Abstract
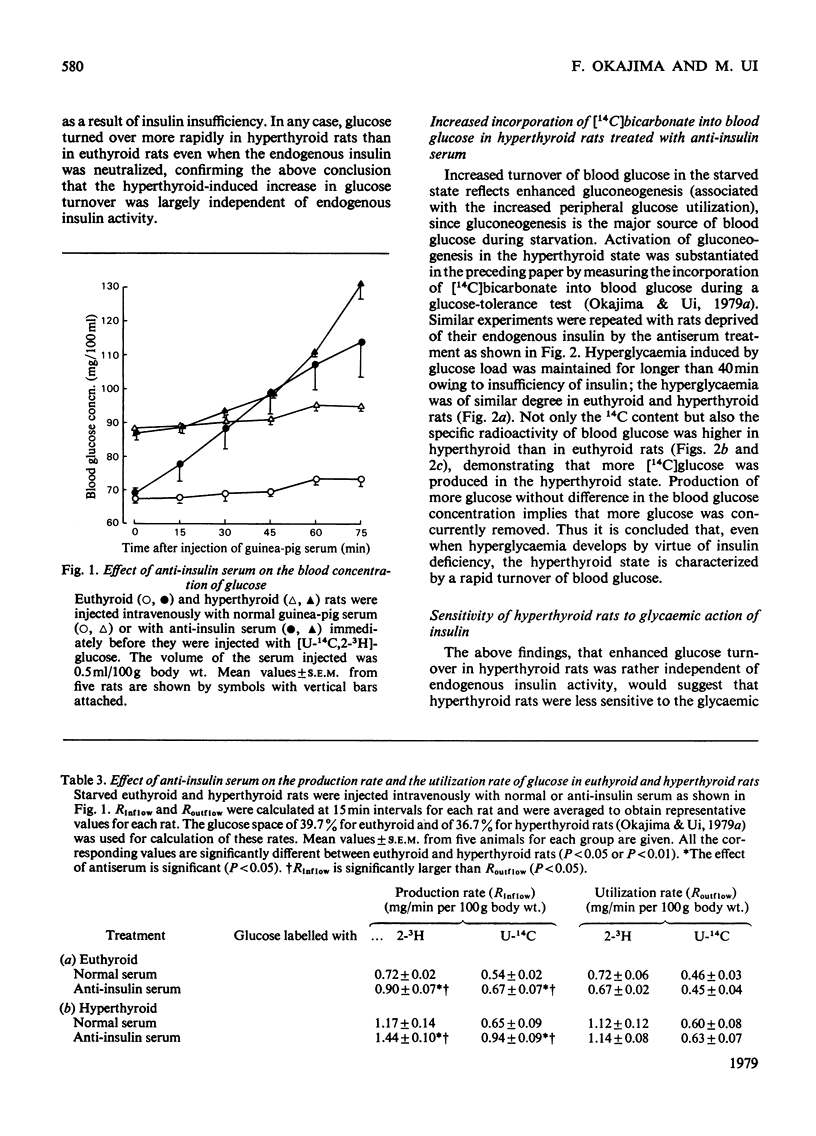
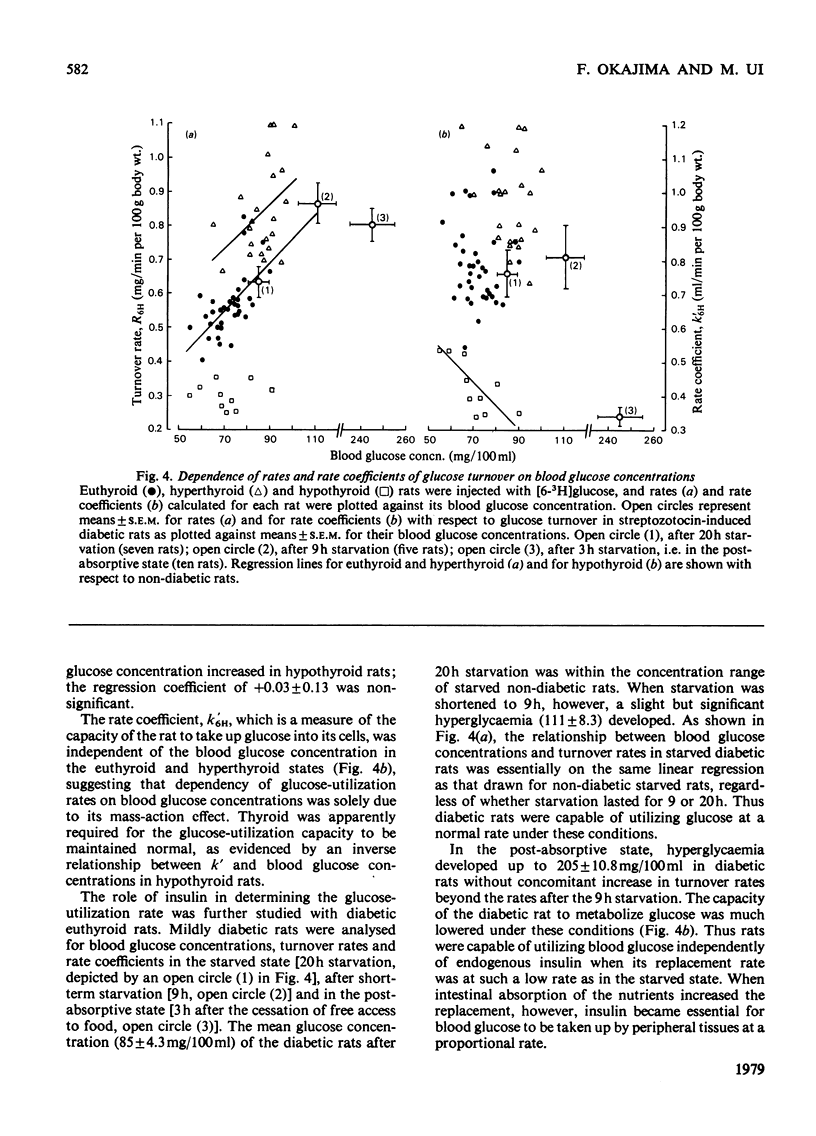
1. Rates and rate coefficients of glucose utilization and replacement (glucose turnover) as well as its recycling were determined in rats by using [U-14C]- and [2-3H]-, [3-3H]- or [6-3H]-glucose. 2. In euthyroid rats, the blood concentration of glucose was 1.5 times and its turnover rate was 2 times as high in the fed state as in the starved state; consequently the rate coefficient, a measure of the capacity of rats to utilize blood glucose, was also higher in the former than in the latter. 3. Induction of mild diabetes by streptozotocin exerted little influence on the content and turnover of blood glucose in the starved state, whereas it caused hyperglycaemia and a decrease in the rate coefficient after feeding. 4. Induction of hyperthyroidism caused increases in rates and rate coefficients of glucose turnover to substantially the same extent whether or not the plasma concentration of insulin was lowered by treatment with streptozotocin or injection with anti-insulin serum. 5. It is concluded that thyroid hormones are capable of enhancing glucose turnover in the starved state independently of endogenous insulin, which plays a significant role in increasing glucose utilization in the fed state.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavagnini F., Peracchi M., Raggi U., Bana R., Pontiroli A. E., Malinverni A., Pinto M. Impairment of growth hormone and insulin secretion in hyperthyroidism. Eur J Clin Invest. 1974 Feb;4(1):71–77. doi: 10.1111/j.1365-2362.1974.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Heath D. F., Frayn K. N., Rose J. G. Glucose turnover in the post-absorptive rat and the effects of halothane anaesthesia. Biochem J. 1977 Mar 15;162(3):653–657. doi: 10.1042/bj1620653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Frayn K. N., Rose J. G. Rates of glucose utilization and glucogenesis in rats in the basal state induced by halothane anaesthesia. Biochem J. 1977 Mar 15;162(3):643–651. doi: 10.1042/bj1620643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura H., Seino Y., Ikeda M., Taminato T., Miyamoto Y. Imparied plasma insulin response to arginine in hyperthyroidism. Important role of the rise of blood glucose in the second phase of insulin release induced by argiinine. Diabetes. 1976 Oct;25(10):961–968. doi: 10.2337/diab.25.10.961. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Accelerated turnover of blood glucose in pertussis-sensitized rats due to combined actions of endogenous insulin and adrenergic beta-stimulation. Biochim Biophys Acta. 1976 Jan 14;421(1):57–69. doi: 10.1016/0304-4165(76)90169-0. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Perfusion of the pancreas isolated from pertussis-sensitized rats: potentiation of insulin secretory responses due to beta-adrenergic stimulation. Endocrinology. 1977 Oct;101(4):1247–1255. doi: 10.1210/endo-101-4-1247. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Spontaneous recovery from streptozotocin-induced diabetes in rats pretreated with pertussis vaccine or hydrocortisone. Diabetologia. 1977 Sep;13(5):521–525. doi: 10.1007/BF01234507. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Joost H. G., Hasselblatt A. Thyroid function and insulin secretion from the perfused pancreas in the rat. Endocrinology. 1976 Jul;99(1):125–129. doi: 10.1210/endo-99-1-125. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Panten U., Hasselblatt A. Thyroxine treatment and insulin secretion in the rat. Diabetologia. 1975 Feb;11(1):49–55. doi: 10.1007/BF00422818. [DOI] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Glucose-turnover values and futile-cycle activities obtained with 14C- and 3H-labelled glucose. Biochem J. 1979 Aug 15;182(2):565–575. doi: 10.1042/bj1820565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Relation of catecholamine actions to thyroid activity in controlling glucose turnover. Biochem J. 1979 Aug 15;182(2):585–592. doi: 10.1042/bj1820585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsetti A., Collard F., Jaffiol C. Abnormalities of carbohydrate metabolism in experimental and clinical hyperthyroidism: studies on plasma insulin and on the A- and B-chains of insulin. Acta Diabetol Lat. 1974 Nov-Dec;11(6):486–492. doi: 10.1007/BF02624588. [DOI] [PubMed] [Google Scholar]
- Renauld A., Pinto J. E., Sverdlik R. C., Foglia V. G. Studies on the effect of hyperthyroidism on the insulin response to hyperglycemia in the dog. Horm Metab Res. 1971 Jul;3(4):247–251. doi: 10.1055/s-0028-1094142. [DOI] [PubMed] [Google Scholar]
- Shikama H., Ui M. Attenuation of epinephrine-induced increase in liver cyclic AMP by endogeneous insulin in vivo. Biochim Biophys Acta. 1976 Sep 24;444(2):461–471. doi: 10.1016/0304-4165(76)90390-1. [DOI] [PubMed] [Google Scholar]
- Shima K., Sawazaki N., Tanaka R., Morishita S., Tarui S. The pancreatic alpha and beta cells responses to 1-arginine and insulin-induced hypoglycaemia in hyperthyroidism. Acta Endocrinol (Copenh) 1976 Sep;83(1):114–122. doi: 10.1530/acta.0.0830114. [DOI] [PubMed] [Google Scholar]
- Skikama H., Ui M. Adrenergic receptor and epinephrine-induced hyperglycemia and glucose tolerance. Am J Physiol. 1975 Oct;229(4):962–966. doi: 10.1152/ajplegacy.1975.229.4.962. [DOI] [PubMed] [Google Scholar]
- Skikama H., Ui M. Metabolic background for glucose tolerance: mechanism for epinephrine-induced impairment. Am J Physiol. 1975 Oct;229(4):955–961. doi: 10.1152/ajplegacy.1975.229.4.955. [DOI] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Kahn C. R., Neville D. M., Jr Insulin binding to liver plasm membranes in the obese hyperglycemic (ob/ob) mouse. Demonstration of a decreased number of functionally normal receptors. J Biol Chem. 1975 Jun 25;250(12):4702–4707. [PubMed] [Google Scholar]


