Abstract
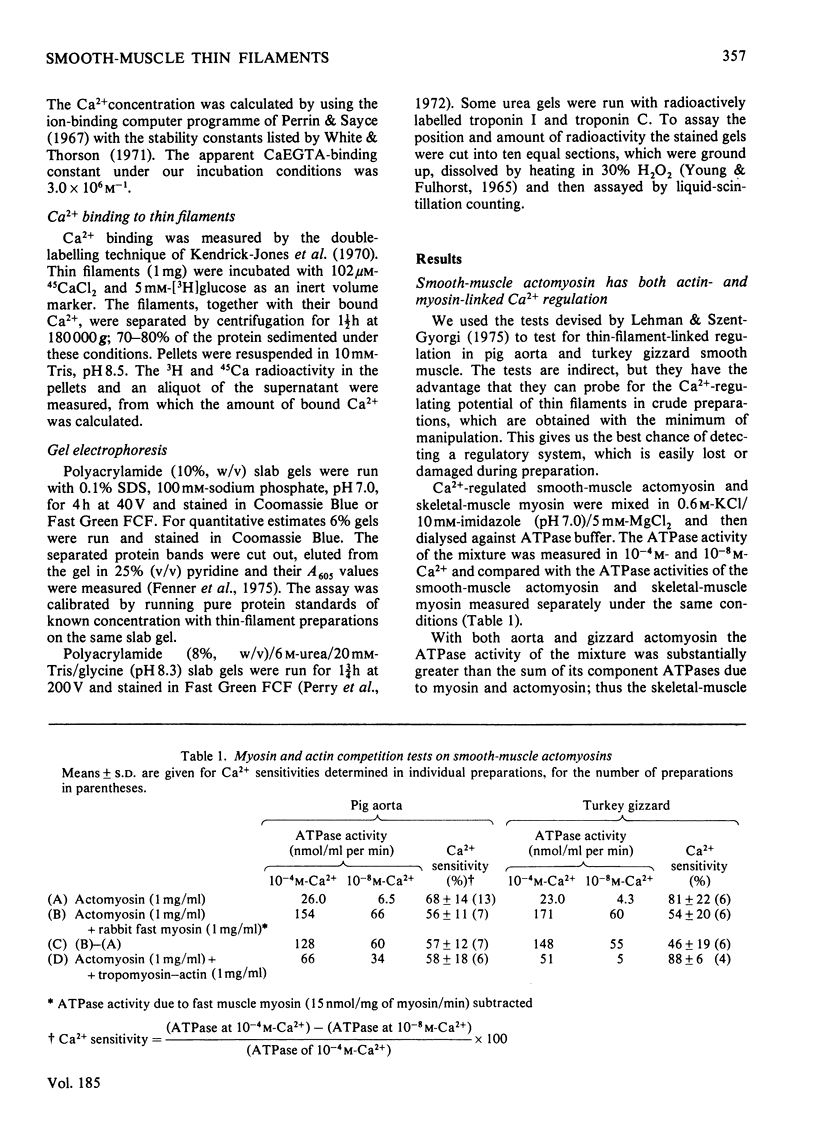
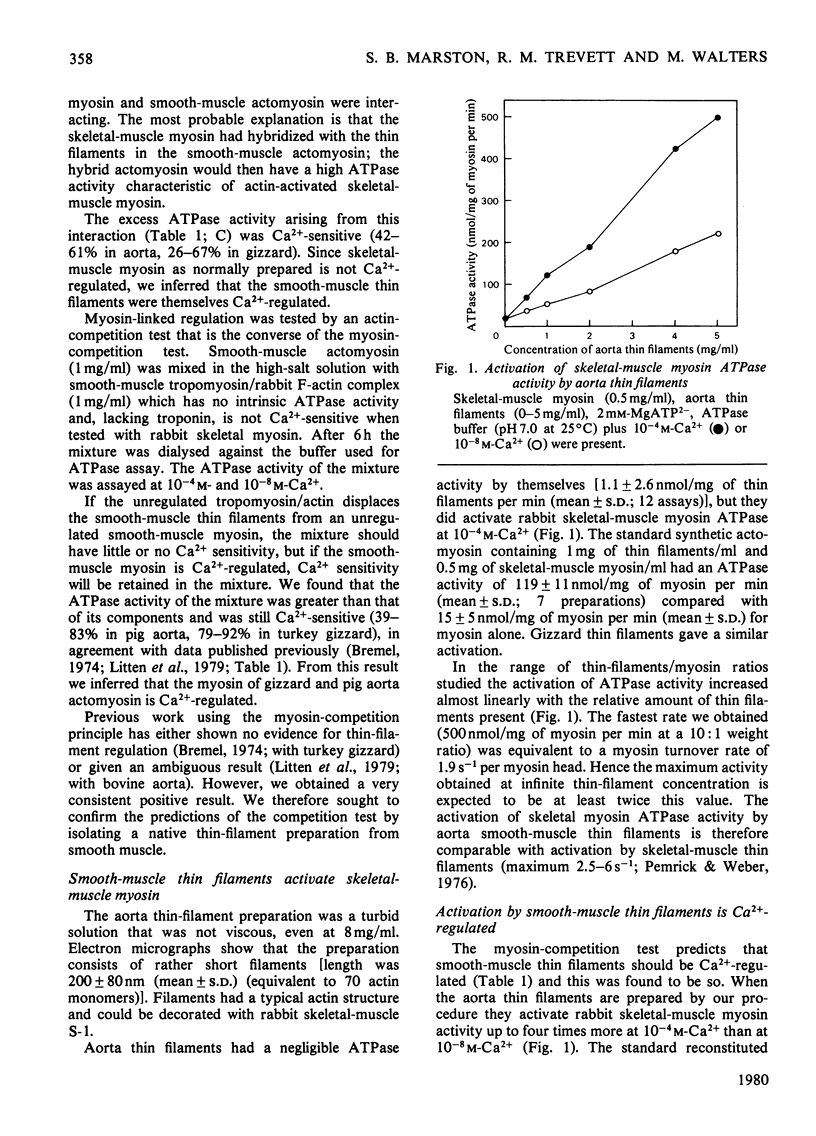
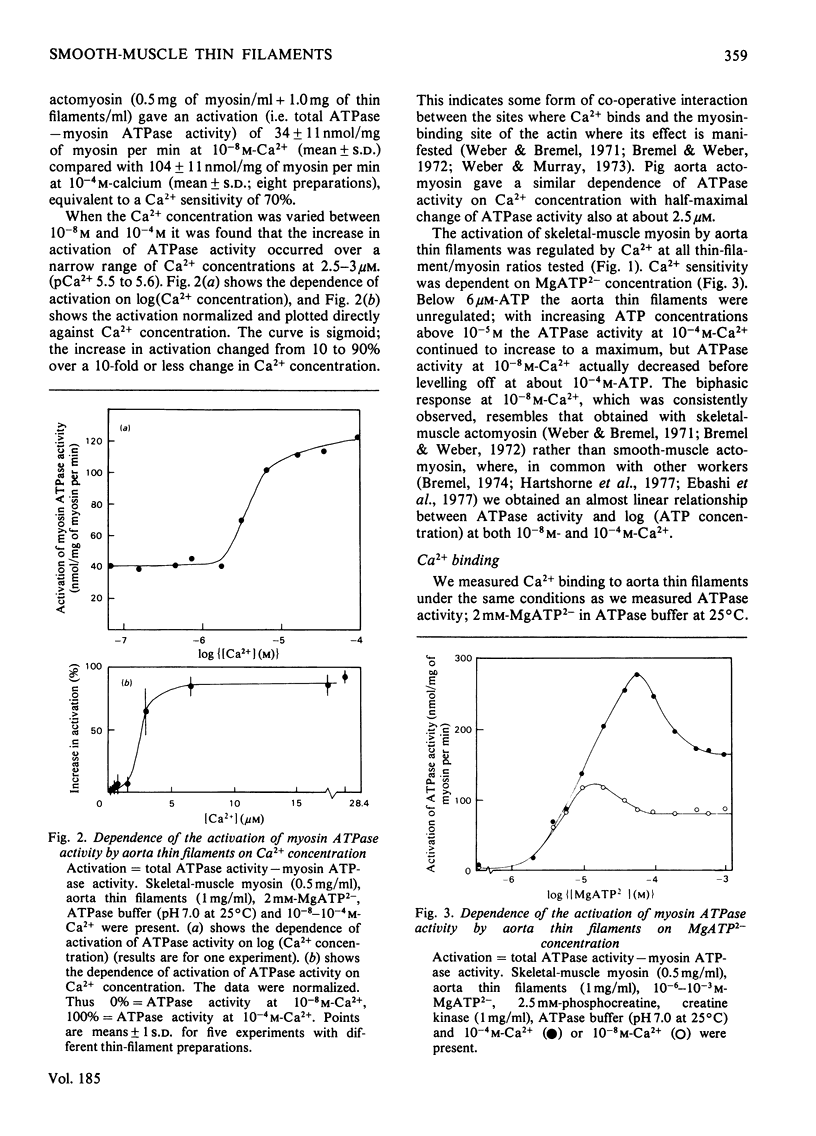
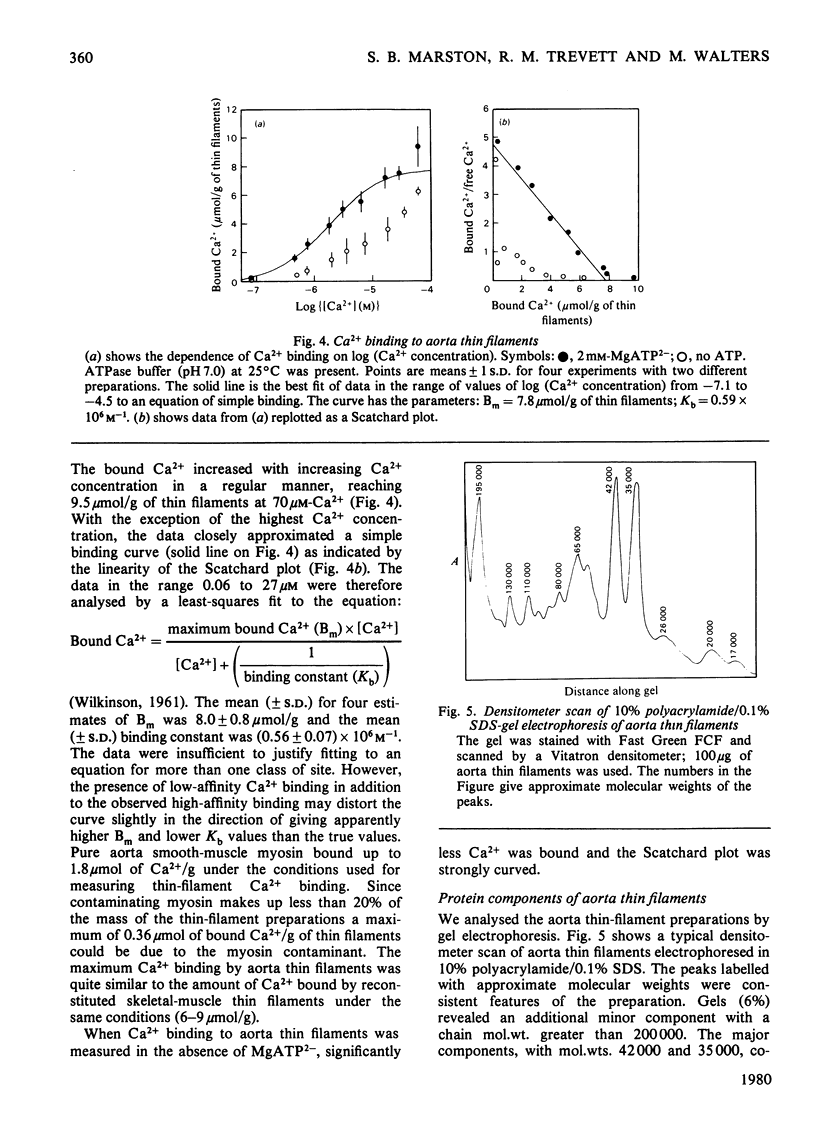
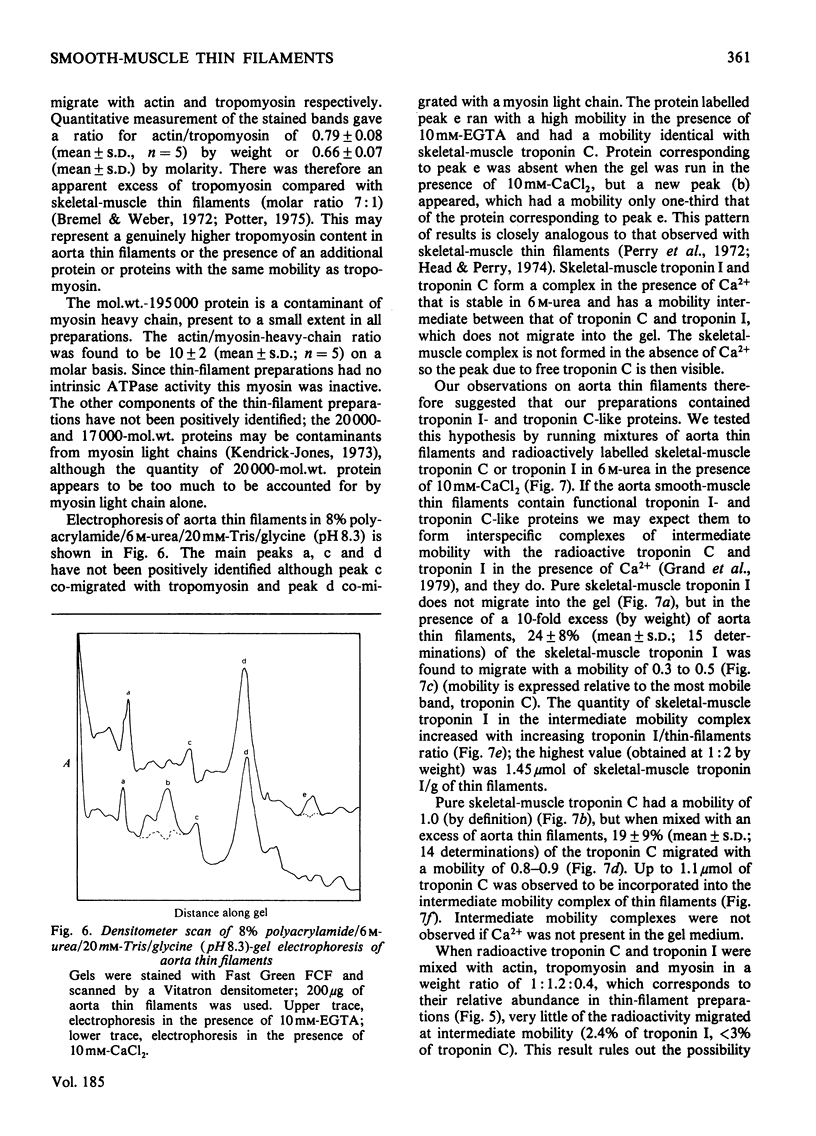
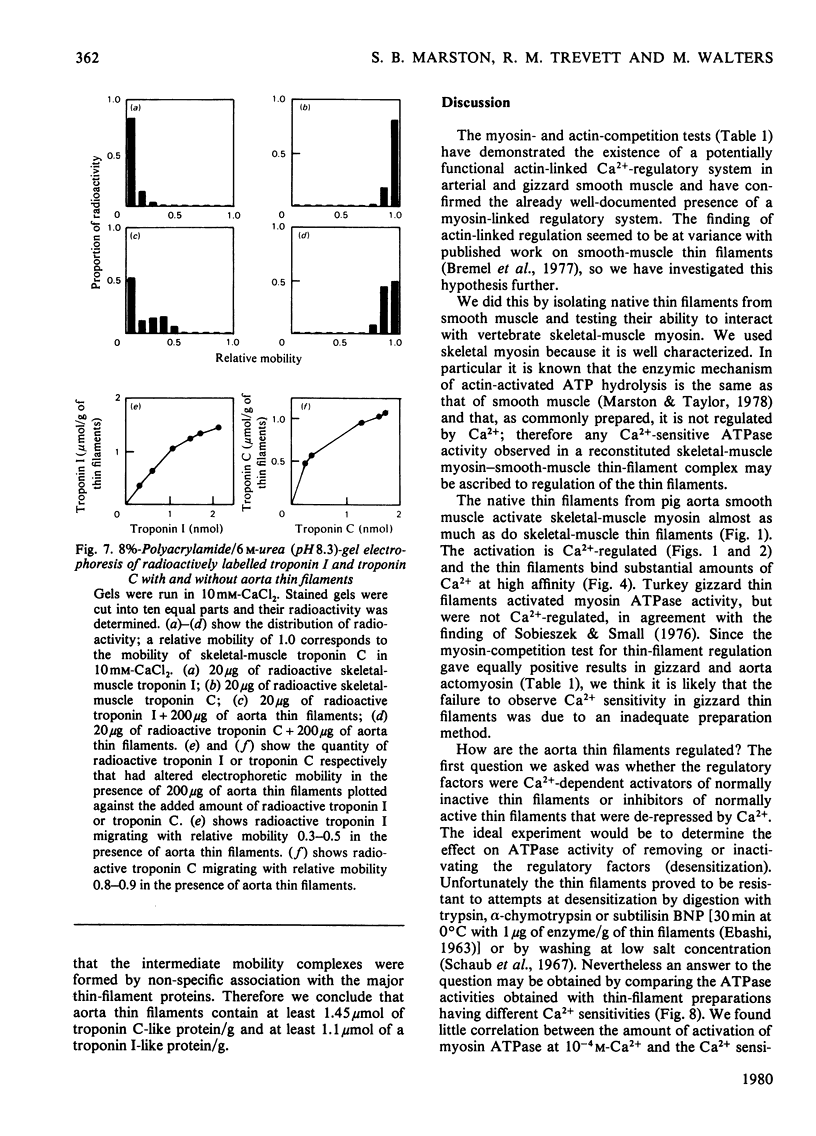
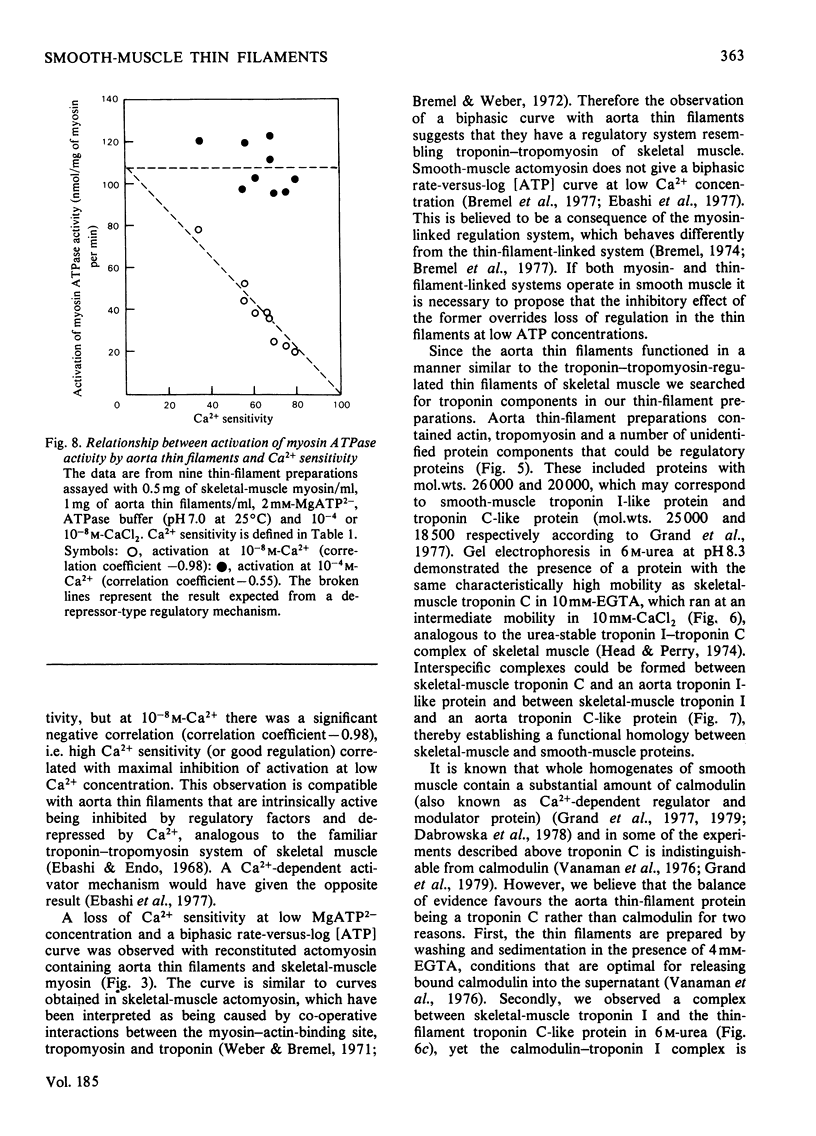
Myosin and actin competition tests indicated the presence of both thin-filament and myosin-linked Ca2+-regulatory systems in pig aorta and turkey gizzard smooth-muscle actomyosin. A thin-filament preparation was obtained from pig aortas. The thin filaments had no significant ATPase activity [1.1 +/- 2.6 nmol/mg per min (mean +/- S.D.)], but they activated skeletal-muscle myosin ATPase up to 25-fold [500 nmol/mg of myosin per min (mean +/- S.D.)] in the presence of 10(-4) M free Ca2+. At 10(-8) M-Ca2+ the thin filaments activated myosin ATPase activity only one-third as much. Thin-filament activation of myosin ATPase activity increased markedly in the range 10(-6)-10(-5) M-Ca2+ and was half maximal at 2.7 x 10(-6) M (pCa2+ 5.6). The skeletal myosin-aorta-thin-filament mixture gave a biphasic ATPase-rate-versus-ATP-concentration curve at 10(-8) M-Ca2+ similar to the curve obtained with skeletal-muscle thin filaments. Thin filaments bound up to 9.5 mumol of Ca2+/g in the presence of MgATP2-. In the range 0.06-27 microM-Ca2+ binding was hyperbolic with an estimated binding constant of (0.56 +/- 0.07) x 10(6) M-1 (mean +/- S.D.) and maximum binding of 8.0 +/- 0.8 mumol/g (mean +/- S.D.). Significantly less Ca2+ bound in the absence of ATP. The thin filaments contained actin, tropomyosin and several other unidentified proteins. 6 M-Urea/polyacrylamide-gel electrophoresis at pH 8.3 showed proteins that behaved like troponin I and troponin C. This was confirmed by forming interspecific complexes between radioactive skeletal-muscle troponin I and troponin C and the aorta thin-filament proteins. The thin filaments contained at least 1.4 mumol of a troponin C-like protein/g and at least 1.1 mumol of a troponin I-like protein/g.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy M. O., Williams D., Sharkey E. M., Hartshorne D. J. A relationship between Ca2+ sensitivity and phosphorylation of gizzard actomyosin. Biochem Biophys Res Commun. 1976 Mar 8;69(1):35–41. doi: 10.1016/s0006-291x(76)80268-9. [DOI] [PubMed] [Google Scholar]
- Bremel R. D. Myosin linked calcium regulation in vertebrate smooth muscle. Nature. 1974 Nov 29;252(5482):405–407. doi: 10.1038/252405a0. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Carsten M. E. Uterine smooth muscle: troponin. Arch Biochem Biophys. 1971 Nov;147(1):353–357. doi: 10.1016/0003-9861(71)90346-8. [DOI] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Sherry J. M., Aromatorio D. K., Hartshorne D. J. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978 Jan 24;17(2):253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- Driska S., Hartshorne D. J. The contractile proteins of smooth muscle. Properties and components of a Ca2+-sensitive actomyosin from chicken gizzard. Arch Biochem Biophys. 1975 Mar;167(1):203–212. doi: 10.1016/0003-9861(75)90457-9. [DOI] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Toyo-Oka T., Nonmura Y. Gizzard Troponin. J Biochem. 1975 Oct;78(4):859–861. doi: 10.1093/oxfordjournals.jbchem.a130976. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Elsden S. R., Gough N. The estimation of creatine and of diacetyl. Biochem J. 1943;37(5):526–529. doi: 10.1042/bj0370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F., Perry S. V. The interaction of the calcium-binding protein (troponin C) with bivalent cations and the inhibitory protein (troponin I). Biochem J. 1974 Feb;137(2):145–154. doi: 10.1042/bj1370145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Mikawa T., Nonomura Y., Ebashi S. Ca2+ regulation in vascular smooth muscle. J Biochem. 1977 Dec;82(6):1793–1796. doi: 10.1093/oxfordjournals.jbchem.a131879. [DOI] [PubMed] [Google Scholar]
- Ito N., Hotta K. Regulatory protein of bovine tracheal smooth muscle. J Biochem. 1976 Aug;80(2):401–403. doi: 10.1093/oxfordjournals.jbchem.a131290. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J. The subunit structure of gizzard myosin. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):183–189. doi: 10.1098/rstb.1973.0021. [DOI] [PubMed] [Google Scholar]
- Lehman W., Szent-Györgyi A. G. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975 Jul;66(1):1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W. Thick-filament-linked calcium regulation in vertebrate striated muscle. Nature. 1978 Jul 6;274(5666):80–81. doi: 10.1038/274080a0. [DOI] [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Solaro R. J., Ford G. D. Nature of the calcium regulatory system of bovine arterial actomyosin. Blood Vessels. 1979;16(1):26–34. doi: 10.1159/000158187. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Mechanism of myosin and actomyosin ATPase in chicken gizzard smooth muscle. FEBS Lett. 1978 Feb 15;86(2):167–170. doi: 10.1016/0014-5793(78)80555-9. [DOI] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Ebashi S. Does phosphorylation of myosin light chain have direct relation to regulation in smooth muscle? J Biochem. 1977 Dec;82(6):1789–1791. doi: 10.1093/oxfordjournals.jbchem.a131878. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Hirata M., Ebashi S., Kakiuchi S. Involvement of an acidic protein in regulation of smooth muscle contraction by the tropomyosin-leiotonin system. J Biochem. 1978 Dec;84(6):1633–1636. doi: 10.1093/oxfordjournals.jbchem.a132290. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Toyo-oka T., Nonomura Y., Ebashi S. Essential factor of gizzard "troponin" fraction. A new type of regulatory protein. J Biochem. 1977 Jan;81(1):273–275. doi: 10.1093/oxfordjournals.jbchem.a131447. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pemrick S., Weber A. Mechanism of inhibition of relaxation by N-ethylmaleimide treatment of myosin. Biochemistry. 1976 Nov 16;15(23):5193–5198. doi: 10.1021/bi00668a038. [DOI] [PubMed] [Google Scholar]
- Perrin D. D., Sayce I. G. Computer calculation of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta. 1967 Jul;14(7):833–842. doi: 10.1016/0039-9140(67)80105-x. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ regulation of muscle contraction. Biochemistry. 1974 Jun 18;13(13):2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- Potter J. D. The content of troponin, tropomyosin, actin, and myosin in rabbit skeletal muscle myofibrils. Arch Biochem Biophys. 1974 Jun;162(2):436–441. doi: 10.1016/0003-9861(74)90202-1. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. The adeonosine-triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Jul;104(1):263–269. doi: 10.1042/bj1040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Ca-regulation of mammalian smooth muscle actomyosin via a kinase-phosphatase-dependent phosphorylation and dephosphorylation of the 20 000-Mr light chain of myosin. Eur J Biochem. 1977 Jun 15;76(2):521–530. doi: 10.1111/j.1432-1033.1977.tb11622.x. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Bremel R. D. Preparation and properties of vertebrate smooth-muscle myofibrils and actomyosin. Eur J Biochem. 1975 Jun 16;55(1):49–60. doi: 10.1111/j.1432-1033.1975.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol. 1977 Jun 5;112(4):559–576. doi: 10.1016/s0022-2836(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Maxwell L. C., Ruegg J. C., Bohr D. F. Preparation and properties of a calcium ion-sensitive actomyosin from arteries. Am J Physiol. 1970 Nov;219(5):1366–1372. doi: 10.1152/ajplegacy.1970.219.5.1366. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., van Bockxmeer F. M. Arterial tropomyosin and a relaxing protein fraction from vascular smooth muscle. Comparison with skeletal tropomyosin and troponin. J Biochem. 1972 Nov;72(5):1075–1080. doi: 10.1093/oxfordjournals.jbchem.a129993. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W., Fulhorst H. W. Recovery of S35 radioactivity from protein-bearing polyacrylamide gel. Anal Biochem. 1965 May;11(2):389–391. doi: 10.1016/0003-2697(65)90030-8. [DOI] [PubMed] [Google Scholar]


