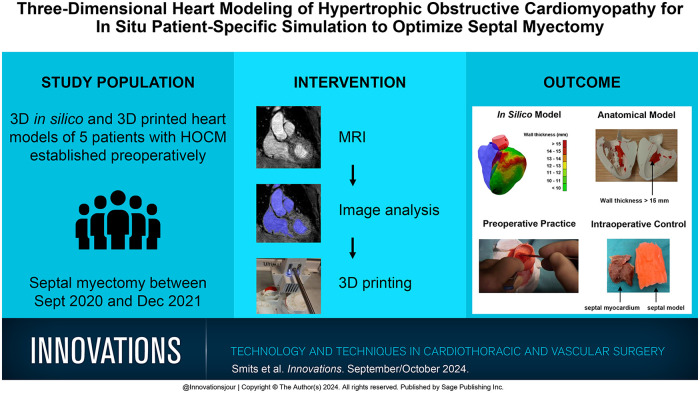
Abstract
Objective:
Hypertrophic obstructive cardiomyopathy (HOCM) develops in at least 1 out of 715 young adults. Patients who are refractory to medical therapy qualify for septal myectomy. Due to anatomy, serious complications such as ventricular septal defect and heart block may occur. Establishing cardiovascular magnetic resonance (CMR)–based 3-dimensional (3D) models as part of preoperative planning and training has the potential to decrease procedure-related complications and improve results.
Methods:
CMR images were used to segment cardiac structures. Left ventricular wall thickness was calculated and projected on top of the in silico model. A 3D model was printed with a red layer indicating a wall thickness exceeding 15 mm and used for preoperative resection planning and patient counseling. To provide preoperative patient-specific in situ simulation, the planned resection volume was replaced with silicone in a second model. For perioperative quality control, resected silicone was compared with resected myocardial tissue. The impact of the models was evaluated descriptively through consultation of both the cardiothoracic surgeon and patients and through patient outcomes.
Results:
Three-dimensional in silico and 3D-printed heart models of 5 patients were established preoperatively. Since the introduction of the models in October 2020, the surgeon feels better prepared, more confident, and less difficulty with making decisions. In addition, patients feel better informed preoperatively.
Conclusions:
Using 3D heart models optimized preoperative planning and training, intraoperative quality control, and patient consultation. Reduction of procedure-related complications and clinical outcome should be studied in larger cohorts.
Keywords: magnetic resonance imaging, virtual surgical planning, in situ simulation, Morrow procedure, 3D printing, simulation-based training
Visual abstract.
Central Message.
Patient-specific 3D heart models for septal myectomy in patients with HOCM optimized preoperative planning and training, intraoperative quality control, and patient consultation. This approach has the potential to improve clinical outcomes of patients in the future.
Introduction
Hypertrophic obstructive cardiomyopathy (HOCM) is an autosomal dominant heritable cardiomyopathy with a thickened interventricular septum exceeding 15 mm and a peak left ventricular outflow tract (LVOT) gradient exceeding 30 mm Hg. 1 HOCM occurs in at least 1 out of 715 young adults.2 –4 When pharmacologic treatment is insufficient, a surgical septal myectomy can be performed 1 to reduce symptoms and improve survival. 5 During a transaortic septal myectomy originally developed by Morrow et al., 6 the bulging part of the basal interventricular septum is resected, as visualized in Figure 1. 7
Fig. 1.
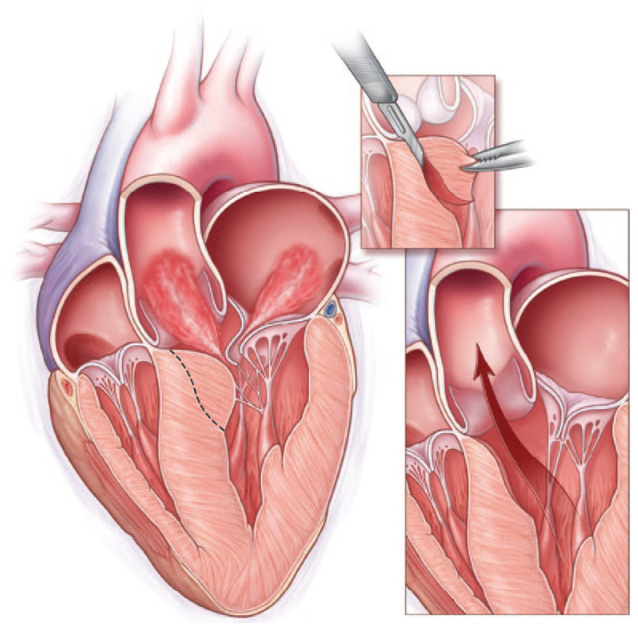
Cross section of a heart with hypertrophic obstructive cardiomyopathy (HOCM) with reduced left ventricular outflow and mitral regurgitation (left). The dotted line represents the incision line for a transaortic septal myectomy (inset) and restoration of outflow after resection of the left basal ventricular septal wall (right). From N Engl J Med, Nishimura RA and Holmes DR Jr, Hypertrophic Obstructive Cardiomyopathy, volume 350, pages 1320–1327, Copyright ©2004 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. 7
Visualization of the complete septal bulge can be challenging. Limited visual control may cause undertreatment, resulting in a residual LVOT gradient or recurrence of symptoms. 8 In contrast, overtreatment resulting in a ventricular septal defect or complete heart block occurs in 1% 9 and ≤5% 1 of cases, respectively. This may be fatal, which makes the impact of these complications significant. These risks might also explain the discrepancy between the incidence of HOCM and the number of Morrow procedures performed. Therefore, a new approach to reduce the incidence of these complications is needed.
Preoperative planning of septal myectomies is not specifically stated in current guidelines; however, diagnostic preoperative transthoracic echocardiography (TTE) 1 and intraoperative transesophageal echocardiography (TEE)10,11 imaging is recommended. Translation of these 2-dimensional (2D) imaging modalities to the intraoperative situation remains challenging. Therefore, a method to provide 3-dimensional (3D) information of the patient’s anatomy is preferable.
State of the Art
Preoperative training in actual patient care with high fidelity and realism is called “in situ simulation.” 12 In the field of congenital heart disease, in situ simulation using patient-specific 3D heart models is of increased interest to improve anatomical insights and to determine the feasibility of and the most suitable approach for surgery.13 –19 A few cases of 3D modeling for HOCM patients have been described.20 –26 Some physical 3D models provide the ability to re-create the surgical view, 26 while others offer preoperative practice for the septal myectomy. In these cases, the optimal resection site can be determined prior to surgery, likely improving the actual procedure. Several studies have compared the resected myocardium with the planned and trained resection, to determine to what extent the planned myectomy was achieved.21,22,24,26 Three of those studies compared volumes with liquid displacement.21,24,26 One study 3D printed the planned resection volume and remaining ventricle separately to visually compare the resected myocardial tissue with the planned resection. 22
Besides the abovementioned advantages of these 3D models for HOCM patients, no visual feedback is provided on the thickness of the interventricular septum and ventricular wall. Since septal thickness guides the extent of the septal myectomy, incorporation of septal and myocardial wall thickness may provide valuable insights accompanied by improved effectiveness and outcomes of patients. Furthermore, using the 3D heart models during preoperative patient counseling has the potential to increase understandability of the disease and procedure for the patient. Until now, only 1 study has incorporated the model in patient counseling. 23 In addition, the 3D models in all abovementioned studies were based on cardiac computed tomography (CT),20 –26 whereas cardiac magnetic resonance (CMR) is the gold standard in the visualization of HOCM. 1 Despite excellent spatial resolution and accurate delineation of myocardium on CT, 27 it does not allow for an eminent blood–myocardium contrast such as CMR. Since accurate visualization ensures more adequate measurements on the location and extent of the hypertrophy, 28 CMR might be preferred over CT for the purpose of creating 3D heart models for HOCM patients.
Therefore, this research focusses on establishing patient-specific 3D heart models based on CMR, to combine the advantages of a surgical view, visual feedback on septal and myocardial thickness, preoperative training opportunities, intraoperative control, and utilization during patient counseling. The hypothesis is that incorporation of these models will increase the effectiveness of the septal myectomy, decrease procedure-related complications, and improve patient counseling.
Methods
This research is described following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 29 This study comprises a prospective cohort study and was approved by the local institutional review board of Medisch Spectrum Twente, Enschede, The Netherlands on December 14, 2021. The study was exempted from the Medical Research Involving Human Subjects Act (K21-42). Informed consent was asked for utilization of patients records for research purposes (the underlying manuscript). Patients who received a septal myectomy between September 2020 and December 2021 in Thorax Centrum Twente (Medisch Spectrum Twente, Enschede, the Netherlands) were included. Informed consent was asked for utilization of patients records for scientific research.
In Silico 3D Heart Model
Preoperative CMR images were acquired in the supine position with a dedicated cardiac 1.5T magnetic resonance imaging (MRI) system (Ingenia/ Ingenia Ambition X, Philips, Best, The Netherlands). An electrocardiography-triggered end-diastolic 3D mDIXON (voxel size 0.57 mm × 0.57 mm × 1.0 mm) or 3D BFTE (voxel size 0.8 mm × 0.8 mm × 0.8 mm) sequence with a diaphragm navigator was performed.
The myocardium of the left and right ventricle, ascending aorta, and left and right coronary arteries were automatically segmented using IntelliSpace portal r10.1.3 (Philips). In addition, papillary muscles, membranous septum, and junction of the aortic and mitral annulus as anatomical landmarks were segmented through manual placement of a seeding point, followed by automatic segmentation based on region growing.
Subsequently, the proximal ascending aorta was virtually resected at the estimated aortotomy site to simulate the surgical view using 3-matic 15.0 (Materialise, Leuven, Belgium). A thickness analysis was performed by calculating the distance between all 2 closest opposite triangles of the left ventricular myocardium. The left ventricular myocardium was additionally segmented into a colored height map with wall thickness regions, as visualized in Figure 2. The 3D thickness analysis was validated with a phantom model with known thickness, as described in the Supplemental Material.
Fig. 2.

In silico heart model with a color map representing the left ventricle myocardium thickness in millimeters. The right ventricle is visualized in transparent blue, the proximal ascending aorta and left ventricular outflow tract are in transparent red, and the origin of the left coronary artery and right coronary artery are in red and blue, respectively. Arrows in complementary colors point toward these structures.
Virtual Surgical Planning
To further increase preoperative surgical insights, the planned myectomy resection site was incorporated into the model using 3-matic 15.0. Resection sites were planned with a minimal distance of 2 mm from the membranous septum and junction of the aortic and mitral annulus as surgical landmarks. Using the coronary arteries as additional landmarks, a U-shaped resection was virtually performed. The thickness of the remaining left ventricular myocardium was determined to ensure a remaining thickness of at least 10 mm.
3D-Printed Anatomical Heart Model
For 3D-printing purposes, the in silico 3D heart model was dissected in half, perpendicular to the anterior mitral valve leaflet. The surface of the left ventricle wall exceeding 15 mm was printed in red and the remaining structures in white, as can be seen in Figure 3. The model was printed with an Ultimaker S5 (Utrecht, The Netherlands) using polylactic acid (PLA) as printing material. A layer thickness of 0.2 mm, infill of 10%, and tree supports for overhanging features inclined more than 60° were used.
Fig. 3.
A 3-dimensional–printed heart model in 2 different views. The red color represents the left ventricle with a wall thickness exceeding 15 mm. Black arrows indicate the left ventricular outflow tract obstruction.
3D-Printed Surgical Training Model
To further optimize preoperative planning, a training model was created to practice the planned resection 1 day before surgery. Ascending aorta, origins of the coronary arteries, LVOT outline, and most of the interventricular septum were isolated from the in silico model. A mold around these parts was created with a 1 mm wall thickness.
The left ventricular myocardium with imprint of the right ventricle of the training model was printed similarly to the 3D-printed anatomical heart model as previously described. The mold was printed in PLA with a layer thickness of 0.1 mm and an infill of 100%, and a printing angle was set per case such that no support was required on the inside of the mold (Fig. 4a–b).
Fig. 4.
(a) (b) The 3-dimensional–printed mold of the ascending aorta, left ventricular outflow tract, coronary arteries, and part of the interventricular septum. Black arrows indicate the position where the mold is opened. (c) Casting material after removal of the mold. (d) Training model consisting of a nonflexible polylactic acid part and a flexible silicon part (red) from a surgical view. (e) Nonsurgical side view of the left ventricle.
To cast the flexible part of the training model, an opening was created in the mold (Fig. 4a–b, black arrow). A 2-component silicon and softener (Eurosil 10 Orange, Schouten Group SynTec, Mijnsheerenland, The Netherlands) was used for casting in a 1:1:1 ratio and stirred for approximately 5 min. The silicone model was left to cure overnight at room temperature (19 °C). Following removal of the mold, the cast was attached to the remaining part of the left ventricle with a 2-component Araldite glue (Huntsman, TX, USA), as visualized in Figure 4c–e.
Prior to surgery, the cardiothoracic surgeon assessed the preoperative TTE and the anatomical heart model. The surgeon combined this information to determine the optimal septal myectomy. Subsequently, this approach was practiced on the flexible training model using an 11-blade scalpel during preoperative in situ simulation, as visualized in Figure 5. Based on the results, the surgeon reassessed the optimal surgical approach.
Fig. 5.

In situ preoperative training of septal myectomy with surgical blade 11 and forceps through simulated transaortic access.
The resected silicone obtained during preoperative training was visually compared intraoperatively with the resected myocardium (Fig. 6b–c).
Fig. 6.
Intraoperative comparison of the resected myocardial tissue (black arrow) to the preoperatively resected model (red arrow). The resected myocardial tissue consists of 2 components, which visualizes the decision of the surgeon to extend the initial excision, partially based on the intraoperative comparison.
Perioperative and Postoperative Parameters
Perioperative and postoperative parameters were collected as part of standard quality registration according to Netherlands Heart Registration definitions. Telephonic consultation with individual patients was performed to determine their shortness of breath with the New York Heart Association (NYHA) classification 30 at follow-up.
No primary or secondary endpoints were defined, as this study has a descriptive nature due to the expected low patient number (high-risk, low-volume surgery).
Statistical Analysis
Descriptive analyses were performed to gain understanding of the collected data. Frequencies, percentages, and number of missing observations/data are provided for categorical variables. Normally distributed continuous variables are expressed as the mean value ± standard deviation, and nonparametric continuous or ordinal variables are expressed as the median (25th to 75th quartile). Analysis of the data collected within this study was exploratory in nature, and no formal hypotheses were tested.
Results
Patient Characteristics and Clinical Outcomes
Five patients were included in this study. Baseline characteristics are listed in Table 1. All patients showed an interventricular septal thickness of ≥15 mm and systolic anterior motion as part of the obstruction mechanism. In addition, all measured LVOT gradients at rest were elevated (≥30 mm Hg). All patients were accepted for a septal myectomy according to the heart team decision. During the course of this study, the heart models increased in complexity (Table 2).
Table 1.
Preoperative Patient Characteristics.
| Variable | Patients (N = 5) |
|---|---|
| Male sex | 5 (100) |
| Age, years | 66 ± 5.7 |
| Length, cm | 174 ± 4.1 |
| Weight, kg | 89 ± 7.7 |
| Interventricular septum thickness, mm | 19 (18–21) |
| LVOT gradient at rest, mm Hg (n = 4) | 81 (47–114) |
| Presence of systolic anterior motion | 5 (100) |
| Preoperative NYHA class | |
| I | 2 (40) |
| II | 2 (40) |
| III | 1 (20) |
| IV | 0 |
Abbreviations: LVOT, left ventricular outflow tract; NYHA, New York Heart Association.
Values are expressed as number of cases (%), mean ± standard deviation, or median (interquartile range).
Table 2.
Use of 3D Heart Models for Surgical Myectomy.
| Type of model | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| In silico heart model | + | + | + | + | + |
| Anatomical heart model | + | + | + | + | + |
| Virtual surgical planning | – | – | + | + | + |
| Surgical training model | – | – | + | + | + |
| Intraoperative control | – | – | + | + | + |
Abbreviations: +, heart model was established; –, heart model was not established; 3D, 3-dimensional.
Table 3 visualizes perioperative measures. Two patients (33%) had a primary indication for coronary artery bypass grafting (CABG) or CABG with aortic valve replacement, with concomitant septal myectomy. Median aortic clamping time and extracorporeal circulation time were 47 (26 to 80) and 84 (45 to 96) min, respectively. Atrial fibrillation, complete heart block, a left bundle branch block, and pacemaker implantation occurred only once as a postoperative complication. One patient passed away postoperatively as a result of a hypercapnic coma in combination with withdrawal of consent for intubation.
Table 3.
Perioperative Characteristics and Postoperative Outcomes.
| Variable | Patients (N = 5) |
|---|---|
| Coronary artery bypass grafting | 3 (60) |
| Mitral valve repair | 1 (20) |
| Aortic valve replacement | 1 (20) |
| Left atrial appendage closure | 1 (20) |
| Extracorporeal circulation time, min | 84 (45–96) |
| Aortic cross-clamp time, min | 47 (26–80) |
| Postoperative complications within 30 days | |
| Atrial fibrillation a | 1 (20) |
| Complete heart block a | 1 (20) |
| CVA with residual injury | 0 |
| CVA without residual injury | 0 |
| Left bundle branch block a | 1 (20) |
| Permanent pacemaker implantation b | 1 (20) |
| Deep sternal wound infection | 0 |
| Mortality | 1 (20) |
| Ventricular septal defect | 0 |
| Myocardial infarction | 0 |
| Rethoracotomy | 0 |
Abbreviation: CVA, cerebrovascular accident.
Values are expressed as number of cases (%) or median (interquartile range).
First manifestation postoperatively.
Including implantable cardioverter defibrillator.
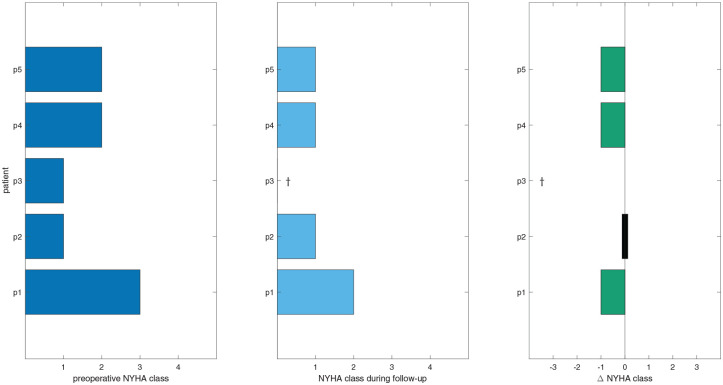
Preoperative NYHA classification, during follow-up, and the difference are visualized in Figure 7. Median follow-up for NYHA classification was 248 (211 to 314) days. A decrease in shortness of breath for symptomatic patients as measured by NYHA classification was observed in 3 of 3 patients (100%).
Fig. 7.
Preoperative NYHA class, NYHA class during follow-up, and the difference in NYHA class. Green values indicate a decrease in NYHA class following surgery. The † symbol indicates the patient is deceased. NYHA, New York Heart Association.
Experiences of Patients and Surgeon
Following each surgery, the value of 3D preoperative planning and practicing was discussed with the cardiothoracic surgeon. The quality of the 3D-printed models was “deemed impressive and improved the preoperative and perioperative patient-specific anatomical insights.” In addition, the “planned resection helped to determine and visualize the resection sites and angles needed.” The ability to preoperatively practice the procedure further increased the ability to re-create the optimal planned resection site. During surgery, the surgeon was able to visually compare resected septal myocardial tissue with resected silicon. In this case, visual comparison contributed to the decision to extend the excision. The surgeon felt “better prepared, more confident, and better supported in decision making.”
Furthermore, all patients felt more informed during preoperative counseling. One patient, including family, anecdotally stated that they felt “significantly better informed after a second counseling using the 3D heart models.” Other relatives explained that “after regular patient information another type of surgery had been expected” compared with the situation after counseling using the 3D heart models.
Discussion
This study presents the development and optimization of in silico and 3D-printed anatomical and training models for preoperative planning and intraoperative quality control of a surgical myectomy. Previously, regular preoperative planning of septal myectomy for HOCM patients included assessing the preoperative TTE and intraoperative TEE images. Nowadays, patients in our institution receive an additional CMR as standard care. This allows for creating 3D patient-specific heart models and providing in situ simulation. Insight into patient-specific anatomy is increased with the anatomical heart models,20 –26 and in combination with in situ simulation of the septal myectomy, the cardiothoracic surgeon feels better prepared and is more confident in performing the surgery.
Intraoperative comparison of the resected myocardial septum with the preoperative simulated resection is a form of quality control on the completeness of the septal myectomy and may lead to extended resection and prevent additional extracorporeal circulation runs. Because inadequate resection of myocardial tissue most often induces persistent or recurrence of LVOT gradients and therefore symptoms, 31 3D heart models also have the potential to increase postoperative quality of life.
Previous research presented 3D heart models as part of preoperative planning for septal myectomy in HOCM patients (Table 4). As can be observed, our study is the first to combine aspects of all models while adding the thickness of the septal and myocardial wall. This increases the potential to improve clinical outcomes of the septal myectomy.
Table 4.
Overview of 3-Dimensional Heart Model Used for Surgical Myectomy.
| Reference | In silico model | Anatomical model | Septal myectomy planning | Septal and myocardial thickness | Training model | Intraoperative assessment |
|---|---|---|---|---|---|---|
| Yang et al. 20 | + | + | – | – | – | – |
| Hermsen et al. 21 | + | + | – | – | + | + |
| Andrushchuk et al. 22 | + | + | + | – | – | + |
| Guo et al. 23 | + | + | – | – | – | – |
| Takayama et al. 24 | + | – | + | – | – | – |
| Sun et al. 25 | + | + | – | – | – | – |
| Wang et al. 26 | + | + | – | – | + | + |
| Present study | + | + | + | + | + | + |
The anatomical model is now standard practice during preoperative patient counseling. The model enables visualization of the condition, explanation of how the septal myectomy will be performed, and why particular procedure-related complications could occur. This allows for true shared decision making between patients, relatives, and the surgical team.
To the best of our knowledge, this was the first study using MRI for the establishment of patient-specific 3D models in HOCM treatment. MRI assesses the extent of myocardial fibrosis as an important marker for increased risk of sudden death, left ventricular function and dimensions, aortic valve physiology, and LVOT acceleration time. In addition, it has the benefits of requiring neither radiation nor iodinated contrast agents. The proposed MRI protocol for establishing the models might also be achieved with CT angiography. The ability of choosing MRI or CT angiography for 3D modeling facilitates widespread implementation in other centers, although using CT angiography with our protocol has not yet been studied.
A step-by-step protocol was created for the establishment of all heart models and is available upon request. This makes incorporation into preoperative care of HOCM patients in other cardiothoracic centers feasible. For optimal implementation, it is recommended to have in-house experts mastering both the medical and technical competencies such as technical medical doctors (technical physicians).
Limitations
We present the results of a small but consecutive patient group, as was expected in low-volume yet highly complex cardiac surgery. We feel our journey on the development and optimization of these 3D patient-specific heart models benefits cardiothoracic surgery practice. A quantitative analysis to assess the effects of complex 3D models on outcome measures needs a larger (multicenter) study including control patients.
The value of each 3D model was discussed after each procedure with both surgeon and patient but was not evaluated systematically in this study. For further research, it is recommended to use a systematic evaluation protocol with a questionnaire and confidence assessment. Nevertheless, the evaluations performed in this explorative study did provide insight into the value from a patient and surgeon perspective.
Intraoperative comparison of resected myocardial tissue and preoperative resected silicon was based on visual inspection. Objective assessment of septal myectomy volume and location might improve intraoperative decision making. Although liquid displacement provides an objective way of assessing resected volume, it fails to provide insights into the anatomical location of the resected myocardium. Further research is necessary to develop objective quality control methods.
The current method to develop the anatomical model in combination with the training model is time-consuming. Obtaining a 3D-printed model takes 2 h of virtual planning and almost 24 h of printing in 2 colors. Using a more sophisticated printer that allows printing 2 or more different materials including elastic materials in 1 printing process might solve the extensive time needed. Curing the silicone training model also takes 24 h. Therefore, it is recommended to fine-tune the process or examine alternative 3D technologies such as virtual reality or augmented reality to decrease costs while obtaining effectiveness. Furthermore, the need for each of the proposed models can be modified for each patient and surgeon. The case complexity and surgical experience might tailor the desired complexity of 3D in situ training by excluding model-specific components. The left, right, and septal ventricular myocardium, papillary muscles, myocardial thickness, LVOT, aortic root, and manual separation of the heart in 2 halves are recommended as minimal components.
Outlook
Previous research showed a significant learning curve 32 to achieve sufficient surgical expertise and experience for the septal myectomy. 24 At this moment, the established 3D models are solely used for preoperative planning of the septal myectomy in our institution. However, they may also be of added value for cardiothoracic surgeons in training.
With the highly complex yet low-volume character of septal myectomy, individual surgical case load is low. An inverse relationship between septal myectomy volume and in-hospital mortality was previously observed. 33 Although we experienced an increase in case load in our institution since the introduction of the 3D models, it might be difficult to further increase surgical volume. Developing hands-on training with the developed models may be used to increase exposure to the procedure and thereby steepen the learning curve.
A transaortic approach of the septal myectomy is the standard approach in our institution. However, in other centers, the apical and mitral approach may be used. For these alternative approaches, the current model can be adapted easily to re-create the respective surgical view.
Basal ventricular obstruction is the most common phenotype and occurs in 70% of HOCM cases. 3 Mid-cavital obstruction septal myectomy might benefit from simulation-based training for this rare presentation. The proposed training methods can be used for all types of myectomy, yet limitations of silicone flexibility and the general use should be studied.
At this moment, an interventricular septal thickness of 10 mm is strived for during septal myectomy 22 to prevent a ventricular septal defect. However, it is unknown which septal thickness is most favorable for a patient. A delicate balance between a favorable septal thickness to achieve physiological flow conditions and low postoperative complication rate is to be presumed. The question is: how far can we go? A first step to an answer might be a fluid dynamics study in which blood flow in an HOCM model is simulated and tested.
Translation of preoperative imaging to the perioperative situation is complex due to cardioplegia use, altering shape, and the dimension of myocardial tissue. Currently, intraoperative instruments to determine real-time septal thickness are lacking. Intraoperative TEE visualization of septal tissue is recommended prior to and after excision, yet image-guided resection remains challenging due to the air barrier between the ultrasound probe and ventricular septum. 6 The perioperative use of intracardiac echocardiography has been investigated, 34 yet limited aortotomy size prevents insertion of additional instruments.
Conclusions
Patient-specific in situ simulation of the Morrow procedure using in silico and 3D-printed anatomical and training models optimized preoperative planning and training, intraoperative quality control, and patient consultation. Whether incorporation of in situ simulation in standard practice will translate into improved patient outcomes needs to be determined in a larger study.
Supplemental Material
Supplemental material, sj-pdf-1-inv-10.1177_15569845241273538 for Three-Dimensional Heart Modeling of Hypertrophic Obstructive Cardiomyopathy for In Situ Patient-Specific Simulation to Optimize Septal Myectomy by Karin C. Smits, Ron G. H. Speekenbrink, Edsko E. G. Hekman, Maaike A. Koenrades, Tijn J. P. Heeringa, Jutta Arens and Frank R. Halfwerk in Innovations
Acknowledgments
The authors would like to thank Jan G. Grandjean, MD, PhD, for his enthusiasm and critical opinions, which have further improved this research. We are grateful for the contribution of Jan van Es, MD, in the segmentation process.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Karin C. Smits  https://orcid.org/0000-0001-6352-5769
https://orcid.org/0000-0001-6352-5769
Maaike A. Koenrades  https://orcid.org/0000-0003-4555-0551
https://orcid.org/0000-0003-4555-0551
Tijn J. P. Heeringa  https://orcid.org/0009-0006-2567-9429
https://orcid.org/0009-0006-2567-9429
Jutta Arens  https://orcid.org/0000-0002-9115-0519
https://orcid.org/0000-0002-9115-0519
Frank R. Halfwerk  https://orcid.org/0000-0003-2928-9728
https://orcid.org/0000-0003-2928-9728
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020; 142: e558–e631. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995; 92: 785–789. [DOI] [PubMed] [Google Scholar]
- 3. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006; 114: 2232–2239. [DOI] [PubMed] [Google Scholar]
- 4. Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015; 65: 1249–1254. [DOI] [PubMed] [Google Scholar]
- 5. Meng X, Liang M, Shi Y, et al. Effects of surgical septal myectomy on survival in patients with hypertrophic obstructive cardiomyopathy. Anatol J Cardiol 2020; 23: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrow AG, Reitz BA, Epstein SE, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of pre and postoperative assessments in 83 patients. Circulation 1975; 52: 88–102. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura RA and Holmes DR Jr.. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004; 350: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 8. Cho YH, Quintana E, Schaff HV, et al. Residual and recurrent gradients after septal myectomy for hypertrophic cardiomyopathy-mechanisms of obstruction and outcomes of reoperation. J Thorac Cardiovasc Surg 2014; 148: 909–916. [DOI] [PubMed] [Google Scholar]
- 9. Robbins RC and Stinson EB.. Long-term results of left ventricular myotomy and myectomy for obstructive hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 1996; 111: 586–594. [DOI] [PubMed] [Google Scholar]
- 10. Marwick TH, Stewart WJ, Lever HM, et al. Benefits of intraoperative echocardiography in the surgical management of hypertrophic cardiomyopathy. J Am Coll Cardiol 1992; 20: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 11. Grigg LE, Wigle ED, Williams WG, et al. Transesophageal Doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and importance in intraoperative decision making. J Am Coll Cardiol 1992; 20: 42–52. [DOI] [PubMed] [Google Scholar]
- 12. Lioce L, Lopreiato J, Downing D, et al. Healthcare simulation dictionary. 2nd ed. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ), 2020. [Google Scholar]
- 13. Noecker AM, Chen JF, Zhou Q, et al. Development of patient-specific three-dimensional pediatric cardiac models. ASAIO J 2006; 52: 349–353. [DOI] [PubMed] [Google Scholar]
- 14. Sodian R, Weber S, Markert M, et al. Pediatric cardiac transplantation: three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg 2008; 136: 1098–1099. [DOI] [PubMed] [Google Scholar]
- 15. Shiraishi I, Yamagishi M, Hamaoka K, et al. Simulative operation on congenital heart disease using rubber-like urethane stereolithographic biomodels based on 3D datasets of multislice computed tomography. Eur J Cardiothorac Surg 2010; 37: 302–306. [DOI] [PubMed] [Google Scholar]
- 16. Kiraly L, Tofeig M, Jha NK, et al. Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair. Interact Cardiovasc Thorac Surg 2016; 22: 238–240. [DOI] [PubMed] [Google Scholar]
- 17. Farooqi KM, Nielsen JC, Uppu SC, et al. Use of 3-dimensional printing to demonstrate complex intracardiac relationships in double-outlet right ventricle for surgical planning. Circ Cardiovasc Imaging 2015; 8: e003043. [DOI] [PubMed] [Google Scholar]
- 18. Ryan JR, Moe TG, Richardson R, et al. A novel approach to neonatal management of tetralogy of Fallot, with pulmonary atresia, and multiple aortopulmonary collaterals. JACC Cardiovasc Imaging 2015; 8: 103–104. [DOI] [PubMed] [Google Scholar]
- 19. Sun X, Zhang H, Zhu K, et al. Patient-specific three-dimensional printing for Kommerell’s diverticulum. Int J Cardiol 2018; 255: 184–187. [DOI] [PubMed] [Google Scholar]
- 20. Yang DH, Kang JW, Kim N, et al. Myocardial 3-dimensional printing for septal myectomy guidance in a patient with obstructive hypertrophic cardiomyopathy. Circulation 2015; 132: 300–301. [DOI] [PubMed] [Google Scholar]
- 21. Hermsen JL, Burke TM, Seslar SP, et al. Scan, plan, print, practice, perform: development and use of a patient-specific 3-dimensional printed model in adult cardiac surgery. J Thorac Cardiovasc Surg 2017; 153: 132–140. [DOI] [PubMed] [Google Scholar]
- 22. Andrushchuk U, Adzintsou V, Nevyglas A, et al. Virtual and real septal myectomy using 3-dimensional printed models. Interact Cardiovasc Thorac Surg 2018; 26: 881–882. [DOI] [PubMed] [Google Scholar]
- 23. Guo HC, Wang Y, Dai J, et al. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: a preliminary study. J Thorac Dis 2018; 10: 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takayama H, Yu SN, Sorabella R, et al. Virtual septal myectomy for preoperative planning in hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2019; 158: 455–463. [DOI] [PubMed] [Google Scholar]
- 25. Sun X, Zhang H, Zhu K, et al. Curved section modeling-based three-dimensional printing for guiding septal myectomy. J Thorac Dis 2018; 10: E535–E537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Guo H, Wang S, et al. Effectiveness of a patient-specific 3-dimensional printed model in septal myectomy of hypertrophic cardiomyopathy. Pak J Med Sci 2020; 36: 1678–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliveira DC, Assuncao FB, Santos AA, et al. Cardiac magnetic resonance and computed tomography in hypertrophic cardiomyopathy: an update. Arq Bras Cardiol 2016; 107: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2012; 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang R, Ma S, Shanahan L, et al. Discovering and identifying New York Heart Association classification from electronic health records. BMC Med Inform Decis Mak 2018; 18: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kotkar KD, Said SM, Dearani JA, et al. Hypertrophic obstructive cardiomyopathy: the Mayo Clinic experience. Ann Cardiothorac Surg 2017; 6: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geske JB, Driver CN, Yogeswaran V, et al. Comparison of expected and observed outcomes for septal myectomy in hypertrophic obstructive cardiomyopathy. Am Heart J 2020; 221: 159–164. [DOI] [PubMed] [Google Scholar]
- 33. Hadaya J, Verma A, Sanaiha Y, et al. Volume-outcome relationship in septal myectomy for hypertrophic obstructive cardiomyopathy. Surgery 2023; 174: 166–171. [DOI] [PubMed] [Google Scholar]
- 34. Williams DM, Nampi RG, Saric M, et al. On-pump intracardiac echocardiography during septal myectomy for hypertrophic cardiomyopathy. JTCVS Tech 2020; 2: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-inv-10.1177_15569845241273538 for Three-Dimensional Heart Modeling of Hypertrophic Obstructive Cardiomyopathy for In Situ Patient-Specific Simulation to Optimize Septal Myectomy by Karin C. Smits, Ron G. H. Speekenbrink, Edsko E. G. Hekman, Maaike A. Koenrades, Tijn J. P. Heeringa, Jutta Arens and Frank R. Halfwerk in Innovations