ABSTRACT
Objectives
Necrotizing enterocolitis (NEC) is a common and sometimes fatal disease affecting premature infants. Elevated formate has been found in the stool of patients with NEC. Sodium formate (NaF) is used to explore the role of formate in the intestinal epithelial injury.
Methods
In this study, 150 mM NaF solution was intraluminally injected in 14-day-old and 28-day-old mice. Mice were sacrificed after 24 h of feces collection, and the blood and small intestinal tissues were collected to detect the pathological damage of intestinal tissue, intestinal permeability, oxidative stress indicators including SOD, HO-1, MDA, and 4-HNE, inflammatory cytokines including IL-1β, TNF-α and IL-6, mitochondrial function such as ATP and PGC-1α in mice intestinal tissue, indicators of the cell death modes including necroptosis-related protein RIPK1 and p-MLKL, and apoptosis- related protein cleaved-caspase-3 and p-AKT (S473).
Results
NaF treatment significantly damaged intestinal epithelial tissue and barrier function, caused mitochondrial dysfunction, manifesting as decreased ATP and PGC-1α levels, increased lipid peroxidation products MDA and 4-HNE, depleted antioxidant enzyme SOD, and upregulated the expression of HO-1. Furthermore, NaF treatment induced inflammatory responses by promoting the release of IL-1β, IL-6 and TNF-α in a development-dependent manner, eventually inducing necroptosis and apoptosis.
Conclusions
Formate may be a source of metabolic intestinal injury contributing to the pathogenesis of NEC in human newborns.
KEYWORDS: Formate, sodium formate, necrotizing enterocolitis, preterm, oxidative stress, inflammation, necroptosis, apoptosis
1. Introduction
Necrotizing enterocolitis (NEC) is a common and sometimes fatal acquired disease affecting premature infants, especially those with very low birth weight and extremely low birth weight. Its incidence is inversely proportional to gestational age [1,2]. Despite decades of research, the precise etiology of NEC remains poorly understood and may be related to many factors, including intestinal immaturity, enteral feeds, intestinal dysbiosis, perinatal ischemic injury, and abnormal inflammatory responses [3–6]. Therefore, most clinical preventive measures and supportive therapies have had limited success, and the morbidity and mortality rates of NEC have decreased minimally [7].
Short-chain fatty acids (SCFAs) are fatty acids with fewer than six carbon (C) atoms, including formate, acetate, propionate, butyrate, and valerate, and are the final products of anaerobic fermentation of indigestible dietary fibers by the human intestinal microbiota and preferred fuel for colonocytes [8,9]. SCFAs, coupled with sodium absorption, are the major mechanisms of salt and water uptake in the colon. In addition, the presence of appropriate SCFAs is important for regulating intestinal osmotic pressure and pH, protecting the intestinal mucosal barrier, and regulating immunity [10]. Therefore, SCFAs are critical for normal colonic physiology. With the completion of gut bacterial colonization and the introduction of enteral feeding formulas, SCFAs are produced in the intestines of newborns. However, overproduction of SCFAs may occur if severe carbohydrate malabsorption and/or bacterial overgrowth exceed the buffering and absorptive capacity of the colon, leading to an increased concentration of SCFAs in the colon [11]. In addition, because of backward reflux across the ileocecal valve and resulting local bacterial overgrowth, the level of SCFAs in the distal ileum may also increase [12]. Existing data suggest that SCFAs in the intestinal lumen can induce concentration- and development- dependent injury to the intestinal mucosa [13,14]. Therefore, although modest quantities of SCFAs are essential for normal colonic function, overproduction or accumulation of intraluminal SCFAs may lead to intestinal mucosal injury and even NEC development, which may be related with vulnerable immature intestinal mucosa, poor gastrointestinal motility and intestinal dysbiosis in premature infants.
Formate or formic acid (FA) has the simplest structure among all SCFAs, and excess formate may be a high-risk factor for NEC. A previous study found that fecal formate levels in patients with NEC at disease onset were higher than those in convalescent and normal newborns and were positively correlated with cytokeratin 8 (K8) [15]. K8 is the main component of intermediate filaments in intestinal glandular epithelial cells and can specifically reflect the extent of intestinal injury. When intestinal mucosal damage occurs, the number of exfoliated epithelial cells in the feces increases, resulting in an increase in fecal K8 levels. Sodium formate (NaF) was found to have a less injuring effect on human intestinal epithelial cell (HIEC-6) than equimolar concentrations of FA in an in vitro pilot experiment (Figure S1); this was likely because FA, as an acid, can change the pH of the cell culture medium. In addition, fecal K8 levels tended to be higher in 150 mM FA-treated mice than in 150 mM NaF-treated mice in the pilot experiment (Figure S2), although the difference was not statistically significant. This may be due to the alkaline environment of the intestine that neutralizes and buffers FA. We used intraluminal injection of 50, 150 and 300 mM NaF to induce intestinal injury in mice in pilot animal experiments, and the results showed that 150 mM NaF was the lowest tested concentration at which K8 levels were significantly increased (Figure S3). To exclude the influence of acid ions and explore the effect and active mechanism of formate in intestinal mucosal injury, 150 mM NaF was chosen as the intervention agent in this study to establish an NEC-like intestinal mucosal injury model to explore the pathogenesis of NEC and provide a theoretical basis for the prevention and treatment of clinical NEC.
2. Materials and methods
2.1. Intestinal mucosal injury mouse model
Forty SPF ICR male mice aged 14 and 28 days were purchased and raised at the Animal Center of Shanxi Medical University and randomly divided into four groups: 14- and 28-day control groups and 14- and 28-day model groups (n = 10/group). Mice were anesthetized by inhalation 3% isoflurane-mixed gas and maintained with 1.5% isoflurane, the abdomen was shaved, and the surgical site was cleaned using 70% alcohol. Laparotomy was performed through the midline of the abdomen with an incision of 1.0 cm. After the ileocecal junction of small intestine was exposed, a sterile NaF (Sigma-Aldrich, 456020, St. Louis, MO, USA) solution (150 mM, 10 µl/g body weight) was injected into the lumen using a 27G needle with the flow aimed toward the proximal small intestine and the abdomen was closed and sutured after gentle massage of the intestine. This model or the effect of this insult is similar to small intestinal bacterial overgrowth. The control group received enteral sham injection without any solution injected into the lumen, and the other operations were the same as the experimental group. Mice were sacrificed by over-anesthesia of 4% isoflurane gas after 24 h of feces collection, and small intestinal tissues were collected and stored at −80 °C for future measurments of various indicators or fixed with 4% paraformaldehyde (Sigma-Aldrich, P6148, USA). At sacrifice, the following scoring criteria reported by Zani, et al. [16] were used to assess macroscopic changes in the intestine: color: 0 normal,1 patchy discoloration, 2 extensive discoloration; consistency: 0 normal,1 moderately friable, 2 extremely friable; dilatation: 0 no dilatation, 1 patchy dilatation, 2 extensive dilatation.
2.2. Histology
According to standard protocol, intestinal tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated, and embedded in paraffin. Subsequently, 8-μm-thick sections were obtained, dewaxed, and stained with hematoxylin–eosin (H&E) (ScyTek Laboratories, Inc., HMM030 and EYB030, Logan, UT, USA). Pathological score of NEC-like intestinal injury were performed by two blinded evaluaters according to the following criteria mentioned in the studies of Casaburi and Wei, et al. [15] and McElroy SJ, et al. [17]: 0, intestinal mucosa and villi are intact and the structure is completely normal; 1, slight submucosal and/or lamina propria separation; 2, moderate submucosal and/or lamina propria separation, submucosal and/or muscular edema; 3, severe submucosal and/or lamina propria separation, submucosal and/or muscular edema, and local villi shedding; 4, intestinal villi are small and accompanied by intestinal necrosis.
2.3. Analysis of intestinal permeability in mice
The mice were orally gavaged with fluorescein isothiocyanate-Dextran (FITC-Dextran, MW 4000, MCE, HY-128868A, USA, 0.44 mg/g body weight). and subsequently sacrificed after 4 h. Before the mice were sacrificed, about 0.5 ml blood was collected by eyeball extripating after anesthetized with 1.5% isoflurane gas, and centrifuged at 4 °C, 3 000 g for 10 min to obtain the serum after coagulation at room temperature for 30 min. Then the serum was diluted with normal saline at 1:4. The FITC-dextran concentration was determined using a Microplate reader (Agilent, BioTek Synergy H1, USA) at an emission wavelength of 480 nm.
2.4. Quantitative detection of adenosine triphosphate (ATP)
Intestinal tissues were lysed with an ATP detection lysate, followed by ultrasonic homogenization. After centrifugation at 4 °C, 12 000 g for 5 min, ATP levels were detected using the ATP detection kit (Beyotime, S0027, China) according to the manufacturer’s instructions.
2.5. Quantitative detections of oxidative stress relative indicators
Superoxide dismutase (SOD) is a powerful endogenous antioxidant, and usually acts as the first line of defense against reactive oxygen species (ROS) [18]. Hemeoxygenase (HO)-1, an inducible isoform of HO usually expressed at low levels under normal conditions, can be induced by various stimuli and plays a critical role in maintaining oxidant/antioxidant homeostasis [19,20]. Malondialdehyde (MDA) has been used as a convenient biomarker for lipid peroxidation for many years, whereas 4-hydroxynonenal (HNE) is currently considered another major biomarker with the highest toxicity [21]. The intestinal tissues were ultrasonically homogenized and centrifuged at 4 °C, 3 000 g for 10 min, and the SOD activity in intestinal tissues was detected using an SOD Detection Kit (Nanjing Jiancheng Bioengineering Institution, A001-3-2, China), and MDA levels were detected the corresponding kit (Beyotime, S0131S, China). The protein expressions of HO-1 and 4-HNE in intestinal tissues were measured by western blot analysis as described below.
2.6. Enzyme-linked immunosorbent assay (ELISA)
Interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α are common pro-inflammatory cytokines released by many immune cells during inflammatory response process, and they have been reported to be elevated in NEC [22]. Part of the intestinal tissues were cut and put into pre-cooled phosphate-buffered saline for ultrasonic homogenization, centrifuged at 4°C, 3000 g for 10 min, and the levels of IL-1β (Boster Bio, EK0394, China), IL-6 (j&l biological, JL20268, China), TNF-α (j&l biological, JL10484, China), and receptor-interacting protein kinase 1 (RIPK1) (j&l biological, JL18522-96 T, China) was detected in the intestinal tissues using the corresponding ELISA kits according to the manufacturer’s protocols.
2.7. Western blot analysis
Intestinal tissues were weighed and homogenized in radio-immunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific, 89901, Rockford, IL, USA) using a polytron homogenizer. The resultant homogenates were used as whole protein lysates for downstream analysis by western blot. The protein concentrations were detected using a BCA Protein Assay Kit (Thermo Fisher Scientific, 23235, USA). Subsequently, samples were loaded into 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (BIO-RAD, 1610174, Hercules, CA, USA) for performing electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes (Amersham Biosciences, 10600023, Buckinghamshire, UK), which were incubated with different primary antibodies at 4 °C overnight according to the proteins detected. The antibodies included anti-β-actin (Santa Cruz Biotechnology, sc517582, Texas, USA, 1:1000); anti-4-HNE (Abcam, ab48506, Cambridge, MA, USA, 1:500), anti-HO-1 (Abcam, ab13243, 1:1000), anti-peroxisome proliferator- activated receptor γ coactivator-1α (PGC-1α) (Abcam, ab77210, 1:500), and anti-Ser and Thr kinase (AKT) S473 (Abcam, ab81283, 1:500); and anti-p-mixed lineage kinase domain like pseudokinase (p-MLKL) (Abcam, ab196436, 1:500) and anti-cleaved caspase 3 (CST, 9664, Boston, MA, USA, 1:500). Subsequently, the membrane was incubated with secondary antibodies such as goat anti-rat (CST, 98164, 1:1000) or goat anti-rabbit antibodies (CST, 91196, 1:1000) at room temperature for 1 h. Protein bands were visualized using an ECL detection kit (Thermo Fisher Scientific, 32109, USA) according to the manufacturer’s protocol and semi-quantitatively analyzed using ImageJ Software (National Institutes of Health, NIH, USA).
2.8. Statistical analysis
All data were analyzed using Graphpad Prism 9.0 software (GraphPad Software Inc., San Diego, CA, USA). Quantitative data conforming to normal distribution and homogeneity of variance, respectively tested by Shapiro–Wilk test and Bartlett test, were presented as means ± standard deviation (SD) and statistically analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test (Graph-Pad Software, CA, USA). Otherwise, Kruskal–Wallis test was used. P < 0.05 was considered statistically significant.
3. Results
3.1. NaF caused the damage of intestinal tissue in a development-dependent manner
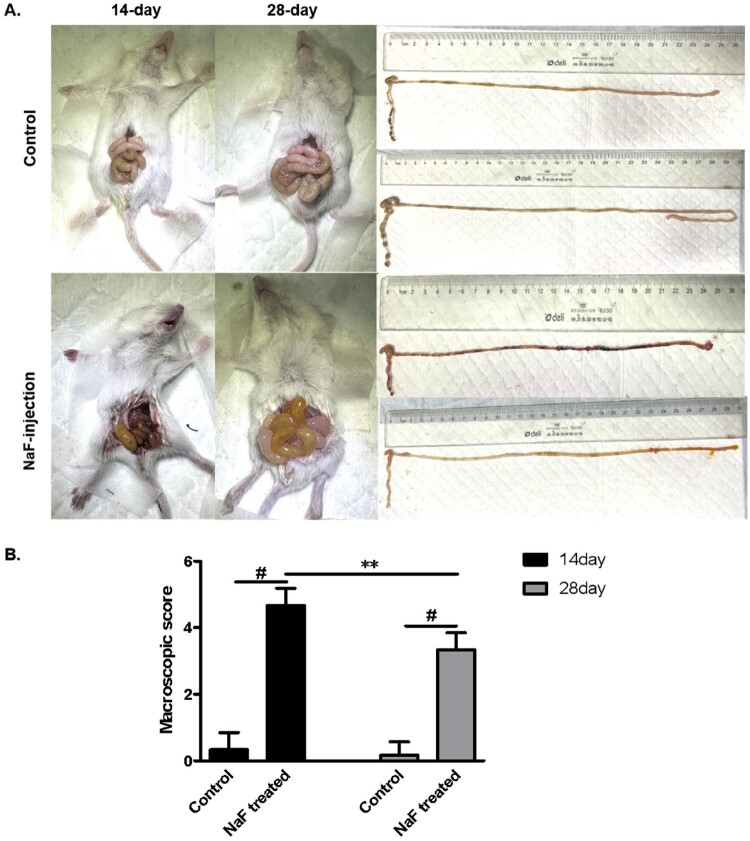
We performed intraluminal injection of 150 mM NaF in 14-day-old and 28-day-old mice (n = 10). Three 14-day-old mice died during the process, and seven 14-day-old and ten 28-day-old mice survived (the survival rates 70% vs 100%) . The basis for diagnosing NEC-like injury in our study is that NEC mice have varying degrees of bloody and loose stools, abdominal distension, and significant pathological changes of intestinal tissue, manifesting as an increased number of exfoliated dead cells in the lumen, disrupted villous structure, shortened villi, and thinning of the mucosal layer. In addition, the mice in the model group has varying degrees of intestinal dilatation, swelling, hemorrhage and necrosis after opening the abdominal cavity. In this study, compared to the control mice, intraluminal injection of NaF caused significant macroscopic changes and NEC-like intestinal tissue injury in pre-weaned mice (14 days) and post-weaned mice (28 days, P < 0.001 or <0.01), and the macroscopic change and pathological change of intestinal tissue were more severe in 14-day mice (P < 0.01 or <0.05, Figures 1, 2A, B).
Figure 1.
Macroscopic changes in 14-day vs. 28-day mice after luminal sodium formate (NaF)-injection. A. Macroscopic images of mouse small intestine. B. Macroscopic score of mouse small intestine (n = 6). (** P < 0.01, # P < 0.001).
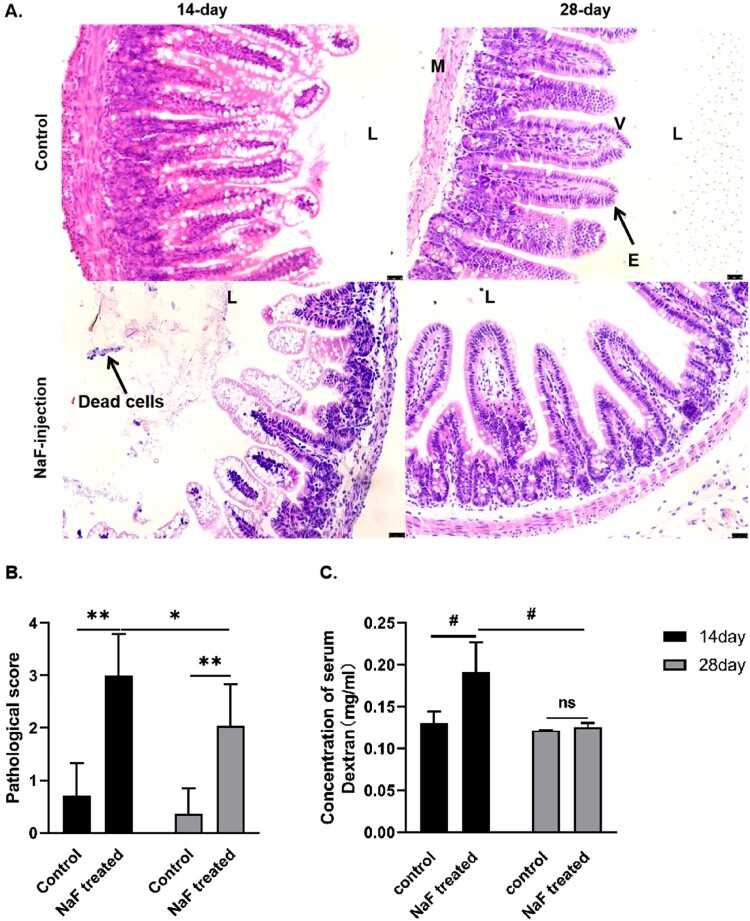
Figure 2.
Increased intestinal epithelial injuries in 14-day vs. 28-day mice after luminal NaF-injection. A. Hematoxylin-eosin (H&E) staining of the mouse small intestinal tissue (magnification ×200, black scale bars indicate a 25 µm). B. Pathological score of mouse small intestinal tissue (n = 6). C. Intestinal permeability measured by serum fluorescein isothiocyanate (FITC)-Dextran levels (n = 6). (* P < 0.05, ** P < 0.01, # P < 0.001). L, lumen; E, epithelial cells; V, villus; M, muscle layer.
To determine the effect of NaF on the intestinal barrier, we measured the levels of FITC-Dextran in mice serum. Compared to the control mice, the serum FITC-Dextran levels of mice in the 14-day model group were significantly increased (P < 0.001). The average level of FITC-Dextran in the 14-day model group was significantly higher than that in the 28-day model group (P < 0.001, Figure 2C), suggesting that 14-day mice have an immature intestinal structure and function and are more susceptible to NaF-induced damage, which also confirms the development-dependent signature in mice.
3.2. NaF induced oxidative stress in intestinal tissue in a development-dependent manner
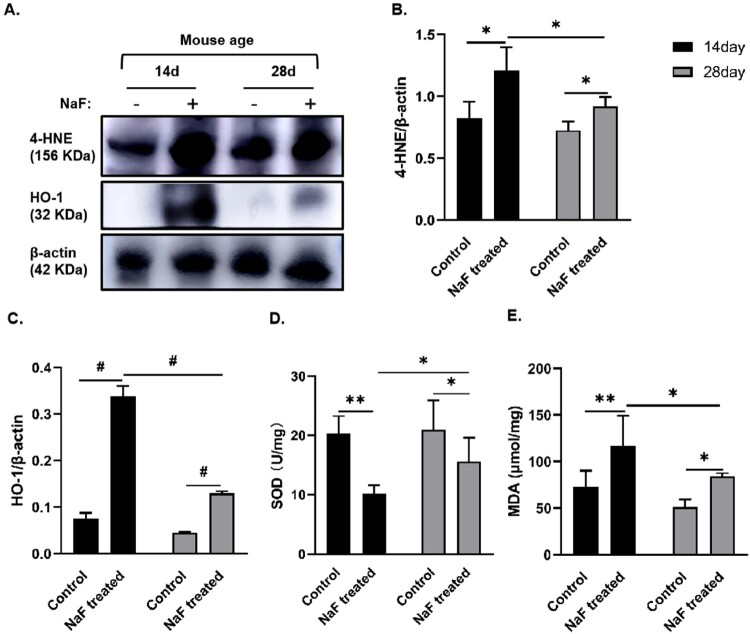
To confirm the conditions of oxidative stress in mouse intestinal tissue, we detected the activity or expression of the antioxidative enzymes SOD and HO-1 and the levels or expression of the lipid peroxidation products MDA and 4-HNE in intestinal tissue. Compared with the corresponding control group, the activity of SOD in the 14-day and 28-day model groups was significantly reduced (respectively P < 0.01 and <0.05), whereas the expression or levels of HO-1, 4-HNE, and MDA were significantly increased (P < 0.05, or <0.01, or <0.001) as shown in Figure 3, indicating that NaF treatment caused oxidative stress, consumed the endogenous antioxidant enzyme SOD, and induced HO-1 expression. Compared to the 28-day model group, the mice in the 14-day model group had lower SOD activity and higher expression or levels of HO-1, MDA and 4-HNE in the intestinal tissues (P < 0.05, or <0.001, Figure 3), indicating that NaF induced oxidative stress in the intestinal tissue in a development-dependent manner. In addition, the trend of HO-1 was consistent with that of the lipid peroxidation products, MDA and 4-HNE, suggesting the inducibility of HO-1.
Figure 3.
NaF induced oxidative stress in intestinal tissue in a development- dependent manner. A–C. Western blot analysis showing the expressions of 4-hydroxynonenal (4-HNE) and heme oxygenase-1 (HO-1) in 14-day and 28-day control and model groups and the statistical comparisons of the relative protein density (n = 3). D. Superoxide dismutase (SOD) activity in intestinal tissue (n = 6). E. Malondialdehyde (MDA) levels in intestinal tissue (n = 6). (* P < 0.05, ** P < 0.01, # P < 0.001).
3.3. NaF triggered inflammatory responses in mice intestinal tissue via development-dependent manner
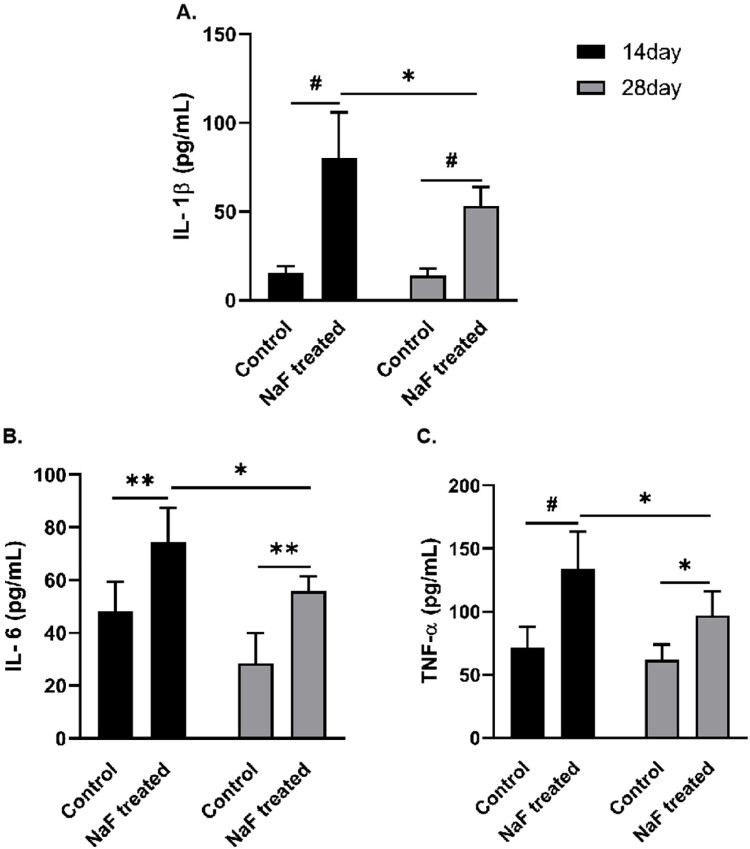
To study the pro-inflammatory effect of NaF, the levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α were detected using ELISA. Compared to the corresponding control groups, NaF treatment significantly increased the levels of IL-1β, IL-6, and TNF-α in intestinal tissues of 14-day and 28-day model groups (P < 0.05, or <0.01, or <0.001) as shown in Figure 4. Notably, the levels of IL-1β, IL-6 and TNF-α were higher in the 14-day model group than in the 28-day model group (P < 0.05, Figure 4), indicating that NaF triggered inflammatory responses in mice intestinal tissue in a development-dependent manner.
Figure 4.
NaF triggered inflammatory responses in intestinal tissues in a development-dependent manner. A–C. The levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α in intestinal tissues (all n = 6). (* P < 0.05, ** P < 0.01, # P < 0.001).
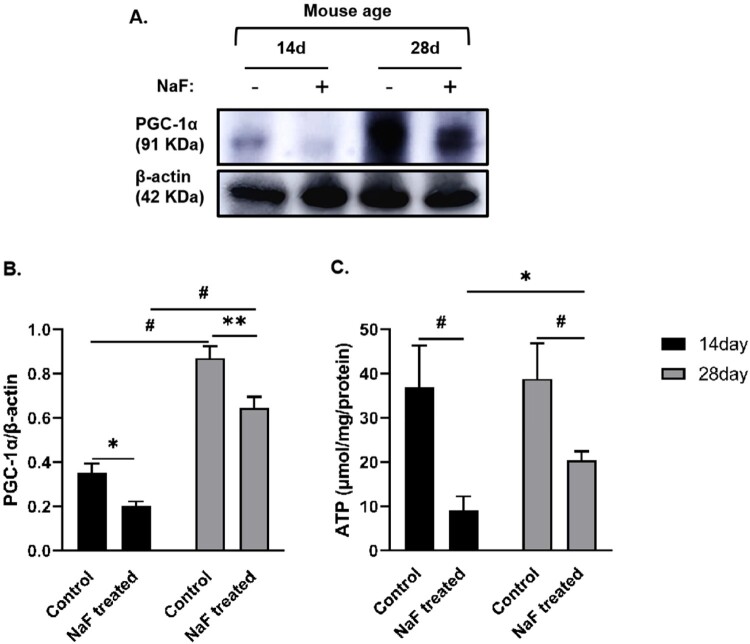
3.4. NaF caused more severe mitochondrial dysfunction in 14-day model mice
PGC-1a regulates mitochondrial biosynthesis, increases mitochondrial oxidative phosphorylation levels, and up-regulates the expression of some proteins. Its expression increases the content of mitochondrial DNA and the number of mitochondria and provides sufficient ATP output for the body [23]. As shown in Figure 5, PGC-1α expression in 14-day control mice was significantly lower than that in 28-day control mice (P < 0.001), indicating that 14-day mice were immature in mitochondrial function and more susceptible to various injuries. To evaluate whether NaF can cause mitochondrial dysfunction, we detected the level or expression of ATP and PGC-1α in intestinal tissue. Compared to the corresponding control group, NaF treatment significantly decreased ATP levels and PGC-1α expression in intestinal tissue, both of which were lower in the 14-day model group than in the 28-day model group (P < 0.05, or <0.001) as shown in Figure 5, suggesting that NaF caused more severe mitochondrial dysfunction in 14-day model mice.
Figure 5.
NaF damaged mitochondrial function in a development-dependent manner. A. Western blot analysis showing peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) expression in intestinal tissues. B. Statistical comparisons of relative protein density in A (n = 3). C. ATP levels in the intestinal tissues (n = 4). (* P < 0.05, ** P < 0.01, # P < 0.001).
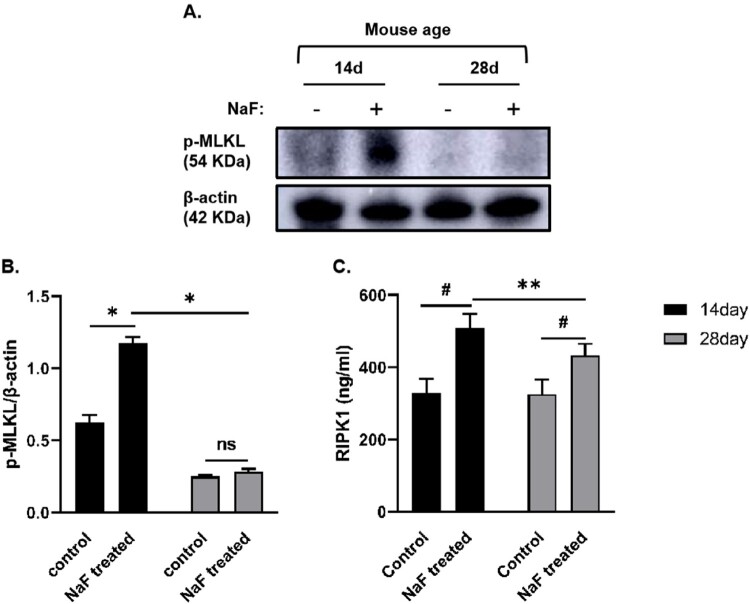
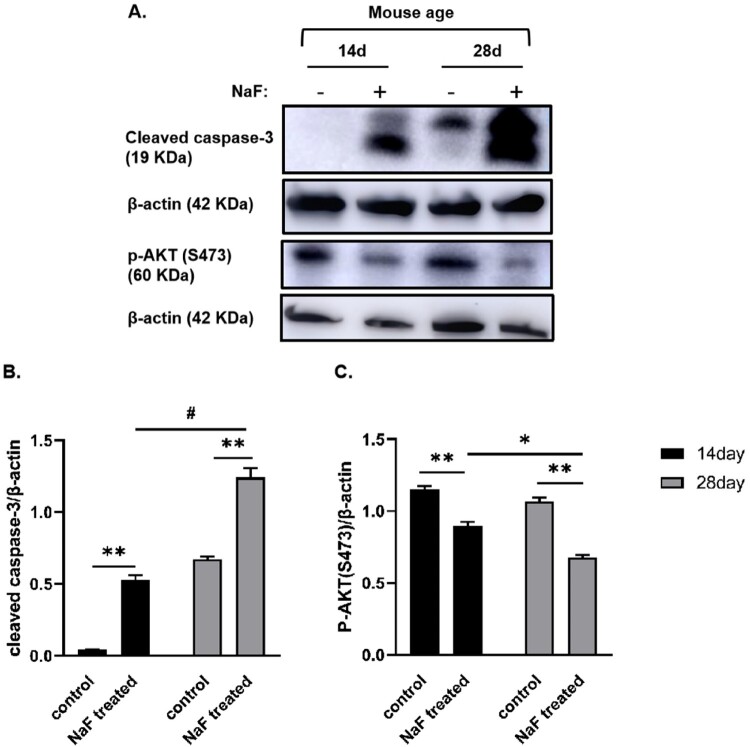
3.5. NaF induced intestinal epithelial cell death via necroptosis and apoptosis in vivo
As shown in Figure 6, compared with the corresponding control groups, the expression or levels of proteins associated with necroptosis activation such as p-MLKL and RIPK1 in the intestinal tissue of 14-day-old model mice were upregulated (P < 0.05 or <0.001), whereas RIPK1 level was significantly increased in 28-day-old model mice (P < 0.001), although there was no significant change in the expression of p-MLKL. As shown in Figure 7, the expressions of cleaved caspase-3, which is the most important terminal cleavage enzyme during apoptosis, were also upregulated in 14-day-old and 28-day-old model mice (P < 0.01), whereas the expressions of p-AKT (S473) inhibiting apoptosis were downregulated (P < 0.01), indicating that NaF treatment induced necroptosis and apoptosis in pre-weaned and post-weaned mice. In addition, our results suggested that NaF mainly induced apoptosis in 28-day mice, whereas necroptosis in 14-day-old mice, by comparing the expression of above 4 proteins associated with necroptosis and apoptosis (P < 0.05, < 0.01, or <0.001), although apoptosis and necroptosis coexisted in the 14-day-old and 28-day-old model mice.
Figure 6.
NaF induced necroptosis in intestinal tissues. A. Western blot analysis showing the expressions of necroptosis-related proteins in intestine tissues in the control and model groups. B Statistical comparisons of relative protein density (n = 3). C. Expression of receptor-interacting protein kinase 1 (RIPK)1 in intestinal tissue in each group (n = 6). (* P < 0.05, ** P < 0.01, # P < 0.001).
Figure 7.
NaF induced apoptosis in intestinal tissues. A. Western blot analysis showing the expressions of apoptosis-related proteins in intestine tissues in the control and model groups. B and C. Statistical comparisons of relative protein density in A (n = 3). (* P < 0.05, ** P < 0.01, # P < 0.001).
4. Discussion
Despite decades of investigations, the etiology of NEC remains unclear. Recently, the intestinal microbiome and its fermentation products, SCFAs, have attracted increasing attention. In this study, a model of NEC-like intestinal mucosal injury was established with luminal NaF treatment to explore the pathogenesis of NEC from mitochondrial dysfunction, which may be due to an imbalance of intestinal flora and metabolites, and can affect various types of cell death. NaF was found to damage mitochondrial function and affect energy metabolism, redox homeostasis, and barrier function of the intestinal epithelium in a development-dependent manner, resulting in necroptosis, apoptosis, and NEC-like injury in mice.
Preterm infants have immature intestines characterized by abnormal absorptive capacity, poor gastrointestinal motility, and an altered gut microbiome [24]. Existing physiological and anatomical differences that lead to intestinal stasis and increased bacterial fermentation of undigested carbohydrates may result in the accumulation of luminal SCFAs, including formate [11,25]. We also found that fecal formate levels in preterm neonates at NEC onset were significantly elevated [15]. Therefore, to elucidate the pathophysiological mechanisms involved, we need to first understand how developmental immaturity of the premature intestine predisposes individuals to intestinal injury and inflammation. It has been reported that the morphology and physiology of the rodent intestine in the second week of life approximates that of the human intestine in the early 3rd trimester [26]. Furthermore, pro-inflammatory responses peak during this time in the murine gut due to immature intestinal barrier function [27,28]. In this study, 14-day-old mice and 28-day-old mice were used to explore whether there is development-dependent intestinal epithelial injury induced by NaF. The reason for choosing 14-day-old and 28-day-old mice for NEC models is that if younger mice were used, they were prone to death leading to experimental failure, if older mice were used, they do not meet the age at which NEC occurs, and if 21-day-old mice were used, the difference may not be significant due to the proximity of the day ages. A previous study showed that in SD rats at postnatal ages of 3-, 9-, and 23-day-old, the severity of mucosal injury induced by the luminal injection of SCFAs decreased as the rats matured [14]. The study showed that, compared to 28-day-old mice, 14-day-old mice had more severe injury and inflammatory response after luminal NaF injection, including significantly intestinal pathological changes, increased intestinal permeability, and elevated levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α. In our previous study, it has been found that the most discriminatory taxa associated with NEC dysbiosis and increased formate production were Enterobacter cloacae and Klebsiella pneumoniae [15]. Lipopolysaccharides (LPS) in the cell walls of these gram-negative bacteria can activate TLR-4 signaling pathway leading to the release of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α, intesinal inflammation and barrier injury, translocation of luminal bacteria, vasoconstriction and intestinal ischemia and NEC [29–31]. This study found that luminal NaF intervention also elevated the levels of IL-1β, IL-6 and TNF-α, indicationg that excess SCFAs such as NaF can lead to intesinal inflammation, and is a high-risk factor for the development of NEC. In summary, these results suggest that luminal NaF could cause NEC-like intestinal epithelial injury in a development-dependent manner, and pre-weaned 14-day-old mice are more susceptible to NaF-induced intestinal injury and are likely to die due to NEC-like intestinal injury, which is consistent with clinical phenomenon that preterm infants are more likely to develop NEC and die.
Mitochondria are key organelles involved in energy metabolism. Normally, mitochondria produce a certain amount of ROS as byproducts of energy metabolism to participate in redox reactions in vivo, which are required for many key cellular functions, such as differentiation, proliferation, and apoptosis [32]. If excess ROS are produced, endogenous antioxidants, such as SOD, can scavenge them, and inducible HO-1 can also be upregulated to maintain cellular homeostasis [19,33]. However, oxidative stress occurs when ROS production exceeds the body’s scavenging capacity, resulting in a redox imbalance. Excessive ROS can interfere with DNA and RNA replication, cause membrane lipid peroxidation, and damage mitochondrial structure and function, leading to less ATP production, increased ROS production, and damage to the antioxidant defense system, which further forms a vicious cycle and aggravates oxidative stress [34]. Formate treatment can inhibit ATP production, increase ROS production, and induce cell death in vitro [15,35]. In this study, luminal injection of NaF caused oxidative stress and mitochondrial dysfunction, manifesting as increased lipid peroxidation products MDA and 4-HNE, upregulated HO-1, decreased SOD activity, and decreased ATP and PGC-1α in the intestinal tissue of mice, which were more significant in the 14-day-old model mice. These results suggested that 14-day-old mice were immature in mitochondrial function and more susceptible to various injuries, and the damaging effect of NaF was development-dependent. Notably, the trend of HO-1 was consistent with that of lipid peroxidation products, suggesting that HO-1 was upregulated by pathological stimuli to maintain the redox balance.
Mitochondria are key regulators of cell death, and mitochondrial dysfunction is involved in apoptosis and necroptosis [36]. Apoptosis is a programmed, sequential, and immunologically silent process that causes barrier dysfunction in bowel diseases, including NEC, and is regarded as a major mechanism of cell death in NEC [37]. However, apoptosis alone is not sufficient to explain the rapid and intense inflammatory responses in NEC. Necroptosis is a newly identified form of pro-inflammatory programmed cell death that is the main form of cell death under various pathological conditions, such as mitochondrial damage and oxidative stress [38]. Necroptosis is usually triggered by the activation of death receptors, such as TNF receptors, followed by the recruitment and activation of RIPK1 and RIPK3, resulting in the phosphorylation, oligomerization, and translocation of MLKL to the plasma membrane and increased membrane permeability and rupture [39,40]. Substantial evidence suggests that necroptosis is involved in the development of intestinal inflammatory diseases, including inflammatory bowel disease (IBD) [41,42] and NEC [43]. A previous study has confirmed that excessive formate in the intestine is associated with NEC occurrence [15]. Wang [44] also showed that the expression of p-MLKL was significantly upregulated in the intestinal tissue of mice exposed to butyrate, which could be inhibited by the necroptosis inhibitor NEC-1, suggesting that butyrate, one of the major forms of SCFAs, can induce intestinal epithelial cell death via necroptosis in vivo. A previous study has confirmed that apoptosis and necroptosis coexist in NEC intestinal epithelial cells and that there is a balance between the two forms of cell death [37]. In this study, NaF treatment significantly increased the expression of p-MLKL and/or the level of RIPK1 associated with necroptosis in model mice, which was more significant in the 14-day-old model mice. However, the expression of the apoptosis-related active protein cleaved caspase-3 in model mice was higher in the 28-day-old mice. These results indicate that NaF treatment mainly induces apoptosis in 28-day-old mice, whereas it induces necroptosis in 14-day-old mice, which may be related to more severe mitochondrial dysfunction and the incomplete initiation of apoptosis caused by immature intestinal barrier function and an imperfect immune system in 14-day-old mice. Necroptosis is accompanied by an inflammatory response, and activation of the RIP3-p-MLKL axis can increase the production of inflammatory cytokines in the intestinal epithelium of patients with IBD, which can change intestinal mucosal permeability [41]. Recent studies have pointed out that the release of the necroptosis effectors MLKL and damage-associated molecular patterns can activate the assembly of the NOD-like receptor protein 3 inflammasome and further expand the inflammatory response [45,46]. Therefore, higher levels of pro-inflammatory cytokines in the intestinal tissue and serum Dextran in the 14-day-old model mice were closely associated with the occurrence of necroptosis in this study. The nuclear factor erythroid 2-related factor 2(Nrf-2)/HO-1 pathway has been found to be associated with the inhibition of oxidative stress, apoptosis [47], and necroptosis [48]. Therefore, HO-1 upregulation induced by NaF treatment in this study may be the feedback protective mechanism initiated by the body in response to noxious stimuli, and the higher expression of HO-1 in 14-day-old mice may be associated with more severe oxidative stress and inflammatory injury of intestinal tissues.
Previous studies have shown that NaF can induce HIEC-6 death through necroptosis in vitro [15], and that NaF can induce autophagy and apoptosis through the c-Jun N-terminal kinase (JNK) signaling pathway in photoreceptor cells in vitro [49]. This study showed that NaF can induce cell death via necroptosis and apoptosis in vivo, and that the difference in the forms of death in vivo and in vitro may be associated with the different intervention concentrations of NaF and the complexity of the body’s regulation of external stimuli. Functional metagenomic findings in a previous study implicated that Enterobacter cloacae and Klebsiella pneumoniae may be major contributors to pyruvate formate lyase and an increase in formate levels [15]. However, there is no direct evidence that alterations in the gut microbiota are associated with increased formate levels in neonates with NEC. In the future, it may be necessary to collect a large number of stool samples from patients with NEC and test for gut flora and SCFAs to identify novel biochemical or metabolic mechanisms underlying NEC.
5. Conclusions
In conclusion, NaF treatment can cause NEC-like intestinal injury via necroptosis and apoptosis in a development-dependent manner in mice by inducing mitochondrial dysfunction, oxidative stress, and inflammatory responses. Therefore, we need to continue exploring the pathogenesis of NEC to develop more effective preventive and therapeutic strategies.
Supplementary Material
Acknowledgements
Thanks to Dr. Yanli Li from the Neurology Laboratory of the First Hospital of Shanxi Medical University for providing experimental equipment during the research.
Funding Statement
This research was supported by Shanxi Scholarship Council of China [grant number 2024-075] and Shanxi Provincial Postgraduate Research and Innovation Project [grant number 2024KY414].
Author contributions
Conceptualization: Jingjing Wei, and Guozhong Tao; Methodology: Yuan Tian, Meiqi Guan, and Jinshu Wei; Formal Analysis: Yuan Tian; Data Curation: Jinshu Wei; Writing-Original Draft Preparation: Yuan Tian; Writing-Review and Editing: Jingjing Wei and Yong Ji; Supervision: Jingjing Wei, Guozhong, and Karl G. Sylvester.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All experiments were conducted under a protocol approved by Laboratory Animal Welfare Ethics Committee of Shanxi Medical University in accordance with Regulations on the Management of Experimental Animals issued and revised by the State Council of China in 2017 and the ARRIVE guidelines (Approval Number: SYDL2024022).
Patient consent for publication
This study did not involve human experiments.
References
- 1.Eaton S, Rees CM, Hall NJ.. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology. 2017;111(4):423–430. doi: 10.1159/000458462 [DOI] [PubMed] [Google Scholar]
- 2.Neu J. Necrotizing enterocolitis: The future. Neonatology. 2020;117(2):240–244. doi: 10.1159/000506866 [DOI] [PubMed] [Google Scholar]
- 3.Denning TL, Bhatia AM, Kane AF, et al. Pathogenesis of NEC: role of the innate and adaptive immune response. Semin Perinatol. 2017;41(1):15–28. doi: 10.1053/j.semperi.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elgin TG, Kern SL, McElroy SJ.. Development of the neonatal intestinal microbiome and its association With necrotizing enterocolitis. Clin Ther. 2016;38(4):706–715. doi: 10.1016/j.clinthera.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 5.Neu J, Pammi M.. Pathogenesis of NEC: impact of an altered intestinal microbiome. Semin Perinatol. 2017;41(1):29–35. doi: 10.1053/j.semperi.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 6.Warner BB, Deych E, Zhou Y, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387(10031):1928–1936. doi: 10.1016/S0140-6736(16)00081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan AT, Ward L, Schibler K, et al. A quality improvement initiative to reduce necrotizing enterocolitis across hospital systems. J Perinatol. 2018;38(6):742–750. doi: 10.1038/s41372-018-0104-0 [DOI] [PubMed] [Google Scholar]
- 8.Martin-Gallausiaux C, Marinelli L, Blottière HM, et al. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49. doi: 10.1017/S0029665120006916 [DOI] [PubMed] [Google Scholar]
- 9.Frolova MS, Suvorova IA, Iablokov SN, et al. Genomic reconstruction of short-chain fatty acid production by the human gut microbiota. Front Mol Biosci. 2022;9:949563. doi: 10.3389/fmolb.2022.949563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Born P, Bauch C, Ulm K, et al. Fecal bacterial activity in symptomatic carbohydrate malabsorption: effect on the fecal short-chain fatty acid ratio. Z Gastroenterol. 2000;38(8):623–626. doi: 10.1055/s-2000-7512 [DOI] [PubMed] [Google Scholar]
- 12.Roland BC, Ciarleglio MM, Clarke JO, et al. Low ileocecal valve pressure is significantly associated with small intestinal bacterial overgrowth (SIBO). Dig Dis Sci. 2014;59(6):1269–1277. doi: 10.1007/s10620-014-3166-7 [DOI] [PubMed] [Google Scholar]
- 13.Lin J. Too much short chain fatty acids cause neonatal necrotizing enterocolitis. Med Hypotheses. 2004;62(2):291–293. doi: 10.1016/S0306-9877(03)00333-5 [DOI] [PubMed] [Google Scholar]
- 14.Nafday SM, Chen W, Peng L, et al. Short-chain fatty acids induce colonic mucosal injury in rats with various postnatal ages. Pediatr Res. 2005;57(2):201–204. doi: 10.1203/01.PDR.0000150721.83224.89 [DOI] [PubMed] [Google Scholar]
- 15.Casaburi G, Wei J, Kazi S, et al. Metabolic model of necrotizing enterocolitis in the premature newborn gut resulting from enteric dysbiosis. Front Pediatr. 2022;10:893059. doi: 10.3389/fped.2022.893059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zani A, Cordischi L, Cananzi M, et al. Assessment of a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg. 2008;18(6):423–426. doi: 10.1055/s-2008-1038951 [DOI] [PubMed] [Google Scholar]
- 17.McElroy SJ, Castle SL, Bernard JK, et al. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am J Pathol. 2014;184(10):2768–2778. doi: 10.1016/j.ajpath.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ighodaro OM, Akinloye OA.. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 2018;54(4):287–293. doi: 10.1016/j.ajme.2017.09.001 [DOI] [Google Scholar]
- 19.Araujo JA, Zhang M, Yin F.. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao M, Qi Z, Deng M, et al. The deubiquitinase USP7 regulates oxidative stress through stabilization of HO-1. Oncogene. 2022;41(33):4018–4027. doi: 10.1038/s41388-022-02403-w [DOI] [PubMed] [Google Scholar]
- 21.Ramana KV, Srivastava S, Singhal SS.. Lipid peroxidation products in human health and disease 2014. Oxid Med Cell Longev. 2014;2014:162414. doi: 10.1155/2014/162414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer CM, Khan AM, Alcorn JL.. Impact of surfactant protein-A on immunomodulatory properties of murine and human breast milk. J Pediatr Gastroenterol Nutr. 2022;75(1):97–103. doi: 10.1097/MPG.0000000000003458 [DOI] [PubMed] [Google Scholar]
- 23.Abu Shelbayeh O, Arroum T, Morris S, et al. PGC-1α Is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants. 2023;12(5):1075. doi: 10.3390/antiox12051075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neu J, Chen M, Beierle E.. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14(3):137–144. doi: 10.1053/j.sempedsurg.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 25.Di Lorenzo M, Bass J, Krantis A.. An intraluminal model of necrotizing enterocolitis in the developing neonatal piglet. J Pediatr Surg. 1995;30(8):1138–1142. doi: 10.1016/0022-3468(95)90006-3 [DOI] [PubMed] [Google Scholar]
- 26.McCracken VJ, Lorenz RG.. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3(1):1–11. doi: 10.1046/j.1462-5822.2001.00090.x [DOI] [PubMed] [Google Scholar]
- 27.Lin PW, Myers LE, Ray L, et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47(8):1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RM, Myers LS, Kurundkar AR, et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180(2):626–635. doi: 10.1016/j.ajpath.2011.10.025. Erratum in: Am J Pathol. 2012 Mar;180(3):1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho SX, Berger PJ, Nold-Petry CA, et al. The immunological landscape in necrotising enterocolitis. Expert Rev Mol Med. 2016;18:e12. doi: 10.1017/erm.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackam DJ, Sodhi CP.. Toll-Like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol. 2018;6(2):229–238.e1. doi: 10.1016/j.jcmgh.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duess JW, Sampah ME, Lopez CM, et al. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes. 2023;15(1):2221470. doi: 10.1080/19490976.2023.2221470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sies H, Belousov VV, Chandel NS, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23(7):499–515. doi: 10.1038/s41580-022-00456-z [DOI] [PubMed] [Google Scholar]
- 33.Chiang SK, Chen SE, Chang LC.. The role of HO-1 and Its crosstalk with oxidative stress in cancer cell survival. Cells. 2021;10(9):2401. doi: 10.3390/cells10092401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loor G, Kondapalli J, Iwase H, et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011;1813(7):1382–1394. doi: 10.1016/j.bbamcr.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng L, Huang J, Feng P, et al. Transcriptomic analysis of formic acid stress response in Saccharomyces cerevisiae. World J Microbiol Biotechnol. 2022;38(2):34. doi: 10.1007/s11274-021-03222-z [DOI] [PubMed] [Google Scholar]
- 36.Bock FJ, Tait SWG.. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100. doi: 10.1038/s41580-019-0173-8 [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Zong H, Chen X, et al. Toll-like receptor 4-mediated necroptosis in the development of necrotizing enterocolitis. Pediatr Res. 2022;91(1):73–82. doi: 10.1038/s41390-021-01457-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Q, Zhang J, Sun Q, et al. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotoxicol Environ Saf. 2020;203:110974. doi: 10.1016/j.ecoenv.2020.110974 [DOI] [PubMed] [Google Scholar]
- 39.Yang M, Chen W, He L, et al. A glimpse of necroptosis and diseases. Biomed Pharmacother. 2022;156:113925. doi: 10.1016/j.biopha.2022.113925 [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Kos R, Garssen J, et al. Molecular insights into the mechanism of necroptosis: The necrosome As a potential therapeutic target. Cells. 2019;8(12):1486. doi: 10.3390/cells8121486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negroni A, Colantoni E, Pierdomenico M, et al. RIP3 AND pMLKL promote necroptosis-induced inflammation and alter membrane permeability in intestinal epithelial cells. Dig Liver Dis. 2017;49(11):1201–1210. doi: 10.1016/j.dld.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 42.Pierdomenico M, Negroni A, Stronati L, et al. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol. 2014;109(2):279–287. doi: 10.1038/ajg.2013.403 [DOI] [PubMed] [Google Scholar]
- 43.Werts AD, Fulton WB, Ladd MR, et al. A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol. 2020;9(3):403–423. doi: 10.1016/j.jcmgh.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K, Tao GZ, Salimi-Jazi F, et al. Butyrate induces development-dependent necrotizing enterocolitis-like intestinal epithelial injury via necroptosis. Pediatr Res. 2023;93(4):801–809. doi: 10.1038/s41390-022-02333-z [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez KD, Davis MA, Daniels BP, et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1β independently of gasdermin-D. J Immunol. 2017;198(5):2156–2164. doi: 10.4049/jimmunol.1601757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriwaki K, Chan FK.. The inflammatory signal adaptor RIPK3: functions beyond necroptosis. Int Rev Cell Mol Biol. 2017;328:253–275. doi: 10.1016/bs.ircmb.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C, Chen RL, Wang Y, et al. Acacetin alleviates myocardial ischaemia/reperfusion injury by inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1 pathway. Pharm Biol. 2022;60(1):553–561. doi: 10.1080/13880209.2022.2041675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao P, Wei Y, Sun G, et al. Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway. J Neuroinflammation. 2022;19(1):269. doi: 10.1186/s12974-022-02633-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Xu SL, Xu WJ, et al. Sodium formate induces autophagy and apoptosis via the JNK signaling pathway of photoreceptor cells. Mol Med Rep. 2016;13(2):1111–1118. doi: 10.3892/mmr.2015.4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.