Abstract
Background
Checkpoint inhibitor-related pneumonitis (CIP) is a rare but serious complication of immune checkpoint inhibitors (ICIs). While it typically occurs within the first few months of treatment, its onset after ICI discontinuation is relatively uncommon. This report presents a case of CIP occurring 2.5 months after cessation of pembrolizumab and reviews the existing literature on CIP after discontinuation of ICIs.
Case presentation
A 77-year-old female with stage IV right lung adenocarcinoma (T4N2M1a) developed pneumonitis 2.5 months after discontinuation of pembrolizumab (following 26 months of initial treatment). Initially suspected as community-acquired pneumonia, the patient received antiviral and antibiotic therapy with progressive deterioration. Microbiological investigations yielded negative results, and consultation suggested lung cancer recurrence. PET-CT revealed heightened metabolic activity in the lungs. Percutaneous lung biopsy demonstrated organizing pneumonia, and NGS testing of biopsy tissue showed no pathogenic organisms. Combined with CT findings and the patient’s history of pembrolizumab use, the diagnosis of checkpoint inhibitor-related pneumonitis (CIP) was established. Short-term steroid therapy resulted in significant improvement.
Conclusions
Integration of clinical presentation, imaging findings, and medication history is crucial for diagnosis. This case underscores the need for vigilance for CIP even after discontinuing ICI therapy. Although this report provides insights into CIP after discontinuation based on a single case and a literature review, further studies involving larger cohorts are warranted to better understand the post-treatment risk of CIP.
Keywords: Lung adenocarcinoma, Immune checkpoint inhibitors, Checkpoint inhibitor-related pneumonitis, Pembrolizumab
Background
Immune checkpoint inhibitors (ICIs) have significantly prolonged the survival of patients and have become the preferred therapeutic option for advanced lung cancer patients [1, 2]. The most widely used ICIs in clinical practice are PD-1/PD-L1 inhibitors. However, with the increasing use of ICIs, immune-related adverse events (irAEs) have also increased. Among them, checkpoint inhibitor-related pneumonitis (CIP) is one of the rare but most severe complications [3]. It has been reported that the incidence of CIP is 3–5%, with a related mortality rate of 10–17% [4, 5]. However, due to the lack of typical clinical manifestations and imaging features, clinical diagnosis is challenging [6]. Additionally, the onset of CIP varies, with reports ranging from several hours to 24 months after the first use of ICIs, with a median onset time of 2–3 months after initial ICI administration [7] and is very rarely seen after treatment has ended. Herein, we present a case of pembrolizumab-related pneumonitis occurring more than 2 months after discontinuation of PD-1 inhibitor pembrolizumab. We also review the relevant literature on post-discontinuation CIP, providing insights for the early identification, diagnosis, and management of CIP following ICI cessation.
Case presentation
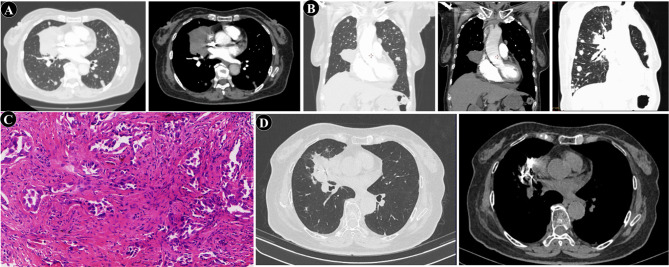
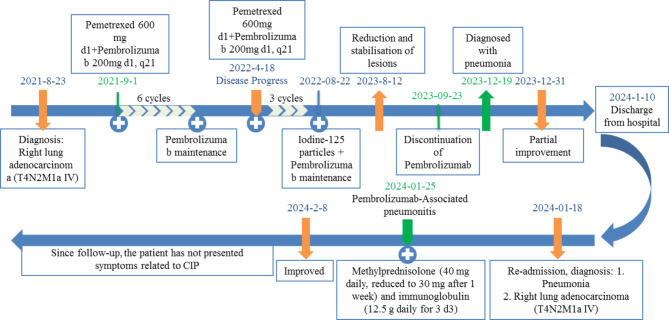
A 77-year-old female patient presented to our hospital with fatigue. She had no history of smoking, chronic cough, asthma, or tuberculosis. The patient had a two-year history of diabetes mellitus, which was managed with oral voglibose (0.2 mg, three times daily). A chest CT scan revealed a pulmonary mass. Subsequent enhanced CT imaging revealed a right lung mass measuring approximately 71 × 51 mm, with multifocal nodules in both lungs. Preliminary assessment suggested metastatic lesions, inflammatory changes in the left lung, localized emphysema in the right lung apex, interstitial alterations in both lungs, and multiple enlarged lymph nodes in the mediastinum (Fig. 1A). Tumor markers NSE and CYFRA21 were elevated at 24.17 ng/ml and 122.76 ng/ml, respectively. CT-guided percutaneous biopsy confirmed adenocarcinoma of the lung (Fig. 1B-C). Immunohistochemistry revealed positivity for TTF-1, NapsinA, and CK7, while P40, CK5/6, CD56, and SYN were negative. The diagnosis was established as stage IV right lung adenocarcinoma (T4N2M1a). One week later, the patient commenced treatment with pemetrexed (600 mg d1) and pembrolizumab (200 mg d1), q21. After 6 cycles, the tumor showed gradual regression, particularly in metastatic lesions, with maintenance therapy of Pembrolizumab. Following approximately four months of maintenance therapy with pembrolizumab monotherapy, the right lung lesion showed an increase in size. Pemetrexed chemotherapy was reintroduced, but after three additional cycles, disease progression continued. The patient’s family opted for the implantation of radioactive iodine-125 seeds. A total of 67 seeds implanted at an activity of 0.7 mCi, delivering a prescribed dose of 110 Gy. Pembrolizumab maintenance therapy was continued. The tumor again began to shrink, and a follow-up CT of the chest one year later demonstrated stability with a reduction in the right hilar lesion, while metastatic foci remained indistinct (Fig. 1D). Pembrolizumab was discontinued after two years of initial treatment (Fig. 2).
Fig. 1.
Radiological and pathological findings during diagnosis and treatment of pulmonary adenocarcinoma. (A) Chest CT on August 24, 2021: Occupying lesion at the right hilum of the lung, with multiple nodules of varying sizes in both lungs. (B) Percutaneous biopsy guided by CT. (C) Pathological of the percutaneous biopsy (×100). (D) Final follow-up chest CT on August 12, 2023: The lesion at the right hilum of the lung has decreased in size and stabilized, with visible aggregation of radioactive iodine-125 particles within the lesion, while metastatic foci remain indistinct
Fig. 2.
Flowchart of Patient Diagnosis and Treatment
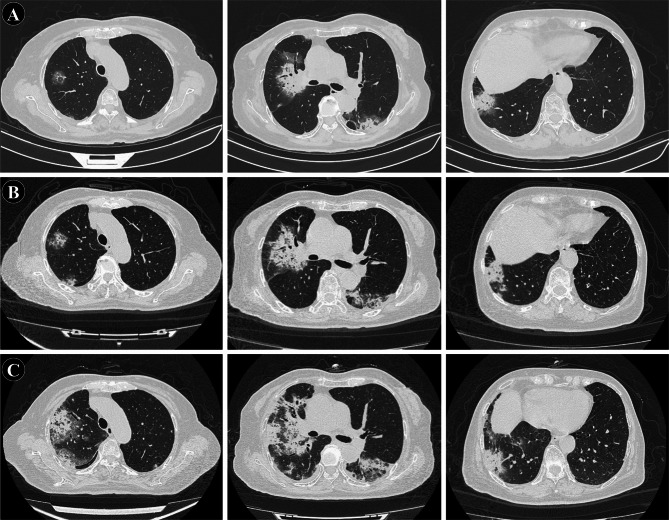
Two and a half months after stopping treatment, the patient presented with fever reaching a peak of 38.8℃, accompanied by fatigue, dry cough, and subsequently, a small amount of white sputum, without hemoptysis. Laboratory investigations including routine blood tests, calcitonin levels, and respiratory pathogen panel were unremarkable, with negative results for COVID-19 nucleic acid and antibody tests, as well as influenza virus antigen. Oral administration of Moxifloxacin and herbal medicine was ineffective. Chest CT revealed bilateral pulmonary infiltrates characterized by multiple peripheral patchy high-density shadows (Fig. 3A). The patient was diagnosed with pneumonia and hospitalized. She received a 14-day course of antibiotics, including ceftazidime, moxifloxacin, ribavirin, and oseltamivir, alongside 7 days of intravenous methylprednisolone (40 mg once daily). A follow-up chest CT showed partial improvement in some lesions (Fig. 3B), and the patient, being asymptomatic, was discharged 10 days later for observation without further medication.
Fig. 3.
Radiological changes during diagnosis and treatment of pneumonia. (A) On December 19, 2023, the patient presented with respiratory symptoms, and chest CT revealed multiple patchy high-density shadows distributed peripherally in both lungs. (B) Follow-up chest CT after antimicrobial treatment on December 31, 2023: Multiple high-density shadows in both lungs with slight reduction in size. (C) Follow-up chest CT on January 18, 2024: Significant increase in size of multiple high-density shadows in both lungs
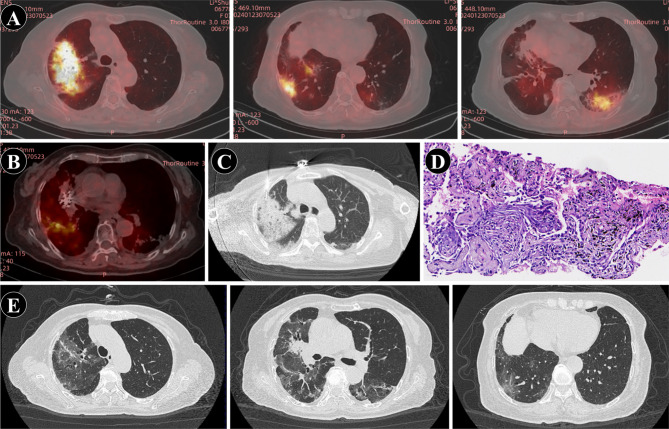
One week after discharge, the patient was readmitted due to worsening cough, accompanied by chest tightness and dyspnea for 4 days. Chest CT revealed a significant enlargement of the previously observed bilateral lung infection (Fig. 3C). On admission, vital signs were notable for a temperature of 37.7℃, heart rate of 92 beats per minute, respiratory rate of 17 breaths per minute, and blood pressure of 110/60 mmHg. Physical examination revealed scattered coarse and moist rales in both lungs, with pronounced involvement of the right lower lung. Laboratory investigations showed elevated white blood cell count (9.6 × 109/L) with predominant neutrophilia, elevated D-dimer levels (8.98 mg/L), and markedly elevated C-reactive protein (159.7 mg/L). Arterial blood gas analysis revealed mild respiratory alkalosis. Notably, tests for COVID-19 and influenza were negative, while serological assays indicated positivity for Mycoplasma pneumoniae IgM antibodies (1.07). Expert consultation at a tertiary care center was sought on three occasions due to concerns about possible disease progression in the context of lung cancer. Further diagnostic evaluations, including PET-CT, lung biopsy, and next-generation sequencing (NGS) analysis, were performed to differentiate between disease progression and infectious pneumonia. PET-CT showed increased metabolic activity (SUV 2.6) in patchy lung lesions (Fig. 4A-B), suggestive of inflammation. Given the rapid radiological changes, disease progression was deemed unlikely. Sputum bacterial culture was negative, fungal smear showed no fungi, and NGS of biopsy specimens revealed no pathogenic microorganisms, excluding infectious pneumonia. CT showed multiple patchy high-density shadows in both lungs (Fig. 4C), which was consistent with the imaging manifestations of CIP outlined in previous studies [8]. Histopathological examination confirmed organizing pneumonia with no evidence of tumor cells, leading to the final diagnosis of pembrolizumab-related pneumonitis (Fig. 4C-D). Antibiotic therapy was discontinued, and the patient was treated with intravenous methylprednisolone (40 mg daily). Due to hyperglycemia, the dose was tapered to 30 mg daily after one week and continued for three days. Concurrently, the patient received intravenous immunoglobulin (12.5 g daily for three consecutive days). Her symptoms gradually improved, and follow-up chest CT revealed significant resolution of the pneumonitis changes (Fig. 4E). As of the latest follow-up, the patient has not exhibited any CIP-related symptoms. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Fig. 4.
Radiological and pathological features during diagnosis and treatment of pulmonary pneumonia. A. PET-CT: Increased metabolic activity in bilateral pneumonia lesions. B. BT-CT: No increased metabolic activity observed in lesions of primary lung cancer implanted with radioactive iodine-125 particles and metastatic foci. C. Percutaneous biopsy guided by CT. D. Histopathological findings of the percutaneous biopsy: Organizing pneumonia (×100). E. Follow-up chest CT on February 8, 2024: Significant improvement and absorption of pneumonia
Literature review
Using the search terms ((“anti-PD1"OR “pembrolizumab” OR “nivolumab” OR “immune checkpoint inhibitor” OR “durvalumab” OR “sintilimab” OR “camrelizumab” OR “toripalimab” OR “tislelizumab”) AND (“cessation” OR " discontinuation”) AND (“pneumonitis” OR “pneumonia”)) in PubMed (as of October 2024), a total of 142 results were retrieved. After excluding duplicates, reviews, meta-analyses, and reports in languages other than Chinese or English, we focused solely on case reports of CIP that occurred after ICI therapy cessation rather than during treatment. Additionally, case series lacking detailed patient data (e.g., age, sex, disease, clinical symptoms, imaging findings, type of ICI, time interval from drug withdrawal to CIP onset, CIP grade, treatments, and outcomes, with more than three key details missing) were excluded. This review identified 10 relevant cases [9–16], and together with the present case, the characteristics of 11 patients are summarized in Table 1.
Table 1.
Clinical data of 11 patients who developed CIP following ICIs treatment cessation
| Author | Patient’s disease | Age | Sex | Clinical Manifestations | Imaging Findings | Type of ICIs | Time interval from drug withdrawal to CIP onset | CIP grade | Treatments | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Kucukarda et al. [9] | Osteosarcoma | 25 | Female | Dyspnea and dry cough | Diffuse interstitial | Atezolizumab | 24 months | 2 | Methylprednisolone | Improved |
| Wu et al. [10] | lung adenocarcinoma | 69 | Male | intermittent Cough, yellow phlegm and fever | Interstitial changes and GGOs | Sintilimab | 6 months | 2 | Methylprednisolone | Improved |
| Diamantopoulos et al. [11] | Melanoma | 62 | Female | Dyspnea and dry cough | Patchy consolidation | Nivolumab | 6 months | 2 | Moxifloxacin and prednisone | Improved |
| Mandala et al. [12] | Melanoma | 64 | Female | Short of breath and dyspnea | Diffuse lesions and nodules | Nivolumab | 8 months | 3 | Widespectrum antibiotics and methylprednisolone | Improved |
| Kimura et al. [13] | Lung adenocarcinoma | 62 | Male | Dyspnea | GGOs | Nivolumab | 7 months | 2 | Prednisolone | Improved |
| Kimura et al. [13] | Squamous cell carcinoma | 68 | Male | / | GGOs | Nivolumab | 8 months | 1 | No treatment | Improved |
| Nakai et al. [14] | Renal cell carcinoma | 50 | Male | Intermittently run a fever | GGOs | Nivolumab | 142 days | 2 | Methylprednisolone and mycophenolate mofetil | Improved |
| Yang et al. [15] | Gastric Cancer | 86 | Male | Asthma with a low-grade fever | Interstitial pneumonia | Pembrolizumab | 2 months | / | Methylprednisolone | Improved |
| Xie et al. [16] | Large-cell neuroendocrine carcinomas | 67 | Male | Dyspnea | / | Pembrolizumab | 2 weeks | / | Improved | |
| Kimura et al. [13] | Lung adenocarcinoma | 69 | Male | Respiratory failure | GGOs | Pembrolizumab | 4 months | 4 | Methylprednisolone | Death |
| This case | Lung adenocarcinoma | 77 | Female | Fever, fatigue, and dry cough | Multiple patchy high-density areas | Pembrolizumab | 2.5 months | 3 | Methylprednisolone | Improved |
CIP: immune checkpoint inhibitor-related pneumonitis; ICIs: immune checkpoint inhibitors; GGOs: ground-glass opacities
Discussion
With the increasing widespread application of ICIs such as PD-1 inhibitor pembrolizumab in clinical lung cancer treatment, irAEs have also increased. Among them, CIP has a relatively low overall incidence rate. However, inadequate clinical awareness can lead to severe or even fatal consequences [17]. This case report describes a patient who developed CIP following treatment with the PD-1 inhibitor pembrolizumab for lung adenocarcinoma. CIP primarily manifests as nonspecific interstitial pneumonitis and often occurs within 0.5 to 24.3 months after the initial use of ICIs, with a median onset time of 2.6 months [18]. For this patient, CIP occurred more than two months after discontinuation of pembrolizumab, which was administered for over 26 months. Given this, a review of the literature on on CIP after discontinuation of ICIs was conducted to summarize the available information.
Reports of CIP occurring after pembrolizumab discontinuation in lung cancer patients are rare. One notable case was reported by Kimura et al. [9], in which a patient with stage IVB lung adenocarcinoma developed pembrolizumab-induced pneumonitis four months after stopping treatment. Importantly, the median interval between treatment cessation and CIP onset was 4.5 months (range: 2 weeks to 24 months), and two patients experienced intermittent CIP recurrence months or even years after discontinuing ICIs [9, 13]. This highlights the need for clinicians to remain vigilant for recurrent CIP. Additionally, three patients with CIP also presented with other irAEs, such as hepatitis and renal dysfunction [14], fulminant myocarditis, myasthenia gravis crisis, hepatic dysfunction [16], and cholestatic hepatitis [15]. Among the 11 patients, three-including the present case-developed grade 3–4 CIP, six developed grade 1–2 CIP, and the severity was not specified in two cases. Steroid therapy was administered to 10 patients, including one who received high-dose methylprednisolone but died from CIP. The remaining 10 patients showed significant improvement.
The clinical symptoms and imaging findings of CIP lack specificity, posing diagnostic and therapeutic challenges, with approximately one-third of patients being asymptomatic at onset [19]. The symptoms in this case, including dyspnea, fever, fatigue, and dry cough, are common clinical manifestations, nonspecific, and largely consistent with previous research reports (Table 1). Among the 11 cases, the most frequent symptoms were dyspnea (5 cases, 45.4%), fever (4 cases, 36.4%), and dry cough (3 cases, 27.3%), while one patient was asymptomatic. Radiological features were described in 10 cases, with common findings including ground-glass opacities (GGOs) in 5 cases, interstitial changes in 2 cases, nodules in 1 case, and consolidation in 1 case. In this case, the patient’s CT scan revealed multiple patchy high-density areas in both lungs, which aligns with the typical radiological presentation of CIP [8]. The most common imaging features of CIP include GGOs, reticular patterns, consolidation, organizing pneumonia-like changes, or nonspecific interstitial pneumonia-like changes [8].
The overall principle of CIP treatment is graded therapy. Currently, there are no specific grading criteria for irAEs in China. Referring to the irAEs management guidelines released by the American Society of Clinical Oncology in 2018 [20], this patient can be classified as grade 3 CIP. According to the guidelines, treatment with glucocorticoids should be initiated, with methylprednisolone at 1–2 mg·kg− 1·d− 1 or equivalent, gradually tapering the dose. However, in this case, the patient has diabetes mellitus, and an increase in steroid dosage led to uncontrolled blood glucose levels, necessitating a reduction in dosage. Steroid are known to promote hyperglycemia, making the exploration of effective CIP treatment alongside diabetes management particularly interesting. Hilder et al. [21] reported that, among 12,142 cancer patients treated with ICIs, 11 had pre-existing diabetes, and 3 of these patients received high-dose steroids for irAE management. They concluded that steroid therapy for irAEs could be safely managed without adverse glycemic consequences through appropriate insulin adjustments and standard care, including diabetes management techniques and insulin therapy. Similarly, in COVID-19 patients with diabetes, studies have shown that effective glycemic control through insulin therapy improves clinical outcomes in those receiving steroids [22]. Pofi et al. recommend frequent adjustments to steroid dosages after initiating therapy, ensuring alignment between steroid dose modifications and glycemic control strategies [23]. In our case, methylprednisolone was reduced from 40 mg to 30 mg due to hyperglycemia, underscoring the importance of close monitoring and timely interventions in such patients. Moreover, steroid dosages may vary depending on the organ affected by irAEs. For example, irAE-induced arthralgia is often managed with lower doses, while colitis or pneumonitis typically requires higher doses [24, 25]. It has been reported that glucocorticoids can control CIP in over 70% of patients, although some may experience pneumonitis recurrence during glucocorticoid tapering, with an increased risk of pneumonitis recurrence upon reinitiating immunotherapy [26]. Therefore, glucocorticoid treatment should not be abruptly discontinued, and it is recommended to gradually taper the dose after symptoms and imaging improvements. For those with poor response to glucocorticoids, combination therapy with intravenous immunoglobulin, mycophenolate mofetil, and cyclophosphamide may be considered. Initially, during the diagnosis of pneumonitis, methylprednisolone 40 mg was administered intravenously once daily, but it was discontinued upon symptom improvement at discharge. However, symptoms recurred and the patient was readmitted eight days later, diagnosed with CIP. Methylprednisolone 40 mg was administered intravenously once daily, with a reduction to 30 mg one week later, along with intravenous immunoglobulin at 12.5 g once daily for three days. Two weeks later, symptoms improved, and most lesions were absorbed on chest CT. Prior literature has also reported the initiation of steroid therapy after ineffective antibiotic treatment, leading to rapid alleviation, thus underscoring the value of steroids in CIP treatment [27, 28].
Additionally, CIP stands as an exclusionary diagnosis, necessitating the exclusion of infectious pneumonia and neoplastic disease progression beyond immunopharmacological history and radiographic manifestations [29]. In this case, 2.5 months after discontinuation of ICIs, the patient developed pneumonitis. Initially presumed to be community-acquired pneumonia, despite administration of antiviral and antibiotic therapies, the condition progressed. Microbiological investigations yielded negative results. Consultation led to consideration of lung cancer recurrence. PET-CT imaging revealed heightened metabolic activity in the pneumonia region. Subsequent percutaneous biopsy disclosed organizing pneumonia. Pathological analysis via NGS of the biopsy specimen detected no pathogenic organisms. Concurrently, in line with characteristic features on chest CT, pneumonitis occurred peripherally in both lungs, with prominence on the right, consistent with CIP. Based on clinical presentation, CIP often manifests as a dry cough or scant white sputum, accompanied by a low-grade fever (around 38℃). In contrast, lung cancer recurrence tends to present with persistent, worsening dry cough, and infectious pneumonia typically produces larger amounts of yellow purulent sputum. Clinical presentations and imaging results can help exclude recurrent cancer or infectious pneumonia.
Conclusion
In conclusion, this report, based on a single case and literature review, highlights that dyspnea and GGOs are the most common clinical and imaging features of CIP. Glucocorticoids remain the cornerstone of treatment; however, the nonspecific nature of CIP symptoms and imaging findings poses significant challenges for early identification and diagnosis. Notably, CIP can occur at any time, even after discontinuation of ICIs, underscoring the importance of long-term vigilance. Clinicians are encouraged to deepen their understanding of the pathogenesis, clinical presentation, radiographic characteristics, and therapeutic strategies for CIP. Close monitoring is particularly crucial for high-risk patients to reduce medication-related risks and improve outcomes. Further research involving larger cohorts is essential to enhance the understanding of CIP and optimize management strategies.
Acknowledgements
None.
Author contributions
HW, YJ, and ML: Presented the ideas and was responsible for the organization and coordination of the study. YJ, PL, JZ, and NF: Collected case information and wrote the original draft. HW and ML: Revised, reviewed and edited the manuscript. All authors contributed to the study and approved the final version of the manuscript.
Funding
None.
Data availability
All data and materials are available for sharing, if needed, by the corresponding author (Mengjie Li, E-mail address: lmjnkdf@163.com).
Declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by Ethics Committee of Qingxian People’s Hospital, approval number [20240504].
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Clinical trial number
not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tan PS, Aguiar P Jr., Haaland B, et al. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer - a systematic review and network meta-analysis of 3024 participants. Lung Cancer. 2018;115:84–8. [DOI] [PubMed] [Google Scholar]
- 2.Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39(9):1040–91. [DOI] [PubMed] [Google Scholar]
- 3.Lin MX, Zang D, Liu CG, et al. Immune checkpoint inhibitor-related pneumonitis: research advances in prediction and management. Front Immunol. 2024;15:1266850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607–16. [DOI] [PubMed] [Google Scholar]
- 5.Suresh K, Naidoo J, Lin CT, et al. Immune Checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao R, Yang F, Yang C, et al. A case report and literature review of immune checkpoint inhibitor-associated pneumonia caused by penpulimab. Front Immunol. 2023;14:1114994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest. 2021;160(2):731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johkoh T, Lee KS, Nishino M, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Chest. 2021;159(3):1107–25. [DOI] [PubMed] [Google Scholar]
- 9.Kucukarda A, Gokmen I, Ozcan E, et al. Recurrent delayed immune-related pneumonitis after immune-checkpoint inhibitor therapy for advanced osteosarcoma. Immunotherapy. 2022;14(6):395–9. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Yin Y, Yan X, et al. Late–onset immune checkpoint inhibitor–related pneumonitis after cessation of sintilimab: a case report and literature review. Exp Ther Med. 2023;25(2):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamantopoulos PT, Gaggadi M, Kassi E, et al. Late-onset nivolumab-mediated pneumonitis in a patient with melanoma and multiple immune-related adverse events. Melanoma Res. 2017;27(4):391–5. [DOI] [PubMed] [Google Scholar]
- 12.Mandala M, Merelli B, Indriolo A, et al. Late-occurring toxicity induced by an immune checkpoint blockade in adjuvant treatment of a stage III melanoma patient. Eur J Cancer. 2018;95:130–2. [DOI] [PubMed] [Google Scholar]
- 13.Kimura H, Sone T, Araya T, et al. Late-onset programmed cell death protein-1 inhibitor-induced pneumonitis after cessation of nivolumab or pembrolizumab in patients with advanced non-small cell lung cancer: a case series. Transl Lung Cancer Res. 2021;10(3):1576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakai Y, Otsuka T, Inoue T, et al. Two cases of delayed onset of immune-related adverse events after discontinuation of nivolumab in patients with metastatic renal cell cancer. IJU Case Rep. 2021;4(5):326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Zhou C, Yuan F, et al. Case Report: severe immune-related cholestatic hepatitis and subsequent pneumonia after pembrolizumab therapy in a geriatic patient with metastic gastric cancer. Front Med (Lausanne). 2021;8:719236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X, Wang F, Qin Y, et al. Case report: fatal multiorgan failure and heterochronous pneumonitis following pembrolizumab treatment in a patient with large-cell neuroendocrine carcinoma of lung. Front Pharmacol. 2020;11:569466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin J, Wu Y, Yang X, et al. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front Immunol. 2022;13:830631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–75. [DOI] [PubMed] [Google Scholar]
- 19.Shannon VR, Anderson R, Blidner A, et al. Multinational association of supportive care in cancer (MASCC) 2020 clinical practice recommendations for the management of immune-related adverse events: pulmonary toxicity. Support Care Cancer. 2020;28(12):6145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilder R, Tsai K, Quandt Z, et al. Safety and efficacy of immune checkpoint inhibitor cancer therapy in patients with preexisting type 1 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1242830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–e10771063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pofi R, Caratti G, Ray DW, et al. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocr Rev. 2023;44(6):975–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldea M, Orillard E, Mansi L, et al. How to manage patients with corticosteroids in oncology in the era of immunotherapy? Eur J Cancer. 2020;141:239–51. [DOI] [PubMed] [Google Scholar]
- 25.Maughan BL, Bailey E, Gill DM, et al. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol. 2017;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Chen S, Yan T, et al. Clinical characteristics, management and prognostic factors of immune checkpoint inhibitor-associated pneumonia in chinese cancer patients. J Coll Physicians Surg Pak. 2024;34(3):302–7. [DOI] [PubMed] [Google Scholar]
- 28.Oda K, Kato K, Nakamura M, et al. Surface marker profiles on lung lymphocytes may predict the mechanism of immune-mediated pneumonitis triggered by tumor-infiltrating lymphocytes in lung cancer patients treated with pembrolizumab. Lung Cancer. 2018;118:171–2. [DOI] [PubMed] [Google Scholar]
- 29.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available for sharing, if needed, by the corresponding author (Mengjie Li, E-mail address: lmjnkdf@163.com).