Abstract
Purpose
As panel testing expands, more individuals with double pathogenic variants (DPVs) in cancer susceptibility genes are likely to be identified. Little is known about the effects of DPVs on cancer phenotype, although this information is crucial for genetic counseling and risk management. We sought to describe the cancer phenotype among individuals with DPVs in cancer susceptibility genes.
Methods
A retrospective study of individuals with DPVs identified through a single testing laboratory from 2012 to 2017 was conducted. DPV combinations were enumerated. For DPV gene combinations that occurred >10 times, cancer histories of individuals with DPVs were compared with cancer histories of controls with a single PV matched by gene.
Results
Among 644 individuals with DPVs, combinations that included the ATM, BRCA1, BRCA2, CHEK2, and PALB2 genes occurred >10 times. There were 8883 matched controls for a single PV in these genes. The median age of first cancer diagnosis was younger with ATM+CHEK2 (43), compared with ATM (47, P = .016) or CHEK2 (47, P = .015) alone. Similar findings were observed when comparing age at first breast cancer (BC) for the ATM+CHEK2 with single-gene controls. Individuals with 2 CHEK2 PVs also were younger at first cancer diagnosis (40) compared with single CHEK2 PV controls (47, P = .0038). This difference was not driven by age at first BC diagnosis among females.
Conclusion
Individuals with ATM+CHEK2 or 2 CHEK2 PVs have a greater cancer burden than single gene controls. These findings can be used to counsel individuals with DPVs and their families and inform cancer screening recommendations.
Keywords: Breast cancer, Genetics, Germline, Hereditary cancer, Multiple pathogenic variants
Introduction
A personal or family history of cancer is an indication for germline genetic testing of cancer susceptibility genes. This testing is often completed through multigene panel testing (MGPT). MGPT has the potential to identify pathogenic or likely pathogenic variants (PVs) in different genes. Although having multiple monogenic hereditary cancer syndromes is not common, the finding can be alarming for individuals and providers alike because it raises many questions about cancer risk and management. The presence of double pathogenic variants (DPVs) in one individual may affect cancer phenotype, age at cancer diagnosis or tumor subtype, which is important for genetic counseling and clinical care. However, because its rarity, studies on individuals with DPVs have been limited by sample size or to a small subset of genes.
Prior studies have found that 1% to 2% of individuals tested for hereditary cancer have >1 PV; however, those with >2 PVs are less frequent.1, 2, 3, 4, 5, 6 Most studies have focused on individuals with both BRCA1 and BRCA2 PVs, previously referred to as double heterozygosity.7 Individuals with both BRCA1 and BRCA2 PVs (BRCA1+BRCA2) were more likely to be diagnosed with breast cancer (BC) than individuals with either a BRCA1 or BRCA2 PV alone.7 Although the age of BC diagnosis was similar to individuals with a BRCA1 PV, individuals with PVs in both BRCA1 and BRCA2 had more estrogen receptor (ER)-positive BCs than individuals with BRCA1 PVs alone.7
There is less known about the effects of DPVs in other genes. In 1 study of 11 individuals with DPVs, almost all had malignancies associated with their respective PVs.2 However, there is a lack of understanding of the tumor biology or epistatic effects of DPVs because most in vivo models of hereditary cancer syndromes have focused on characterizing PVs in a single cancer susceptibility gene.8
Herein, we describe the landscape of DPVs identified on MGPT and enumerate the most frequent DPVs gene combinations. We delineate the phenotype of individuals with DPVs by gene combinations, sex, cancer type, and age of diagnosis for BC and first cancer. We compare cancer histories of individuals with DPVs with controls with a single PV matched by gene. For females with DPVs associated with BC and a personal history of BC, we report the hormone receptor (HR) subtype. The goal of this work is to provide information for genetic counseling of individuals with DPVs.
Materials and Methods
A retrospective study was conducted of individuals who were tested by MGPT for personal or family history of cancers between March 2012 and March 2017 on panels of varying sizes (5-67 genes) at a single diagnostic laboratory. A full gene list and NCBI reference numbers are provided in the supplement (Supplemental Table 1). Patients with monoallelic PVs in genes associated predominantly with autosomal recessive or biallelic cancer predisposition syndromes (BLM, FANCC, MUTYH, RAD50, and XRCC2) were excluded from this analysis entirely because of limited evidence of their cancer associations in the heterozygous state. The CHEK2 p.I157T, p.S428F, and p.T476M variants and APC p.I1307K were defined as lower-risk PVs and separated from other PVs.9,10 Cases with more than 2 PVs were also excluded from the study. Individuals with biallelic PVs in genes associated with autosomal recessive cancer predisposition were only included if they also had an additional PV in another gene (eg, biallelic MUTYH and BRCA2). Based on the most frequent DPV combinations, control cohorts were selected, including individuals with a single PV in ATM, BRCA1, BRCA2, CHEK2, and PALB2. The control cohorts were tested on a 67-gene panel, and controls were selected if they had a PV in the gene of interest and were negative for PVs in the other 66 genes. The 67-gene panel covers all the genes on the smaller panels used for this study.
Clinical characteristics, including cancer history, sex, and age at testing for each tumor diagnosis, were obtained from test requisition forms and clinical documentation, including pedigrees and chart notes, when provided. Variant interpretation was performed according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology guidelines.11 Pathogenic and likely pathogenic variants were denoted as PVs in this study. When DPVs were identified in the same gene, phase was categorized as likely/confirmed in trans, likely/confirmed in cis, or phase unknown. Likely/confirmed trans was applied to PVs in the same gene that are recurrent and are known to occur on different haplotypes (likely) or when trans phase was molecularly confirmed (confirmed).12
Descriptive statistics for individuals stratified by DPV combination were summarized, and included sex, median age of first cancer (interquartile range [IQR]), proportion and 95% confidence intervals (CI) of female individuals with BC, individuals with non-BCs, individuals with more than 1 cancer diagnosis, and reported BC HR subtype. Individuals were sorted by their DPVs and analyzed when >10 had the same combination (eg, ATM+BRCA2, BRCA1+CHEK2). Comparisons were made with single-PV controls matched by gene using t tests and Wilcoxon rank sum, when applicable, for median age at BC diagnosis in female individuals and median age at first cancer diagnosis and using Fisher’s exact test for number of primary tumors (>2 cancers). All statistical tests were two-sided. With exception of the CHEK2+CHEK2 gene combination, adjustments for multiple tests were made according to the Bonferroni method and a P value of less than .025 was considered statistically significant. Because CHEK2+CHEK2 was compared with only monoallelic CHEK2, P value of less than .05 was considered statistically significant. All analyses were conducted with R v.4.0.4.
Results
Cohort demographics
Among 653 individuals with multiple PVs (MPVs), 544 (83.3%) (95% CI, 80.3%-86.0%) were female, 440 (67.4%) (95% CI, 63.7%-70.9%) were White, and the median age of testing was 53 (IQR: 19; Table 1). Most individuals with MPVs had a reported cancer diagnosis: 52.8% (95% CI, 49.0%-56.6%) had a single cancer diagnosis and 27.9% (95% CI, 24.6%-31.4%) had >2 cancer diagnoses, whereas 117 (17.9%) (95% CI, 15.2%-21.0%) were unaffected by cancer at the time of genetic testing. Among the 8883 individuals serving as matched controls for ATM, BRCA1, BRCA2, CHEK2, and PALB2, most were female (93.0%) (95% CI, 92.4%-93.5%), White (64.7%) (95% CI, 63.7%-65.7%), and had a personal history of cancer (79.6%) (95% CI, 78.8%-80.4%). Among the controls, the median age of genetic testing was 52 (IQR: 19), similar to those with DPVs.
Table 1.
Characteristics of MPV cohort and single-gene controls
| Sample Characteristic | MPVs |
Single-PV Controls |
Total Controls N (%) |
||||
|---|---|---|---|---|---|---|---|
| N (%) |
ATM N (%) |
BRCA1 N (%) |
BRCA2 N (%) |
CHEK2 N (%) |
PALB2 N (%) |
||
| Total N | 653 | 1367 (15.4) | 2535 (28.5) | 2668 (30) | 1605 (18.1) | 708 (8) | 8883 (100) |
| Sex | |||||||
| Female | 544 (83.3) | 1275 (93.3) | 2407 (95.0) | 2425 (90.9) | 1475 (91.9) | 676 (95.5) | 8258 (93.0) |
| Male | 109 (16.7) | 92 (6.7) | 128 (5.0) | 243 (9.1) | 130 (8.1) | 32 (4.5) | 625 (7.0) |
| Race, ethnicity, and ancestry | |||||||
| African American/Black | 19 (2.9) | 58 (4.2) | 228 (9.0) | 234 (8.8) | 24 (1.5) | 45 (6.4) | 589 (6.6) |
| Ashkenazi Jewish | 59 (9.0) | 74 (5.4) | 185 (7.3) | 146 (5.5) | 67 (4.2) | 21 (3.0) | 493 (5.5) |
| Asian | 14 (2.1) | 36 (2.6) | 127 (5.0) | 136 (5.1) | 16 (1.0) | 35 (4.9) | 350 (3.9) |
| Hispanic | 25 (3.8) | 63 (4.6) | 223 (8.8) | 180 (6.7) | 45 (2.8) | 46 (6.5) | 557 (6.3) |
| White | 440 (67.4) | 936 (68.5) | 1433 (56.5) | 1604 (60.1) | 1299 (80.9) | 475 (67.1) | 5747 (64.7) |
| Other/Unknown | 96 (14.7) | 200 (14.6) | 339 (13.4) | 368 (13.7) | 154 (9.6) | 86 (12.1) | 1147 (12.9) |
| Number of primary cancer(s) | |||||||
| 0 | 117 (17.9) | 271 (19.8) | 430 (17.0) | 465 (17.4) | 316 (19.7) | 107 (15.1) | a |
| 1 | 345 (52.8) | 762 (55.7) | 1542 (60.8) | 1621 (60.8) | 895 (55.8) | 448 (63.3) | a |
| ≥2 | 182 (27.9) | 299 (21.9) | 490 (19.3) | 506 (19.0) | 365 (22.7) | 144 (20.3) | a |
| Unknown | 9 (1.4) | 35 (2.6) | 73 (2.9) | 76 (2.8) | 29 (1.8) | 9 (1.3) | a |
| Median age at genetic testing (IQR) | 53 (19) | 52 (19) | 49 (20) | 53 (21) | 53 (20) | 53 (19) | 52 (19) |
| Median age at first cancer diagnosis (IQR) | 46 (16) | 47 (17) | 44 (16) | 49 (18) | 47 (15) | 48 (16) | a |
| Breast cancer among females | |||||||
| Yes | 321 (59.0) | 832 (65.3) | 1483 (61.6) | 1529 (63.1) | 1023 (69.4) | 524 (77.5) | a |
| No | 215 (39.5) | 410 (32.2) | 854 (35.5) | 829 (34.2) | 428 (29.0) | 143 (21.2) | a |
| Unknown | 8 (1.5) | 33 (2.6) | 70 (2.9) | 67 (2.8) | 24 (1.6) | 9 (1.3) | a |
| Median age at first breast cancer diagnosis (IQR) among females | 47 (16) | 46 (15) | 41 (15) | 46 (16) | 47 (15) | 48 (15) | a |
IQR, interquartile range; MPV, multiple PVs in one individual (adjective); MPVs, multiple PVs in one individual (plural noun); PV, pathogenic or likely pathogenic variant.
Not analyzed because of heterogeneity.
Distribution of DPVs
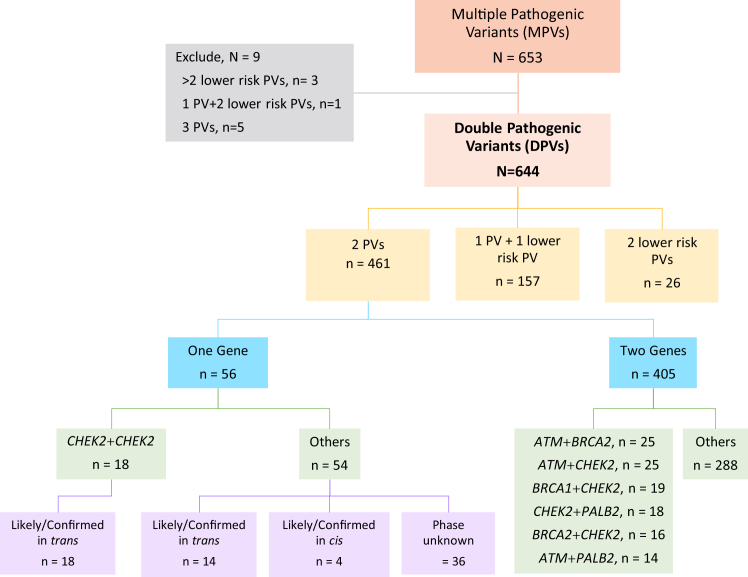
We excluded 9 (1.3%) individuals with >2 PVs from the analysis: 5 with 3 PVs, 3 with >2 lower-risk PV, and 1 with 1 PV and 2 lower-risk PVs. Of the remaining 644 individuals with DPVs, 157 (24.3%) had 1 PV with 1 lower-risk PV, and 26 individuals (4%) had 2 lower-risk PVs only (Figure 1). There were 461 cases (71.6%) with DPVs in moderate- or high-penetrance cancer susceptibility genes. Of these, 405 (62.3%) individuals had PVs in different genes, and 56 (8.7%) had PVs in the same gene. Among individuals with PVs in the same gene, 18 had 2 PVs in CHEK2: 9 were confirmed trans and 9 were likely trans because they were combinations of founder PVs that have been described independently (eg, c.1100del and c.444+1G>A; Figure 1).
Figure 1.
Flow DiagramofDPVCohort. DPVs, double pathogenic or likely pathogenic variants in one individual (plural noun); MPVs, multiple pathogenic or likely pathogenic variants in one individual (plural noun); PV, pathogenic or likely pathogenic variant (single noun); PVs, pathogenic or likely pathogenic variants (plural noun).
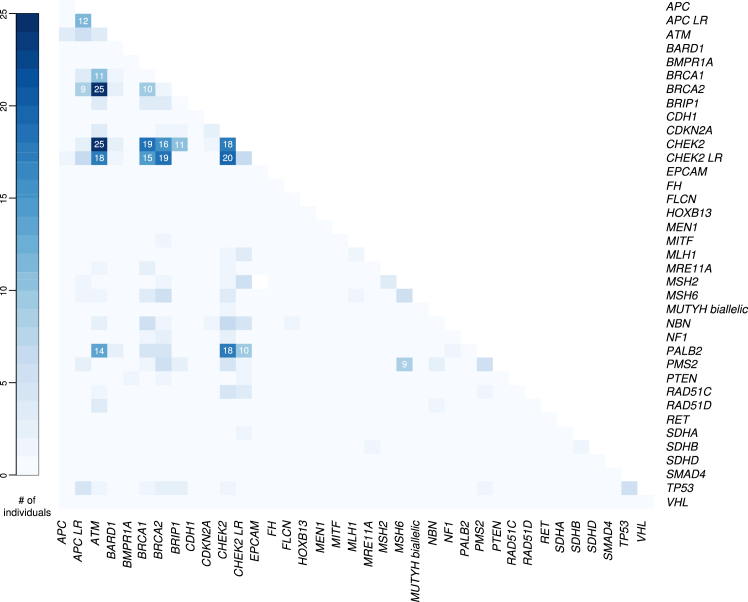
The most frequent recurrent DPVs were enumerated, and cancer histories are reported (Figure 2, Table 2). The combination of DPVs in ATM+CHEK2 (n = 25) and ATM+BRCA2 (n = 25) were equally frequent. CHEK2 in combination with BRCA1 (n = 19), CHEK2 (n = 18), PALB2 (n = 18), and BRCA2 (n = 16) was the next most frequent finding. All individuals with the combination of CHEK2+CHEK2 PVs were females, most had BC (94.4%) and multiple primary tumors (61.1%). PVs in BRCA1 in combination with BRCA2 were detected in 10 individuals (Figure 2, Table 2).
Figure 2.
Heatmap of DPVGeneCombinations.
Table 2.
Cancer phenotype of individuals with DPVs by gene combination
| DPVs | Total N/F/M |
N with Cancer F (% of Females)/M (% of Males) |
N of ≥2 Primary Cancer F (%)/M (%) |
Median (IQR) Age of 1st Cancer | N of Female BC (%)a | Median (IQR) Age of Female BC | N of all Cancer Excluding Female BCb (%) |
|---|---|---|---|---|---|---|---|
| ATM+BRCA2 | 25/19/6 | 14 (56.0) 4 (16.0) |
4 (16.0) 1 (4.0) |
49.5 (19.5) | 11 (57.9) | 44 (14) |
2 Male BC (33.3 of M) 1 PC (16.7 of M) 3 OV (15.8 of F) 1 CRC (4.0) 1 Mel (4.0) 1 TC (4.0) |
| ATM+CHEK2 | 25/23/2 | 22 (88.0) 1 (4.0) |
6 (24.0) 0 (0.0) |
43 (10.5) | 20 (87.0) | 42.5 (9.5) | 1 CC (4.3) 1 OV (4.3 of F) 1 VulC (4.3 of F) 1 KidC (4.0) |
| BRCA1+CHEK2 | 19/17/2 | 10 (52.6) 2 (10.5) |
4 (21.1) 0 (0.0) |
44 (10.5) | 9 (52.9) | 45 (9) |
1 Male BC (50.0 of M) 1 PC (50.0 of M) 2 OV (11.8 of F) 2 EC (11.8 of F) 1 LC (5.3) 1 Mel (5.3) |
| CHEK2+CHEK2 | 18/18/0 | 17 (94.4) 0 (0.0) |
11 (61.1) 0 (0.0) |
40 (15) | 17 (94.4) | 45.5 (11) | 3 TC (16.7) 2 BlC (11.1) 2 CRC (11.1) 2 LK (11.1) 1 Mel (5.6) 1 OV (5.6 of F) 1 SarC (5.6) 1 SIC (5.6) 1 EC (5.6 of F) |
| CHEK2+PALB2 | 18/18/0 | 17 (94.4) 0 (0.0) |
5 (27.8) 0 (0.0) |
47 (18) | 17 (94.4) | 47 (18) | 1 OV (5.6 of F) 1 TC (5.6) 1 SIC (5.6) 1 LK (5.6) |
| BRCA2+CHEK2 | 16/15/1 | 11 (68.8) 0 (0.0) |
2 (12.5) 0 (0.0) |
45 (13) | 10 (66.7) | 43.5 (8.75) | 1 OV (6.7 of F) 1 LP (6.3) |
| ATM+PALB2 | 14/12/2 | 11 (78.6) 1 (7.1) |
4 (28.6) 0 (0.0) |
51.5 (11) | 9 (75.0) | 49 (10) | 3 OV (25.0 of F) 1 EC (8.3 of F) 1 PaC (7.1) |
| ATM+BRCA1 | 11/10/1 | 9 (81.8) 1 (9.1) |
3 (27.3) 1 (9.1) |
44 (13) | 4 (40.0) | 39.5 (7.25) |
1 PC (100.0 of M) 4 OV (40.0 of F) 2 TC (18.2) 1 EC (10.0 of F) 1 GC (9.1) 1 KidC (9.1) |
| BRCA1+BRCA2 | 10/9/1 | 7 (70.0) 1 (10.0) |
1 (10.0) 0 (0.0) |
39.5 (18.25) | 5 (55.6) | 33 (11) | 2 OV (22.2 of F) 1 CRC (10.0) |
Counts and percentages of males are italicized. BC, breast cancer; BlC, bladder cancer; CC, cervical cancer; CRC, colorectal cancer; DPVs, double pathogenic or likely pathogenic variants in 1 individual (plural noun); EC, endometrial cancer; GC, gastric cancer; KidC, kidney cancer; LC, lung cancer; LK, leukemia; LP, Lymphoma; Mel, melanoma; OV, ovarian cancer; PaC, pancreatic cancer; PC, prostate cancer; SarC, sarcoma; SIC, small intestine cancer; TC, thyroid cancer; VulC, vulvar cancer.
The percentage represents those among females.
The percentage for OV, EC, CC, and VulC represents those among females. The percentage for PC and male BC represents those among males.
Cancer histories of frequent DPV combinations compared with matched controls
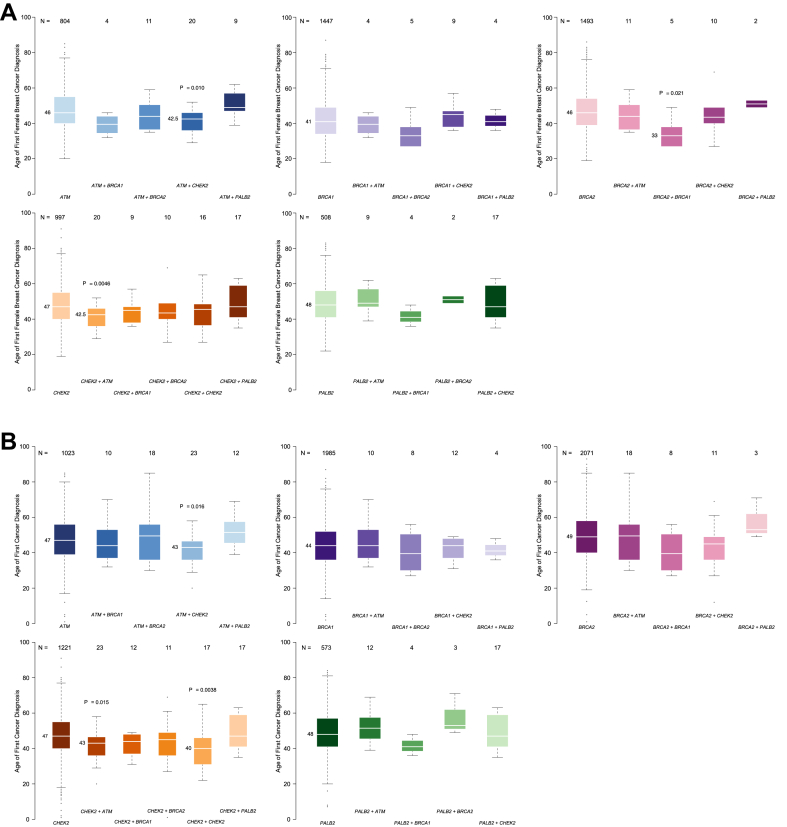
For each of the above gene combinations, the median age of first BC diagnosis among female individuals was also compared with single-PV controls matched by gene (ATM, BRCA1, BRCA2, CHEK2, and PALB2, Figure 3A). The median age of first BC diagnosis was younger for individuals with combined ATM+CHEK2 (42.5, IQR: 9.5) than it was for controls with PVs in either ATM (46, IQR: 15; P = .01, statistically significant after Bonferroni correction) or CHEK2 (47, IQR: 15; P = .0046, statistically significant after Bonferroni correction). Among the 9 female individuals with PVs in BRCA1+BRCA2, the median age at BC diagnosis was 33 (IQR: 11), which was significantly younger than age at BC diagnosis among the BRCA2 control cohort (46, IQR: 16; P = .02, statistically significant after Bonferroni correction), but not statistically significantly different from the BRCA1 control cohort (41, IQR: 15; P = .1). Differences in median age at first BC were not different between individuals with 2 CHEK2 PVs (45.5) compared with those with a single CHEK2 PV (47, IQR: 16; P = .15). Other gene combinations (ATM+BRCA1, ATM+BRCA2, ATM+PALB2, BRCA1+CHEK2, BRCA1+PALB2, BRCA2+CHEK2, BRCA2+PALB2, CHEK2+PALB2) did not differ in median age of first BC diagnosis compared with each of their respective matched single-PV controls (Figure 3A).
Figure 3.
A. Median age of first breast cancer (BC) diagnosis. With exception of the CHEK2+CHEK2 combination, P values are statistically significant after the Bonferroni correction if P < .025. When CHEK2+CHEK2 was compared with monoallelic CHEK2, the P value is statistically significant if P < .05. B. Median age of first cancer diagnosis. With exception of the CHEK2+CHEK2 combination, P values are statistically significant after the Bonferroni correction if P < .025. When CHEK2+CHEK2 was compared with monoallelic CHEK2, the P value is statistically significant if P < .05.
The median age of first cancer diagnosis was also compared by DPV combination with matched controls (Figure 3B, Table 2). The median age of first cancer diagnosis was younger for individuals with ATM+CHEK2 (43, IQR: 10.5) compared with ATM alone (47, IQR:17; P = .016, statistically significant after Bonferroni correction) and compared with CHEK2 alone (47, IQR:15; P = .015, statistically significant after Bonferroni correction). Individuals with 2 CHEK2 PVs also had a younger age first cancer diagnosis (40, IQR: 15) compared with single CHEK2 PV controls (47, IQR: 15; P = .0038). Although the median age of first cancer diagnosis for individuals with PVs in BRCA1+BRCA2 (39.5) was younger than BRCA1 (44, IQR:16) and BRCA2 (49, IQR:18), these differences were not statistically significant. Likewise, the other examined gene combinations (ATM+BRCA1, ATM+BRCA2, ATM+PALB2, BRCA1+CHEK2, BRCA1+PALB2, BRCA2+CHEK2, BRCA2+PALB2, CHEK2+PALB2) were not associated with younger age of first cancer compared with their respective gene matched controls (Figure 3B). The most frequent gene combination among male individuals was ATM+BRCA2 (n = 6), too few to make meaningful comparisons with sex-matched controls.
The number of primary tumors was also assessed in individuals with the most frequent DPV combinations compared with matched single-PV controls (Supplemental Table 2). Individuals with combined CHEK2+CHEK2 PVs were more likely to have multiple primary cancers (>2) compared with individuals with a single CHEK2 PV (OR 5.21; 95% CI.83-15.97; P <.001). Other DPV gene combinations did not demonstrate statistically significant differences in the number of individuals with ≥2 primaries compared with matched single-PV controls.
Breast cancer HR subtype
Among the 544 females with DPVs, 59.0% (321) had a reported BC diagnosis (Table 1). Of the 321 BC cases in females, HR subtype was reported for 65.4% (210/321, Supplemental Table 3), and HER2 receptor status was reported for 51.7% (166/321, Supplemental Table 3). Most BC cases were ER- and/or progesterone receptor (PR)-positive (ER/PR+) 76.7% (161/210), and 22.38% were HER2-positive (37/166). ER/PR+ BC was the most common HR subtype reported with PVs in CHEK2+CHEK2 (100.0% among the 12 BCs with ER/PR status provided), ATM+CHEK2 (93.3%, 14/15), BRCA1+CHEK2 (85.7%, 6/7), and BRCA2+CHEK2 (85.7%, 6/7; Supplemental Table 4). HER2 status was available for 11 breast tumors in CHEK2+PALB2 heterozygotes and none was HER2-positive. Among DPVs, 34 cases were triple negative (Supplemental Table 3), 16 of whom had a BRCA1 PV in combination with another PV.
Discussion
The likelihood of identifying DPVs in a patient is increasing because of the widespread use of multigene panel testing for cancer susceptibility.1, 2, 3, 4, 5 However, there have been little data on the cancer phenotypes of individuals with DPVs. This study provides insights on the frequency of DPVs and more frequent PV gene combinations while also delineating the associated cancer histories compared with control cohorts matched by gene.
Overall, among individuals with DPVs, the median ages of BC diagnosis and first cancer diagnosis were similar to individuals with a single matched PV, with a few notable exceptions. First was the combination of DPVs in ATM+CHEK2. Here, PVs in 2 moderate risk genes were associated with younger age BC diagnosis than having a single PV in either moderate risk gene (ATM or CHEK2) alone. The statistically significant difference in the age of cancer diagnosis with ATM+CHEK2 compared with a single PV in ATM or CHEK2 alone is almost entirely attributable to the difference in the age of BC diagnosis.
Next, the median age at BC diagnosis with PVs in BRCA1+BRCA2 (33), although younger than the median age at diagnosis in BRCA1 controls (41), was not statistically different. When compared with median age at BC diagnosis among BRCA2 controls (46), BC was diagnosed over a decade earlier, and this was statistically significant. Further investigation is needed to elucidate whether the differences observed are because of the presence of a BRCA1 variant or if differences are due to the added PV load. Among the females with PVs BRCA1+BRCA2 and BC, 55.5% (5/9) had ER/PR+ tumors compared with 23.5% of tumors with BRCA1 and 50.8% of tumors with BRCA2. These findings inform clinical management and are similar to prior work from the CIMBA consortium.7 In that report, there were significantly more ER+ BCs (42.9%) with BRCA1+BRCA2 than a single BRCA1 PV (24.0%) and significantly fewer ER+ BCs than with a single BRCA2 PV (76.5%), suggesting that the BRCA1+BRCA2 combination influences tumor biology.7 In this context, it is unclear if chemoprevention with tamoxifen, raloxifene, or an aromatase inhibitor would affect the BC risk in individuals with the combination of BRCA1+BRCA2. Another focused family analysis indicated that individuals with both BRCA1+BRCA2 PVs had higher cancer incidence than individuals with PVs in either gene alone and surmised this group might derive greater benefit from more intensive screening or risk-reducing interventions.13
Biallelic (confirmed or likely trans phase) CHEK2 was associated with younger ages at first cancer diagnosis compared with a single PV in CHEK2. Although median age at first BC was also younger for biallelic CHEK2, this was not statistically significant. However, a significantly younger age of BC diagnosis among biallelic CHEK2 has previously been reported by our group and others in a larger cohort.9,14 The median age of first cancer diagnosis for CHEK2+CHEK2 was younger than median age of first BC—suggesting that females with CHEK2+CHEK2 have a risk of a non-BC at a younger age than BC. Moreover, individuals with biallelic CHEK2 PVs were more likely to have ≥ 2 primary cancers compared with single CHEK2 PV heterozygotes. These findings suggest that genetic counseling and management of cancer risks with biallelic CHEK2, when confirmed in trans, are distinct from the identification of a single CHEK2 PV alone. Individuals with multiple primary cancers have a high rate of a PVs in cancer susceptibility genes15; however, it is unknown whether individuals with DPVs are more likely to have multiple primary cancers than individuals with a single PV.
Unexpectedly, most BCs that occurred in the context of PVs in BRCA1+CHEK2 were also ER/PR+ (86%). This finding should be validated in large, independent data sets. Potential epistatic effects of DPVs will need to be studied in vivo before making definitive assertions regarding biological impact or claims of synergistic cancer risks.
Finally, prior work has indicated that CHEK2-associated breast tumors are more likely to be HER2-positive than those in the general population. In this cohort, there was a higher portion of HER2 positive tumors with the CHEK2+CHEK2 (40%) and BRCA1+CHEK2 (29%) combinations compared with CHEK2 alone (26%); there were no HER2-positive tumors with the CHEK2+PALB2 combination (0/11). Further study of these differences could be important to better understanding the pathogenesis of HER2+ BC.
Limitations of this study include a highly selected testing cohort, predominantly comprised of White female individuals with a personal history of BC. One challenge in the field of cancer genetics is that historically there has been an emphasis on hereditary cancer genetic testing for females with BC, and although guidelines for testing have broadened, testing among males remains underutilized.16, 17, 18, 19, 20 Most individuals had germline genetic testing because of their personal diagnosis of BC; thus, DPV gene combinations relating to non-BC susceptibility syndromes were infrequent. There were too few males to allow for meaningful comparisons of the cancer phenotype or age at cancer onset among males with DPVs and the single-PV controls as we did for BC among females. To mitigate the effects of testing bias, phenotype comparisons were made to matched controls with a single PV in from the same testing cohort and therefore subject to the same testing bias as subjects with DPVs. Another limitation comes from the nature of the study. Because germline DPVs are rare, we were limited by small numbers of recurrent DPV combinations for phenotype analysis. To address this, we restricted our analysis to DPV combinations that occurred in >10 subjects. Our analyses excluded individuals with DPVs that had a lower-risk pathogenic variant, namely, the CHEK2 p.I157T, p.S428F, and p.T476M variants9 and APC I1307K,10 although the combination of 1 PV and a lower-risk PV comprised 24.0% (157/653) of the overall cohort. Among the 156 patients included in our detailed phenotype analysis, 14 (8.9%) had the 9-gene panel; thus, it is possible (1.4% probability based on our findings in this study) that they could have a third pathogenic variant potentially altering their overall cancer burden. Notably, we focused on breast cancer phenotype and most high-risk breast cancer genes are included in small panels.
The approach to individuals with DPVs in cancer susceptibility genes are multifold. Genetic counseling should be prioritized and cascade testing of family members performed to inform cancer risks. Because medical management of DPVs in different genes can be nuanced, it is critical to evaluate whether the associated cancer risks are overlapping (eg, each PV is associated with BC susceptibility), whether there are additional, mutually exclusive risks (eg, 1 PV associated with BC and 1 PV associated with colorectal cancer), or whether there is a combination of overlapping and additional risks. More data and additional functional studies are needed to make accurate predictions about the role of DPVs in cancer development or progression. Until we better understand the implications of DPVs on cancer pathogenesis and risk, assumptions about synergistic effects of double PVs should not be made as they are not necessarily predictable.
Notable findings from this work include that age of cancer onset is younger among individuals with ATM+CHEK2 and CHEK2+CHEK2. We also confirmed prior work showing age at BC onset with BRCA1+BRCA2 is similar to BRCA1 alone, yet with a HR subtype that mimics BRCA2. Exploratory analyses suggest certain combinations of PV in BC susceptibility genes may be associated with unusual and unexpected BC HR subtypes. Further studies are needed to characterize the age-specific cancer risks and tumor subtypes with DPVs to inform screening and preventative care.
Data Availability
All data generated or analyzed during this study have been de-identified and are included in this published article.
Orcid
Huma Q. Rana: http://orcid.org/0000-0001-8749-3618
Conflict of Interest
Carolyn Horton, Min-Tzu Lo, Dr Polfus, Cassidy Carraway, Parichehr Hemyari, and Colin Young report employment at Ambry Genetics, a commercial lab. Min-Tzu Lo reports current employment at Monogram Biosciences. Judy E. Garber reports research collaboration with Ambry Genetics and Invitae (no compensation). Huma Q. Rana reports research collaboration with Invitae and Ambry Genetics (no compensation). All other authors declare no conflicts of interest.
Acknowledgments
This article was presented previously: Horton C, Lo MT, Yussuf A, Young C, Carraway C, Agaoglu NB, Bychkovsky BL, Rana HQ. Double Jeopardy? A Closer Look at Cancer Histories of Individuals with Multiple Germline Pathogenic Variants. ASCO, Chicago, IL, 2022. J Clin Oncol 40, 2022 (suppl 16; abstr 10514). Selected for poster discussion; presented by Brittany Bychkovsky.
Funding
None.
Author Information
Conceptualization: N.B.A., B.L.B., C.H., H.Q.R.; Data Curation: N.B.A., B.L.B., C.H., M.-T.L., C.C., P.H., C.Y., M.E.R., H.Q.R.; Formal Analysis: N.B.A., B.L.B., C.H., M.-T.L., L.P., P.H., C.Y., M.E.R., H.Q.R.; Investigation: N.B.A., B.L.B., C.H., M.-T.L., L.P., P.H., C.Y., M.E.R., H.Q.R.; Methodology: N.B.A., B.L.B., C.H., M.-T.L., L.P., C.C., P.H., C.Y., M.E.R., H.Q.R.; Project Administration: C.H., H.Q.R.; Visualization: N.B.A., B.L.B., C.H., M.-T.L., P.H., C.Y., M.E.R., R.S., J.E.G., H.Q.R.; Writing-original draft: N.B.A., B.L.B., C.H., M.-T.L., H.Q.R.; Writing-review and editing: N.B.A., B.L.B., C.H., M.-T.L., P.H., C.Y., M.E.R., R.S., J.E.G., H.Q.R.
Ethics Declaration
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Western institutional review board and an exemption for a waiver of consent for the de-identified data was granted.
Footnotes
The Article Publishing Charge (APC) for this article was paid by Ambry Genetics.
Nihat B. Agaoglu and Brittany L. Bychkovsky are co–first authors. These authors contributed equally to this manuscript.
Additional Information
The online version of this article (https://doi.org/10.1016/j.gimo.2024.101829) contains supplemental material, which is available to authorized users.
Additional Information
References
- 1.Neben C.L., Zimmer A.D., Stedden W., et al. Multi-gene panel testing of 23,179 individuals for hereditary cancer risk identifies pathogenic variant carriers missed by current genetic testing guidelines. J Mol Diagn. 2019;21(4):646–657. doi: 10.1016/j.jmoldx.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Agaoglu N.B., Doganay L. Concurrent pathogenic variations in patients with hereditary cancer syndromes. Eur J Med Genet. 2021;64(12) doi: 10.1016/j.ejmg.2021.104366. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal E.T., Bernhisel R., Brown K., Kidd J., Manley S. Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer Genet. 2017;218-219:58–68. doi: 10.1016/j.cancergen.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Sokolenko A.P., Bogdanova N., Kluzniak W., et al. Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res Treat. 2014;145(2):553–562. doi: 10.1007/s10549-014-2971-1. [DOI] [PubMed] [Google Scholar]
- 5.Megid T.B.C., Barros-Filho M.C., Pisani J.P., Achatz M.I. Double heterozygous pathogenic variants prevalence in a cohort of patients with hereditary breast cancer. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.873395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaDuca H., Polley E.C., Yussuf A., et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebbeck T.R., Friebel T.M., Mitra N., et al. Inheritance of deleterious mutations at both BRCA1 and BRCA2 in an international sample of 32,295 women. Breast Cancer Res. 2016;18(1):112. doi: 10.1186/s13058-016-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahid S., Lipkin S. Mouse models of inherited cancer syndromes. Hematol Oncol Clin North Am. 2010;24(6):1205–1228. doi: 10.1016/j.hoc.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bychkovsky B.L., Agaoglu N.B., Horton C., et al. Differences in cancer phenotypes among frequent CHEK2 variants and implications for clinical care-checking CHEK2. JAMA Oncol. 2022;8(11):1598–1606. doi: 10.1001/jamaoncol.2022.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ukaegbu C., Levi Z., Fehlmann T.D., et al. Characterizing germline APC and MUTYH variants in Ashkenazi Jews compared to other individuals. Fam Cancer. 2021;20(2):111–116. doi: 10.1007/s10689-020-00198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browning S.R., Browning B.L. Haplotype phasing: existing methods and new developments. Nat Rev Genet. 2011;12(10):703–714. doi: 10.1038/nrg3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidemann S., Fischer C., Engel C., et al. Double heterozygosity for mutations in BRCA1 and BRCA2 in German breast cancer patients: implications on test strategies and clinical management. Breast Cancer Res Treat. 2012;134(3):1229–1239. doi: 10.1007/s10549-012-2050-4. [DOI] [PubMed] [Google Scholar]
- 14.Rainville I., Hatcher S., Rosenthal E., et al. High risk of breast cancer in women with biallelic pathogenic variants in CHEK2. Breast Cancer Res Treat. 2020;180(2):503–509. doi: 10.1007/s10549-020-05543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bychkovsky B.L., Lo M.T., Yussuf A., et al. Prevalence and spectrum of pathogenic variants among patients with multiple primary cancers evaluated by clinical characteristics. Cancer. 2022;128(6):1275–1283. doi: 10.1002/cncr.34056. [DOI] [PubMed] [Google Scholar]
- 16.Jeong G.W., Shin W., Lee D.O., et al. Uptake of family-specific mutation genetic testing among relatives of patients with ovarian cancer with BRCA1 or BRCA2 mutation. Cancer Res Treat. 2021;53(1):207–211. doi: 10.4143/crt.2020.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins V., Halliday J., Warren R., Williamson R. Assessment of education and counselling offered by a familial colorectal cancer clinic. Clin Genet. 2000;57(1):48–55. doi: 10.1034/j.1399-0004.2000.570107.x. [DOI] [PubMed] [Google Scholar]
- 18.Holloway S.M., Bernhard B., Campbell H., Cetnarskyj R., Lam W.W. Inequality of use of cancer genetics services by members of breast, ovarian and colorectal cancer families in South East Scotland. Fam Cancer. 2008;7(3):259–264. doi: 10.1007/s10689-008-9184-x. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan S., Won N.Y., Dotson W.D., Wright S.T., Roberts M.C. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28(12):1631–1644. doi: 10.1038/s41431-020-00725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz J., Ramón y Cajal T., Torres A., et al. Uptake of predictive testing among relatives of BRCA1 and BRCA2 families: a multicenter study in northeastern Spain. Fam Cancer. 2010;9(3):297–304. doi: 10.1007/s10689-009-9313-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study have been de-identified and are included in this published article.