso A., Revelli A., Gennarelli G., et al., “Controlled Ovarian Stimulation and Progesterone Supplementation Affect Vaginal and Endometrial Microbiota in IVF Cycles: A Pilot Study,” Journal of Assisted Reproduction and Genetics 37 (2020): 2315–2326, 10.1007/s10815-020-01878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Reschini M., Benaglia L., Ceriotti F., et al., “Endometrial Microbiome: Sampling, Assessment, and Possible Impact on Embryo Implantation,” Scientific Reports 12 (2022): 8467, 10.1038/s41598-022-12095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kyono K., Hashimoto T., Kikuchi S., et al., “A Pilot Study and Case Reports on Endometrial Microbiota and Pregnancy Outcome: An Analysis Using 16S rRNA Gene Sequencing Among IVF Patients, and Trial Therapeutic Intervention for Dysbiotic Endometrium,” Reproductive Medicine and Biology 18 (2019): 72–82, 10.1002/rmb2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Inversetti A., Zambella E., Guarano A., et al., “Endometrial Microbiota and Immune Tolerance in Pregnancy,” International Journal of Molecular Sciences 24 (2023): 2995, 10.3390/ijms24032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brosens J. J., Bennett P. R., Abrahams V. M., et al., “Maternal Selection of Human Embryos in Early Gestation: Insights From Recurrent Miscarriage,” Seminars in Cell & Developmental Biology 131 (2022): 14–24, 10.1016/j.semcdb.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu F. T., Yang S., Yang Z., et al., “An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women With Recurrent Spontaneous Abortion,” Microbiology Spectrum 10 (2022): e0046222, 10.1128/spectrum.00462-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu Y., Ko E. Y., Wong K. K., et al., “Endometrial Microbiota in Infertile Women With and Without Chronic Endometritis as Diagnosed Using a Quantitative and Reference Range‐Based Method,” Fertility and Sterility 112 (2019): 707–717, 10.1016/j.fertnstert.2019.05.015. e1. [DOI] [PubMed] [Google Scholar]
- 112. Moreno I., Codoñer F. M., Vilella F., et al., “Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure,” American Journal of Obstetrics and Gynecology 215 (2016): 684–703, 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 113. Chen W., Wei K., He X., et al., “Identification of Uterine Microbiota in Infertile Women Receiving In Vitro Fertilization With and Without Chronic Endometritis,” Frontiers in Cell and Developmental Biology 9 (2021): 693267, 10.3389/fcell.2021.693267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cicinelli E., Vitagliano A., Loizzi V., et al., “Altered Gene Expression Encoding Cytochines, Grow Factors and Cell Cycle Regulators in the Endometrium of Women With Chronic Endometritis,” Diagnostics (Basel) 11 (2021): 471, 10.3390/diagnostics11030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Punzón‐Jiménez P. and Labarta E., “The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review,” Journal of Assisted Reproduction and Genetics 38 (2021): 2519–2541, 10.1007/s10815-021-02247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Winters A. D., Romero R., Gervasi M. T., et al., “Does the Endometrial Cavity Have a Molecular Microbial Signature?,” Scientific Reports 9 (2019): 9905, 10.1038/s41598-019-46173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mao X., Chen H., Peng X., et al., “Dysbiosis of Vaginal and Cervical Microbiome Is Associated With Uterine Fibroids,” Frontiers in Cellular and Infection Microbiology 13 (2023): 1196823, 10.3389/fcimb.2023.1196823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in figshare at https://pubmed.ncbi.nlm.nih.gov/.


