Abstract
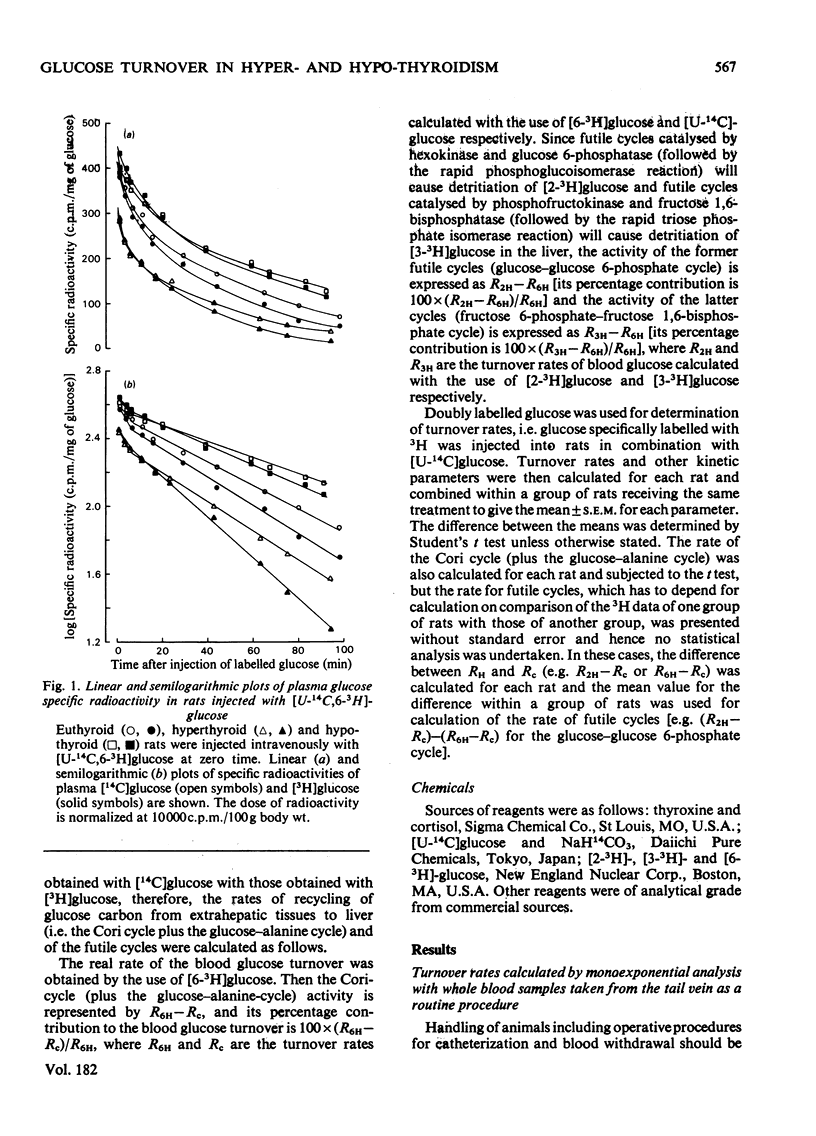
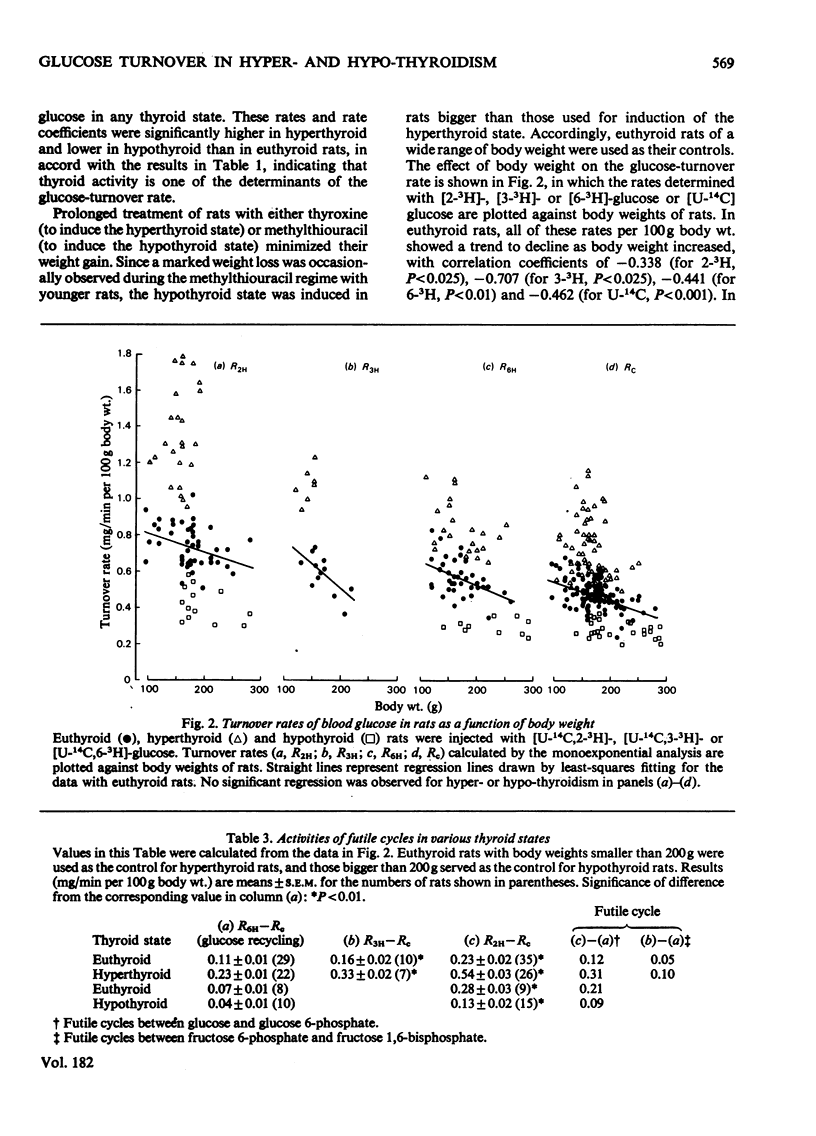
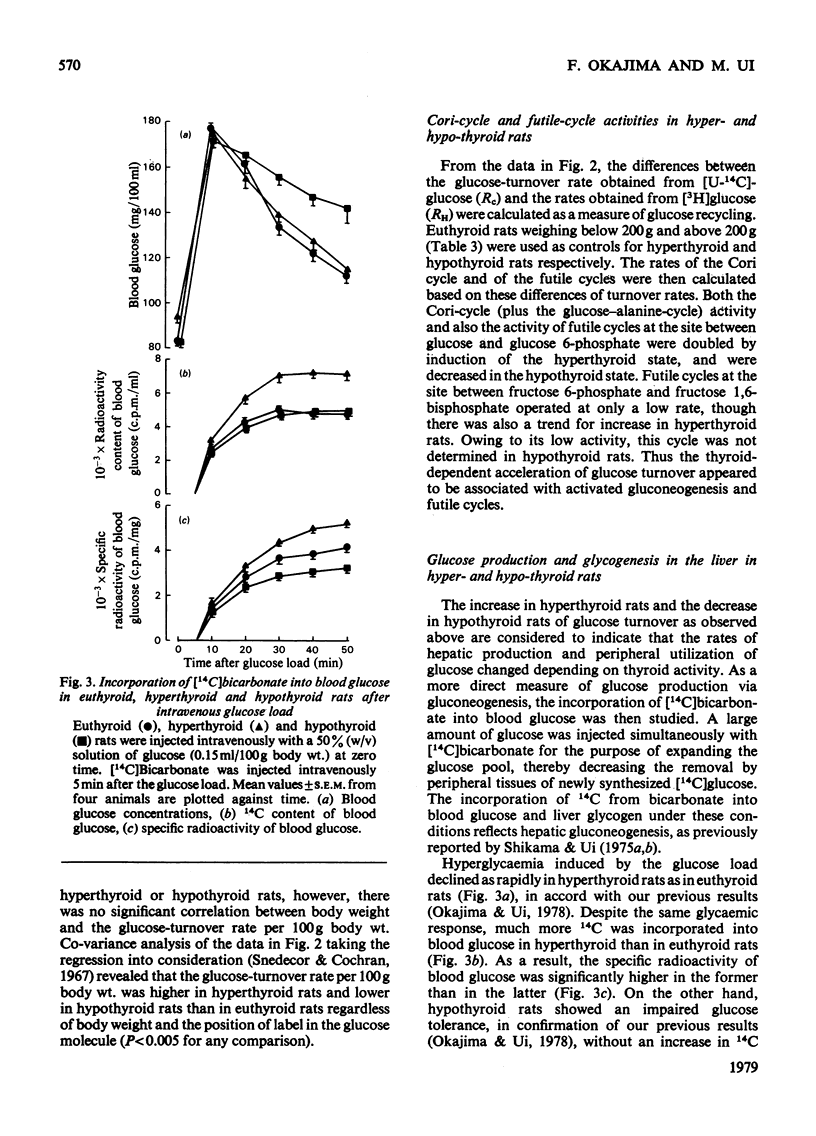
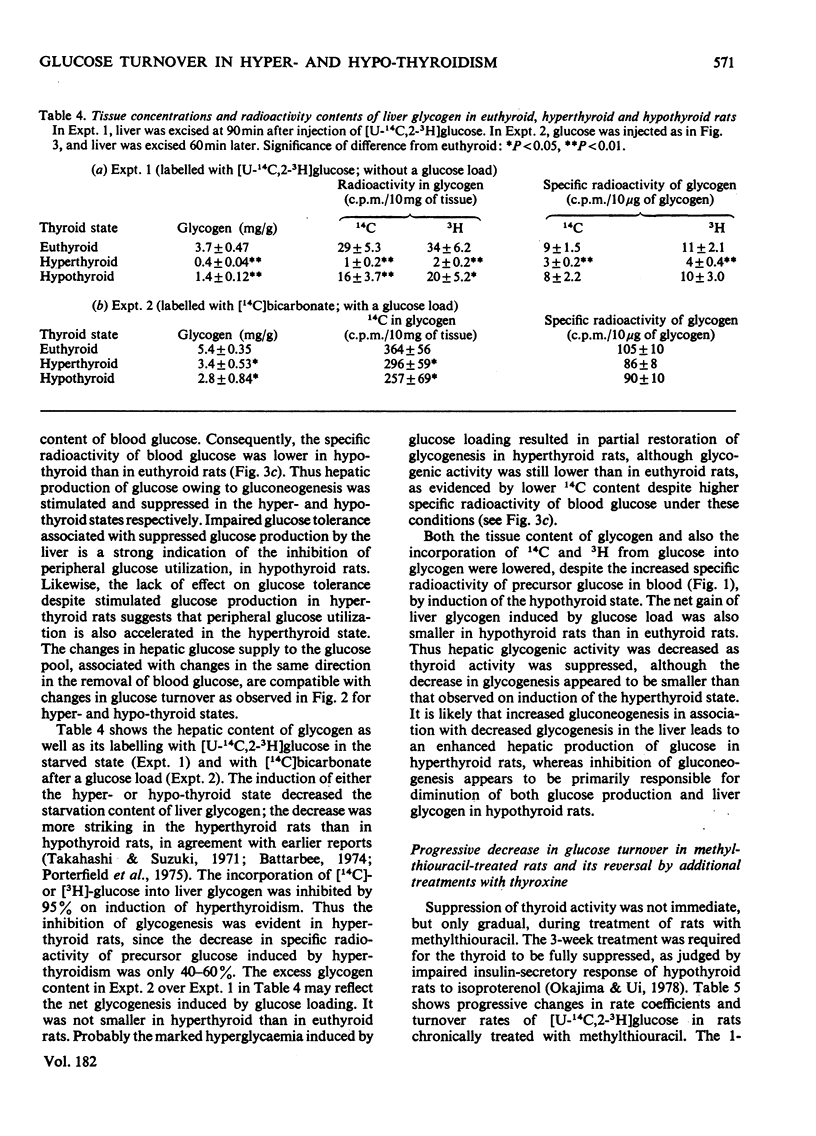
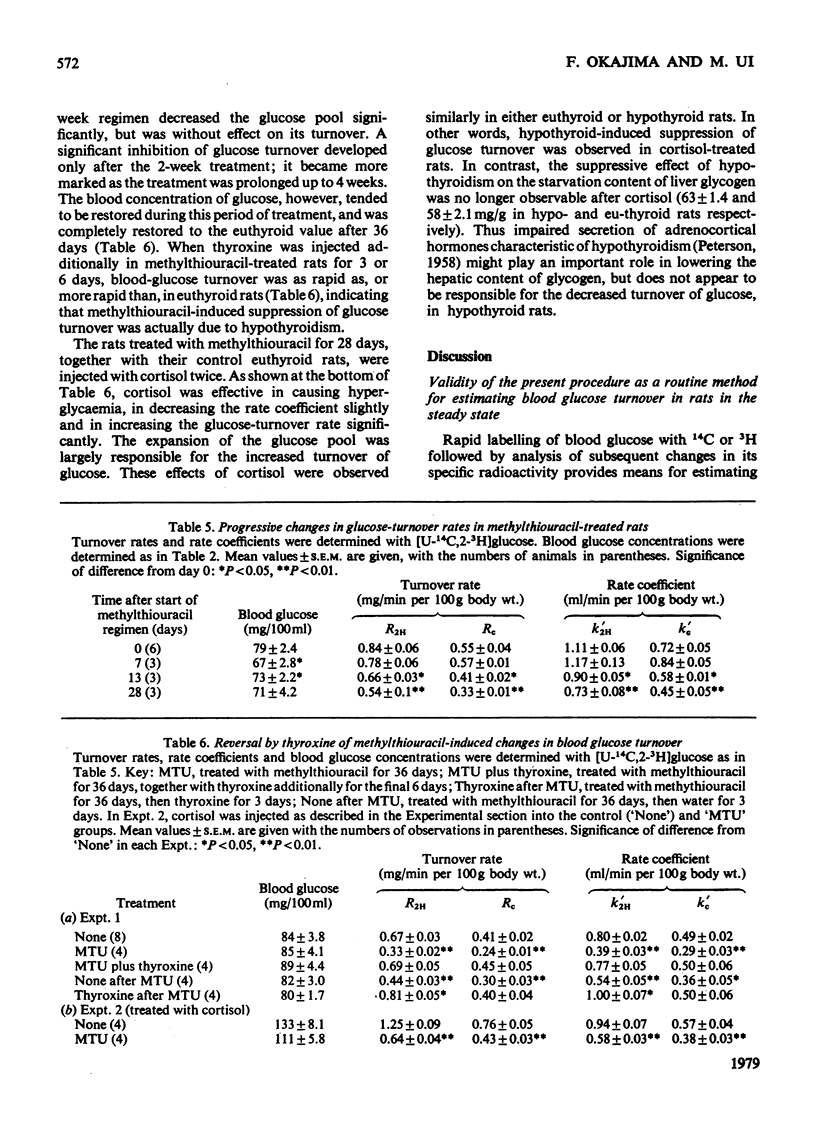
1. A trace amount of glucose labelled with 14C uniformly and with 3H at position 2, 3 or 6 was injected intravenously into starved rats to measure the turnover rate of blood glucose. 2. Reliable estimates were made based on the semilogarithmic plot of specific radioactivity of the glucose contained in whole blood samples taken from the tail vein. 3. Glucose turned over more rapidly in hyperthyroid and more slowly in hypothyroid than in euthyroid rats. The percentage contribution of glucose recycling (determined from the difference in replacement rates between [U-14C]glucose and [6-3H]glucose) to the glucose utilization increased on induction of hyperthyroidism. 4. Futile cycles between glucose and glucose 6-phosphate (determined from the difference between replacement rates of [2-3H]glucose and [6-3H]glucose) were activated and inactivated by induction of hyperthyroid and hypothyroid states respectively. 5. The hepatic content of glycogen was much lower in hyper- and hypo-thyroid than in euthyroid rats. The enhanced glucose production in hyperthyroid rats resulted from not only activationof hepatic gluconeogenesis but also diversion of the final product of gluconeogenesis from liver glycogen to blood glucose. In hypothyroidism, the inhibition of gluconeogensis led to suppression of both glucose production and glycogenesis in the liver.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Morrison A., Gottlieb B., Bjerknes C., Rathgeb I., Steele R. Alteration by fasting of the effects of methylprednisolone on carbohydrate metabolism in the normal dog. Metabolism. 1974 Apr;23(4):369–374. doi: 10.1016/0026-0495(74)90055-9. [DOI] [PubMed] [Google Scholar]
- Battarbee H. D. The effects of thyroid state on rat liver glucose-6-phosphatase activity and glycogen content. Proc Soc Exp Biol Med. 1974 Nov;147(2):337–343. doi: 10.3181/00379727-147-38337. [DOI] [PubMed] [Google Scholar]
- Belo P. S., Romsos D. R., Leveille G. A. Determination of glucose-utilization in the dog with [2-3H], [6-3H]-, and [U-14C]glucose. Proc Soc Exp Biol Med. 1976 Jul;152(3):475–479. doi: 10.3181/00379727-152-39421. [DOI] [PubMed] [Google Scholar]
- Brockman R. P., Bergman E. N., Pollak W. L., Brondum J. Studies of glucose production in sheep using (6-3H)glucose and (U-14C)glucose. Can J Physiol Pharmacol. 1975 Dec;53(6):1186–1189. doi: 10.1139/y75-164. [DOI] [PubMed] [Google Scholar]
- Brunelle P., Bohuon C. Baisse de la triiodothyronine serique avec l'age. Clin Chim Acta. 1972 Nov;42(1):201–203. doi: 10.1016/0009-8981(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Dunn A., Chenoweth M., Schaeffer L. D. Effect of adrenalectomy on glucose turnover, the Cori cycle, and gluconeogenesis from alanine. Biochim Biophys Acta. 1969 Feb 18;177(1):11–16. doi: 10.1016/0304-4165(69)90058-0. [DOI] [PubMed] [Google Scholar]
- Dunn A., Chenoweth M., Schaeffer L. D. Estimation of glucose turnover and the Cori cycle using glucose-6-t-14C. Biochemistry. 1967 Jan;6(1):6–11. doi: 10.1021/bi00853a002. [DOI] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Forichon J., Jomain M. J., Dallevet G., Minaire Y. Reversible and irreversible glucose disposal in dogs: influence of fasting and cold exposure. Metabolism. 1976 Aug;25(8):897–902. doi: 10.1016/0026-0495(76)90122-0. [DOI] [PubMed] [Google Scholar]
- Freedland R. A., Krebs H. A. The effect of thyroxine treatment on the rate of gluconeogenesis in the perfused rat liver. Biochem J. 1967 Sep;104(3):45P–45P. [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Frayn K. N., Rose J. G. Glucose turnover in the post-absorptive rat and the effects of halothane anaesthesia. Biochem J. 1977 Mar 15;162(3):653–657. doi: 10.1042/bj1620653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Frayn K. N., Rose J. G. Rates of glucose utilization and glucogenesis in rats in the basal state induced by halothane anaesthesia. Biochem J. 1977 Mar 15;162(3):643–651. doi: 10.1042/bj1620643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F. The effect of scald injury upon the distribution of glucose between red cells and plasma and upon the turnover of glucose in red cells in the rat. Br J Exp Pathol. 1973 Aug;54(4):359–367. [PMC free article] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Mak D. 3H-2-glucose in turnover studies. Can J Physiol Pharmacol. 1970 Oct;48(10):732–734. doi: 10.1139/y70-105. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Norwich K. H. Validity of the rates of production and utilization of metabolites as determined by tracer methods in intact animals. Fed Proc. 1974 Jul;33(7):1841–1848. [PubMed] [Google Scholar]
- Hue L., Hers H. G. On the use of (3H, 14C)labelled glucose in the study of the so-called "futile cycles" in liver and muscle. Biochem Biophys Res Commun. 1974 Jun 4;58(3):532–539. doi: 10.1016/s0006-291x(74)80453-5. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M., Borkow I. Estimation of glucose turnover in the dog with glucose-2-T and glucose-U- 14 C. Am J Physiol. 1972 Mar;222(3):710–712. doi: 10.1152/ajplegacy.1972.222.3.710. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M. Effect of catecholamines and methylprednisolone on carbohydrate metabolism of dogs. Metabolism. 1972 Jan;21(1):48–59. doi: 10.1016/0026-0495(72)90019-4. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Borkow I. Effect of catecholamines and dibutyryl-cyclic-AMP on glucose turnover, plasma free fatty acids, and insulin in dogs treated with methylprednisolone. Can J Physiol Pharmacol. 1972 Oct;50(10):999–1006. doi: 10.1139/y72-144. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Accelerated turnover of blood glucose in pertussis-sensitized rats due to combined actions of endogenous insulin and adrenergic beta-stimulation. Biochim Biophys Acta. 1976 Jan 14;421(1):57–69. doi: 10.1016/0304-4165(76)90169-0. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz J., Rostami H., Dunn A. Evaluation of glucose turnover, body mass and recycling with reversible and irreversible tracers. Biochem J. 1974 Jul;142(1):161–170. doi: 10.1042/bj1420161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka M., Ui M. Activation of the Cori cycle by epinephrine. Am J Physiol. 1977 Feb;232(2):E145–E155. doi: 10.1152/ajpendo.1977.232.2.E145. [DOI] [PubMed] [Google Scholar]
- Kusaka M., Ui M. Tracer kinetic analysis of Cori cycle activity in the rat: effect of feeding. Am J Physiol. 1977 Feb;232(2):E136–E144. doi: 10.1152/ajpendo.1977.232.2.E136. [DOI] [PubMed] [Google Scholar]
- Marecek R. L., Feldman J. M. Steady state glucose dynamics in hyperthyroid rabbits. Res Commun Chem Pathol Pharmacol. 1973 Mar;5(2):493–504. [PubMed] [Google Scholar]
- Menahan L. A., Wieland O. The role of thyroid function in the metabolism of perfused rat liver with particular reference to gluconeogenesis. Eur J Biochem. 1969 Aug;10(1):188–194. doi: 10.1111/j.1432-1033.1969.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Minor role of endogenous insulin in thyroid-dependent changes in glucose turnover. Biochem J. 1979 Aug 15;182(2):577–584. doi: 10.1042/bj1820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Relation of catecholamine actions to thyroid activity in controlling glucose turnover. Biochem J. 1979 Aug 15;182(2):585–592. doi: 10.1042/bj1820585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. E. The influence of the thyroid on adrenal cortical function. J Clin Invest. 1958 May;37(5):736–743. doi: 10.1172/JCI103659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield S. P., Whittle E., Hendrich C. E., Little R. C. Hypoglycemia and glycogen deficits in fetuses of hypothyroid pregnant rats. Proc Soc Exp Biol Med. 1975 Jul;149(3):748–753. doi: 10.3181/00379727-149-38891. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Wals P., Katz J. Metabolism of [5-T]fructose by isolated liver cells. J Biol Chem. 1975 Nov 25;250(22):8642–8646. [PubMed] [Google Scholar]
- Sestoft L., Bartels P. D., Fleron P., Folke M., Gammeltoft S., Kristensen L. O. Influence of thyroid state on the effects of glycerol on gluconeogenesis and energy metabolism in perfused rat liver. Biochim Biophys Acta. 1977 Aug 25;499(1):119–130. doi: 10.1016/0304-4165(77)90234-3. [DOI] [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- Singh S. P., Snyder A. K. Effect of thyrotoxicosis on gluconeogenesis from alanine in the perfused rat liver. Endocrinology. 1978 Jan;102(1):182–187. doi: 10.1210/endo-102-1-182. [DOI] [PubMed] [Google Scholar]
- Skikama H., Ui M. Adrenergic receptor and epinephrine-induced hyperglycemia and glucose tolerance. Am J Physiol. 1975 Oct;229(4):962–966. doi: 10.1152/ajplegacy.1975.229.4.962. [DOI] [PubMed] [Google Scholar]
- Skikama H., Ui M. Metabolic background for glucose tolerance: mechanism for epinephrine-induced impairment. Am J Physiol. 1975 Oct;229(4):955–961. doi: 10.1152/ajplegacy.1975.229.4.955. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Suzuki M. Effects of various thyroid states on the metabolism of adenine nucleotides and of glycogen in rat liver. FEBS Lett. 1971 Jan 25;12(4):221–224. doi: 10.1016/0014-5793(71)80025-x. [DOI] [PubMed] [Google Scholar]
- Tibbling G. Glycerol turnover in hyperthyroidism. Clin Chim Acta. 1969 Apr;24(1):121–130. doi: 10.1016/0009-8981(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Winnick S. Response of hepatic glucokinase and glucose-6-phosphatase activities in juvenile and adult hyperthyroid mice. Endocrinology. 1970 Jul;87(1):124–128. doi: 10.1210/endo-87-1-124. [DOI] [PubMed] [Google Scholar]
- Yajima M., Ui M. Gluconeogenesis in epinephrine-induced hyperglycemia. Am J Physiol. 1974 Jul;227(1):1–8. doi: 10.1152/ajplegacy.1974.227.1.1. [DOI] [PubMed] [Google Scholar]


