ABSTRACT
Background
Early‐phases clinical trials (Phases 1 and 2) have evolved from a traditional assessment of toxicity to an adaptive approach based on patients' medical needs and access to effective new therapies. The global risks, benefits, and relevance of early‐phases clinical trials participation for patients with hematological malignancies remain poorly evaluated.
Patients and Methods
All early‐phases clinical trials participations for patients with hematological malignancies, from 2008 to 2023, in a tertiary academic center in Europe, were reviewed. Patient's demographics, tumor type categories, therapeutic responses, mortality, overall survival (OS), and investigational product (IP) were assessed.
Results
Over the period 2008–2023, 736 patients participating in 92 different early‐phases clinical trials, were analyzed. The most common tumor categories were diffuse large B‐cell lymphoma (n = 253; 34.4%), acute myeloid leukemia/myelodysplastic syndrome (n = 164; 22.3%) and multiple myeloma (n = 100; 13.6%). The median OS was 14.8 (95% CI: 12.4–17.9) months and response rate 31.9%, including complete responses in 13.5% of patients. By tumor categories, the highest and lowest median duration of OS were observed for patients with Hodgkin lymphoma (99.8; [95% CI: 47.0‐not reached] months) and peripheral T‐cell lymphoma (8.9 [95% CI: 5.3–12.0] months), respectively. The on‐protocol and treatment‐related mortality rates were 5.43% and 0.54%, respectively. Overall response rate was 29.1% including 13.5% of complete response. Overall, 202 (27.5%) patients received an IP later approved by the health authorities, and those patients had better OS (18.2 months vs. 12.1 months HR: 1.160 [95% CI; 0.6977–1.391], p = 0.0283).
Conclusion
In conclusion, patients with hematologic malignancies who have participated in early‐phases clinical trials over the past 15 years have achieved variable therapeutic response rates, acceptable risk/benefit ratio and potentially significant therapeutic advantages. This study provides framework material for hematologists to further discuss clinical trial participation with their patients.
Keywords: benefit risk assessment, clinical protocols, clinical trial, hematological malignancies, Phase 1, survival analysis
1. Introduction
Phase 1 clinical trials are defined as first clinical trials in human to evaluate drug safety and objective is to determine the Phase 2 recommended dose [1, 2, 3]. Patients were traditionally referred to Phase 1 trials when they have no alternative effective therapy or when standard treatment has failed [4]. Subsequently, patients screened were heavily pretreated and enrollment criteria very selective [5]. Inclusion in Phase 1 trial may raise ethical questions on its benefit for patients [3, 6]. More recently since 2000–2010‐decade, Phase 1 trials tend to adaptive designing, including additional objectives, such as response and survival [2, 3, 4, 7, 8, 9, 10, 11].
Most studies evaluating large cohorts of patients in Phases 1 clinical trials are in the field of solid tumors [3, 8, 9, 12, 13, 14, 15, 16, 17]. Few data are available to evaluate patients in Phases 1–2 treated specifically for hematological malignancies. We previously reported patients with hematological malignancies participating in early‐phase clinical trials have intrinsic disease characteristics different from those of solid tumors [18, 19].
Furthermore, medical need is a human health science concept often used in medical and economic studies to underline diseases with weak therapeutic opportunities and poor prognosis [20]. By this concept, each disease can be in competition with other diseases for health authorities and medico economic system, who must respond to the medical needs of an entire population. Rare cancers can be an unfair source of research and benefit for patients [20, 21, 22]. As the design of clinical trials for anticancer drugs is strongly evolving toward an adaptive approach for the direct benefit of patients, real‐life data are valuable for assessing what are the real medical needs not covered by available therapeutics [7, 23]. We lack real life data on patients participating in early‐phases clinical trials, with hematological cancers.
Phases 1–2 studies typically have small numbers and short follow‐up, and the potential benefit of participating in these studies may appear small and poorly understood. Here, we propose a study in a large patient population, with longer follow‐up, and retrospective over more than 10 years, to investigate the benefits and risks of participating in Phases 1–2 trials for patients with hematological cancer. This study aims to report a comprehensive evaluation of safety and efficacy data and assessment of risks and benefits for patients with hematological cancer who participated in early‐phases clinical trials from 2008 to 2023.
2. Patients and Methods
2.1. Objectives
Primary objective of the study was to describe the data from Phases 1–2 in a large population of patients with hematological malignancies to evaluate the risk benefit of participation in a clinical trial. Secondary objectives were to report on clinical characteristics by different tumor types of hematological cancers. We retrospectively investigate characteristics of patients participating in early‐phases (Phases 1 and 2) clinical trials at Institute Gustave Roussy, France, and aggregating data from Phases 1 and 2, over the last 15 years (from 2008 to 2023).
2.1.1. Patients
All consecutive adult patient (above 18‐year‐old at time of inclusion in the considered clinical trials) and treated for a hematological malignancy in an early‐phases clinical trial (Phase 1 or 2) in the Innovative Therapeutics and Early Trials Department at Institute Gustave Roussy from January 1st, 2008, until January 1st, 2023, were retrospectively reviewed. All data about patient's demographics, tumor type, investigational treatment, toxicity of investigational treatment, efficacy of investigational treatment and overall survival (OS) outcomes were collected. The classification of clinical trials according to therapeutic class and the NCT numbers of each clinical trial are summarized in Table S1.
2.1.2. Endpoint Definitions
Toxicities of investigational treatments were assessed according to the National Cancer Institute (NCI)—Common Toxicity Criteria and was collected from the medical chart of the patients at Institute Gustave Roussy. Safety was analyzed by on‐protocol mortality, by treatment related mortality and mortality rates in the first 30 and 90 days of the protocol and investigated treatment‐related death rate (Table 1). The on‐protocol mortality rate was defined as death as the reason for end of protocol regardless of causality with the treatment. Response rate to investigational treatments was evaluated by established criteria approved by the protocol for each trial and was collected from the medical chart of the patients at Institute Gustave Roussy. Hematological malignancy diagnosis was established according to WHO criteria in agreement at the time of clinical trial entry. Overall, eight categories of tumor types were distinguished with: diffuse large B‐cell (DLBCL) lymphoma, acute myeloid leukemia and myelodysplastic syndrome (AML/MDS), multiple myeloma (MM), indolent lymphoma (IL), peripheral T‐cell lymphoma (PTCL), Hodgkin lymphoma (HL), mantle cell lymphoma (MCL), and myelofibrosis (MF). Indolent type lymphomas included follicular lymphoma, chronic lymphocytic leukemia, Waldenstrom's disease, and marginal zone lymphoma. The last category (other) of all other tumor types included hairy cell leukemia and chronic myelomonocytic leukemia.
TABLE 1.
Mortality data in patients with hematological cancers, participants in early‐phases clinical trials (Phases 1 and 2), between 2008 and 2023.
| Tumor type categories (n patients) | All patients (736) | DLBCL (253) | AML/MDS (164) | Myeloma (100) | Indolent lymphoma (78) | PTCL (45) | Hodgkin (40) | MCL (32) | MF (20) | Other (4) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality ≤ Day 30 | 20 (2.72%) | 9 (3.56%) | 7 (4.26%) | 0 | 1 (1.28%) | 2 (4.44%) | 0 | 1 (3.12%) | 0 | 0 |
| Mortality ≤ Day 90 | 101 (13.72%) | 39 (15.41%) | 38 (23.17%) | 4 (4.00%) | 3 (3.84%) | 9 (20.00%) | 1 (2.5%) | 6 (18.75%) | 1 (5.00%) | 0 |
| Death on‐protocol, as reason of end of protocol | 40 (5.43%) | 1 (0.39%) | 38 (23.17%) | 0 | 1 (1.28%) | 0 | 0 | 0 | 0 | 0 |
| Death related to study drug | 4 (0.54%) | 1 (0.39%) | 2 (1.21%) | 0 | 1 (1.28%) | 0 | 0 | 0 | 0 | 0 |
Note: The table reports the overall mortality rates in the first 30 and 90 days after the start of the protocol, mortality during the protocol (regardless of causality with the protocol treatment) and mortality due to toxic events related to the protocol treatment. Data presented are n (%) unless otherwise stated. Percentages are expressed compared with the total number of patients in each disease category.
Abbreviations: AML, acute myeloid leukemia; DLBCL, diffuse large B cell lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, Myelofibrosis; PTCL, peripheral T cell lymphoma.
2.1.3. Data Analysis, Statistical Analysis, and Ethics
Continuous variables were reported as medians and interquartile ranges (IQR), while categorical variables were reported as numbers and proportions. OS was estimated using the Kaplan–Meier method and survival curves were compared using the log‐rank test (Kaplan and Meier, 1958). OS was measured from the day of the first investigational treatment administration until death of any causes, and censoring patients still alive at the date of last follow‐up. The cutoff date for the OS analysis was February 7th, 2023. Data from all Phases 1–2 trials were aggregated and analyzed retrospectively and globally over the last 15 years (2008–2023). We will assess treatment responses according to whether the investigational product (IP) was approved by the FDA or EMA at the time of data analysis (data analysis date February 7th, 2023). Univariate and multivariate analyses assessing the impact of variables on OS were performed using Cox regression models. The variables selected were those used most often in clinical trials at the time of clinical study entry. The sodium balance was also tested for its simple use (blood ionogram), availability data and the known prognostic value of hyponatremia in patients with cancer [24, 25]. The proportional hazard assumption was validated, and all significant variables (p < 0.05) were included in the multivariate analysis. Multivariate analysis was performed by Cox regression, followed by backward stepwise selection. Hazard ratios (HR) are given with 95% confidence interval (CI). Statistical analyses were performed using the STATA software (STATA 15.0 Corporation, College Station, TX). Ethics approvals were obtained from local institutional review boards for the data collection collected from the medical chart of the patients at Institute Gustave Roussy.
3. Results
3.1. Patient Characteristics
From 2008 to 2023, 736 patients with hematological malignancies were enrolled in early‐phases clinical trials at Institute Gustave Roussy. At clinical trial entry time, median age (range) of patients was 66 (19–88), and 59.8% were male. The most frequent hematological malignancies categories were diffuse large B cell lymphoma (DLBCL) (n = 253; 34.4%), AML/MDS (n = 164; 22.3%), and MM (n = 100; 13.6%). Mean age was homogeneous in the main categories DLBCL, AML/MSD, and myeloma (66, 66, and 69 years old, respectively). We just observe exception in patients with HL who were younger (mean age 34 years). Patients in AML/MDS category had the lowest hematological blood count parameters (hemoglobin, platelet, and absolute neutrophil count) at clinical trial inclusion (Table 2).
TABLE 2.
Characteristics of adult patients included and treated in early‐phases clinical trials (Phases 1 and 2) for hematological malignancies, between 2008 and 2023. Categorical data are presented are n (%) unless otherwise stated. Continuous data are presented by median [IQR], unless otherwise stated.
| Tumor type categories | All patients | DLBCL | AML/MDS | Myeloma | Indolent lymphoma | PTCL | Hodgkin | MCL | MF | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients (%) |
736 (100.0%) |
253 (34.4%) |
164 (22.3%) |
100 (13.6%) |
78 (10.6%) |
45 (6.1%) |
40 (5.4%) |
32 (4.3%) |
20 (2.7%) |
4 (0.5%) |
| Age at inclusion (years) [range] | 66 [19;88] | 66 [22;87] | 69 [23;86] | 66 [44;82] | 69 [43;82] | 63 [25;88] | 34 [19;81] | 67 [45;81] | 68 [52;76] | 67 [63;84] |
| Gender | ||||||||||
| Male | 440 (59.8) | 151 (59.7) | 86 (52.4) | 53 (53.0) | 53 (68.0) | 30 (66.7) | 26 (65.0) | 24 (75.0) | 13 (65.0) | 4 (100) |
| Female | 296 (40.2) | 102 (40.3) | 78 (47.6) | 47 (47.0) | 25 (32.0) | 15 (33.3) | 14 (35.0) | 8 (25.0) | 7 (35.0) | 0 |
| Biological parameters | ||||||||||
| Hemoglobin (g/dL) | 11.3 [9.8;12.8] | 11.4 [10.2;12.7] | 9.9 [8.8;11.2] | 11.3 [10.3;12.6] | 13 [11.4;14] | 11.7 [10.2;13] | 13 [11.4;13.8] | 11.9 [11.3;13.7] | 10.4 [8.8;12.5] | 13 [12.3;15] |
| Platelets (G/L) | 157 [88;224] | 188 [130;253] | 49 [21;109] | 176 [117;224] | 153 [112;185] | 165 [91;245] | 210 [164;257] | 175 [133;218] | 148 [100;219] | 117 [72;188] |
| ANC (G/L) | 2.9 [1.3;4.8] | 4.0 [2.5;6.1] | 0.4 [0.1;1.5] | 2.6 [1.6;3.6] | 3.4 [1.8;4.5] | 3.6 [1.9;5.8] | 4.9 [3.5;6.5] | 3.8 [1.9;5.1] | 3.2 [2.0;9.5] | 1.6 [1.1;1.9] |
| ALC (G/L) | 0.9 [0.6;1.5] | 0.7 [0.4;1.1] | 0.9 [0.6;1.4] | 1.2 [0.8;1.6] | 1.6 [0.9;3.0] | 0.8 [0.4;1.1] | 1.4 [1.0;1.7] | 1.0 [0.5;1.3] | 1.0 [0.6;1.5] | 1.0 [0.7;1 6] |
| LDH (UI/L) | 229 [183;322] | 258 [204;346] | 215 [174;330] | 185 [143;233] | 218 [186;272] | 254 [191;406] | 205 [169;259] | 211 [181;278] | 487 [304;762] | 186 [162;196] |
| Albumin (g/L) | 39 [36;42] | 40 [37;43] | 39 [35;41] | 38 [34;42] | 41 [38;44] | 40 [36;43] | 39 [34;43] | 40 [36;44] | 40 [35;43] | 40 [40;43] |
| Sodium imbalance deficit (mmol/L) | 1 [0;3] | 1 [0;3] | 2 [0;3] | 2 [0;3.9] | 0 [−1;2] | 1 [−0.8;3] | 0.4 [−0.7;2] | 1 [0;2] | 2 [0;5] | 2 [0.5;2.5] |
Abbreviations: ALC, absolute lymphocyte count; AML, acute myeloid leukemia; ANC, absolute neutrophil count; DLBCL, diffuse large B cell lymphoma; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; PTCL, peripheral T cell lymphoma.
3.2. Treatments Investigated in Early‐Phases Clinical Trials for Patients With Hematological Malignancies
Overall, 736 patients participated in 92 different clinical trials. The median number of patients included per clinical trial was 4 [range 1–44]. The investigated therapeutic products from clinical trials belonged to 15 different therapeutic areas variously distributed according to tumor type categories (Figure 1). The most frequent therapeutic classes investigated were epigenetic (n = 236 pts., 32.1%), immuno‐oncology (n = 107 pts., 14.5%), protein degrader (n = 105 pts.; 14.3%), targeted therapy (n = 61 pts.; 8.3%), and anti‐apoptotic protein inhibitor class (n = 56 pts.; 7.6%).
FIGURE 1.
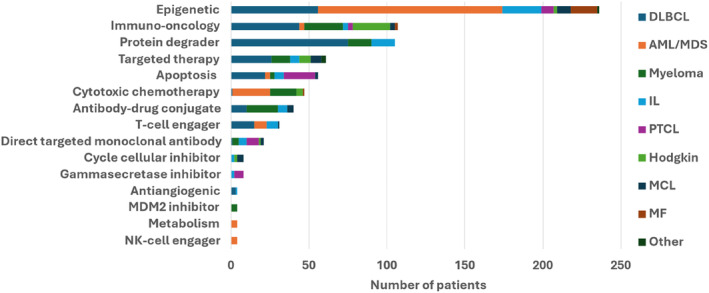
Area of therapeutic investigation (by class of drugs investigated), in early‐phases of clinical trials (Phases 1 and 2), in patients with hematological cancers, between 2008 and 2023 (n = 736 patients). AML, acute myeloid leukemia; DLBCL, diffuse large B cell lymphoma; IL, indolent lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; PTCL, peripheral T cell lymphoma.
3.3. Risk and Safety for Participation in Phase 1 Clinical Trials
On‐protocol mortality rate was 5.4% in the overall population. Patients treated for AML/MDS had the higher mortality on‐protocol (23.2%) while for other hematological indications mortality rate during protocol remained low between 0% and 1.3%. Deaths related to investigated treatment occurred in 0.54% of patients in the overall population.
3.4. Response to Investigated Therapies and Benefit From Participation in Phase 1 Clinical Trials
A total of 684 out of 736 patients (92.9%) had available data to evaluate therapeutic responses (Table 3 and Table S2). The overall response rate (ORR) for all tumor types was 31.9%, including complete responses (CR) achieved in 13.5% of patients and partial responses in 18.5% of patients. Therapeutic response rates varied across disease categories as shown in Figure 2. OS was significantly higher in patients responding to treatments (median OS = 30.4 months [95% CI; 24.3–48.9] vs. 9.6 months, [95% CI; 8.5–11.0]; HR: 0.45 [95% CI; 0.37–0.57], p < 105) (Figure 3). By tumor type categories, ORR were the highest in AML/MDS (49.4%) and MM (33.0%) category. Conversely and across all categories of diseases, low ORR was observed for MCL (9.4%) and PTCL (15.6%) (Figure 2 and Table 3). Of the 736 patients, 202 (27.2%) participated in a clinical trial who evaluated a therapy that was later approved by health authorities FDA or EMA. ORRs and OS were significantly improved in patients who received a treatment later approved (respectively for ORR: 54.5% vs. 23.3%, p < 0.0001 and for OS: 18.2 months vs. 12.1 months, HR: 0.79 [95% CI; 0.65–0.95], p = 0.0128) (Table 3 and Figure 3).
TABLE 3.
Therapeutic response rate of patients with main category of hematological cancers (DLBCL, AML/MSD, and myeloma) participating in early‐phases clinical trials (Phases 1 and 2) from 2008 to 2023 and depending on whether the experimental product was subsequently approved by the health authorities (Food and Drug Administration or the European Medicines Agency). Data presented are n (%) unless otherwise stated. For AML, CR corresponds to CR and CRi (complete response with incomplete count recovery).
| Tumor type categories (n patients) | All patients (736) | DLBCL (253) | AML/MDS (164) | Myeloma (100) |
|---|---|---|---|---|
| Evaluable patients for efficacy a | 684 (92.9) | 236 (93.3) | 149 (90.9) | 96 (96.0) |
| Best overall response (complete and partial) b | 235 (31.9) | 60 (23.7) | 81 (49.4) | 33 (33.0) |
| Complete response b | 99 (13.5) | 26 (10.3) | 45 (27.4) | 11 (11.0) |
| Partial response b | 136 (18.5) | 34 (13.4) | 36 (22.0) | 22 (22.0) |
| IP thereafter approved a | 202 (27.5) | 35 (13.8) | 97 (59.5) | 43 (43.0) |
| Evaluable patients for efficacy a | 195 (96.3) | 34 (97.1) | 92 (94.9) | 43 (100) |
| Best overall response (complete and partial) b | 110 (54.5) | 12 (34.3) | 68 (70.1) | 17 (39.5) |
| Complete response b | 54 (26.7) | 7 (20.0) | 34 (35.1) | 8 (18.6) |
| Partial response b | 56 (27.7) | 5 (14.3) | 34 (35.1) | 9 (20.9) |
Abbreviations: AML, acute myeloid leukemia; DLBCL, diffuse large B cell lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; PTCL, peripheral T cell lymphoma.
Percentages are expressed compared with total number of patients in each disease subcategory.
Percentages are expressed compared with total number of evaluable patients in each disease subcategory.
FIGURE 2.
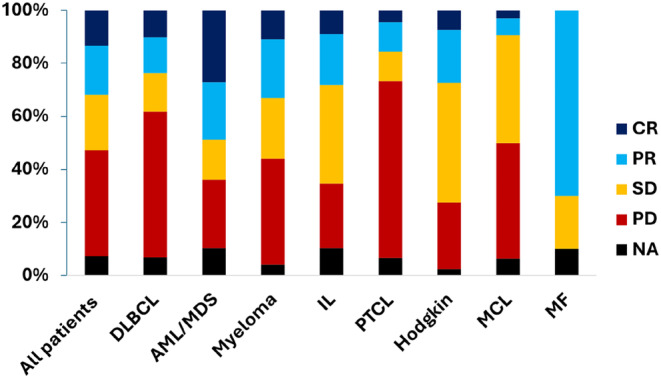
Histogram stacked at 100%, showing the distribution of therapeutic response rates, according to tumor type categories, in patients with hematological cancer participating in early‐phases clinical trials (Phases 1 and 2), between 2008 and 2023. Responses were evaluated by the investigators in each protocol and according to the criteria of each disease and each protocol. For acute leukemia, the complete responses included in this figure the complete response (CR) and the complete responses with incomplete count recovery (CRI). AML, acute myeloid leukemia; CR, complete response; Cri, complete response incomplete count recovery; DLBCL, diffuse large B cell lymphoma; IL, indolent lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; NA, not available; PD, progressive disease; PR, partial response; PTCL, peripheral T cell lymphoma; SD, stable disease.
FIGURE 3.
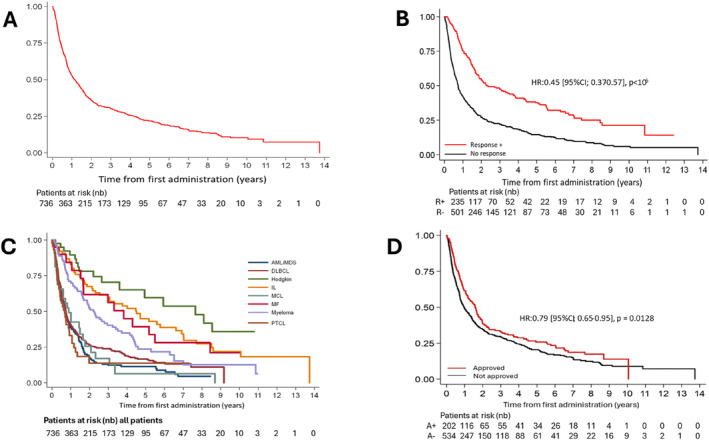
Overall survival of patients with hematological cancers, included in early‐phases clinical trials, between 2008 and 2023, in all patients (Panel A), according to objective response to treatment (Panel B [Objective responses positive were partial response and complete response]), according to the tumor type category (Panel C) and according to treatment approval or not (Panel D). AML, acute myeloid leukemia; DLBCL, diffuse large B cell lymphoma; IL, indolent lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; PTCL, peripheral T cell lymphoma.
3.5. Overall Survival of Patients Participating in Phase 1 Clinical Trials
With a median patient follow‐up of 78.0 months (95% CI [70.4–86.7]), the median OS for all patients was 14.8 months (95% CI; 12.4–17.9) (Figure 3). The estimated OSs at 1, 3, and 5 years were of 55% (95% CI; 51–58), 31% (95% CI; 28–31), and 23% (95% CI; 19–26), respectively. By tumor type categories, the longest median OS were observed in HL (99.8 months [95% CI; 47.0‐NR], IL [58.8 months, 95% CI; 33.1–86.4], and MF [49.9 months 95% CI; 19.8–71.6]). The poorest OSs were observed in PTCL (8.9 months [95% CI; 5.3–12.0]), AML/MDS (9.0 months [95% CI; 6.3–11.2]), DLBCL (9.6 months, [95% CI; 8.4–11.0]), and MCL (11.6 months [95% CI; 3.8–21.5]) (Figure 3).
Predictors of OS were analyzed, and the results are shown in Table S3. In a univariate Cox model, age, elevated LDH, hypoalbuminemia, lymphopenia, neutropenia, anemia, thrombocytopenia, and negative sodium imbalance were significantly associated with the risk of death. In the corresponding multivariate model, all previous variables, except hypoalbuminemia (p = 0.076), remained significantly associated with the risk of death (Table S3).
4. Discussion
We report here a large cohort of patients with hematologic malignancies participating in early‐phases clinical trials. Compared with the main other scientific articles on early‐phases solid tumor trials [3, 8, 9, 12, 13, 14, 15, 16, 17], we observe similarities and distinctions. The main similarity was the safety data, as based on death related to treatment also called toxic deaths. Toxic deaths frequency was 0.54% in our study, which was similar frequency reported in other retrospective study of early‐phases clinical trials for solid tumors; ranging between 0.47% [13] and 0.67% [15]. Compared with solid tumor clinical trials, we interestingly observed in our study for hematological cancers higher response rates: the ORR in our study was 31.9%, while ORR were in Phase 1 for solid tumor between 3.8% [9] and 12.2% [15]. Our study emphasizes that in Phase 1 clinical trials, patients with hematologic malignancies respond more favorably to the treatments investigated and this results are important to calibrate further methods in study design and for discussions between physicians and patients about objective and benefits to participate in such clinical trials.
In our study, we report the outcome of patients with hematologic malignancy participating in early‐phases clinical trial and obtaining ORRs of 31.9%. We observed that this ORR was particularly high in the AML/MDS tumor category (49%), that could partially be explained by the Phase 1 trials and results with IDH2 and IDH1 inhibitors [26, 27], which have greatly improved disease control and patient OS in the last 10 years.
Compared to solid tumors, significant differentiating parameters were biological characteristics of patients at the time of study entry. These biological abnormalities were intrinsically due to the hematological disease and must be taken into consideration particularly when protocols include both patients with solid tumors and hematological cancers.
On‐protocol mortality rate, defined as death as the reason for end of protocol regardless of causality with the treatment, and called on‐study death rate, was 5.43% in our study. For comparison, Chihara et al. found an on‐study death rate of 8.0%, in a population of patients treated in Phase 1 for both solid and hematological tumors [15]. Our study found that on‐study death rate was higher in patients with AML/MDS reaching 23.2% of patients, which could reflect the life‐threatening condition of the disease.
Expectations for patients participating in clinical trials and for physicians who could propose participation to clinical trials should be continuously based firstly on benefits for patients and this benefit evaluation should be basically evaluated on safety and therapeutic advantages. We demonstrate in our study that benefits could be consistent with ORR of 31.9% of participating patients, that is much higher than historical results from retrospective analysis from Phase 1 solid tumors studies [28, 29]. We also observed that 27.5% of patients benefited from an investigational product which was subsequently approved by the health authorities FDA or EMA. These data confirm Phase 1 should be a valid therapeutic option and modern Phase 1 can now be deemed as definitive and valid opportunities for new anticancer treatment options [11, 30].
Some recent medical economic studies on cancer drug development highlighted many inequalities in research between different tumor types and especially for rare cancers [21, 22, 31]. Medical needs assessment should be regularly updated to design the most appropriate clinical trials [32]. Our study on Phase 1 clinical trials for patients with hematological malignancies provides comprehensive real data from clinical practice and could help to better assess unmet medical needs in hematological malignancies. For example, in our study we observed a median age of 66 years, homogeneous across the different tumor categories except for patients with Hodgkin lymphoma (HL) which concerns young patients (median age was 34 years). The young age of patients with HL could accentuate the current significant medical need for these patients. Patients with AML, although they can benefit from IDH inhibitors with significant response rates, remain in our study with poor OS (median OS was 9 months) and AML remain a significant unmet medical need. Among patients with non‐HLs, PTCL patients have poorest duration of OS (median OS was 8.9 months) and very low ORRs to the investigated treatment (ORR 15.6%). Although the recent therapeutic advances with epigenetic drugs for PTCL [33, 34, 35, 36], the search for innovative approaches should intensify in PTCL field to obtain better results in outcome of patients.
Our study could guide and help with possible recommendations to clinicians for the allocation of their patients and eligibility for Phases 1 and 2 trials. Based on our study, we could recommend to intensify clinical research of new drugs as a priority for patients with relapsed or refractory pathologies with the most unmet medical needs and poor outcomes, e.g., for diseases such as PTCL, acute myeloid leukemia, diffuse large B‐cell lymphoma, and MCL. Since these patients have a poor prognosis, they should benefit as a priority from new research and new treatments. We can also emphasize that based on our study, certain pathologies such as Hodgkin's lymphoma or IL are associated with a prolonged median survival, which suggests that disease control can be prolonged and therefore that anti‐lymphoma treatments and innovative approaches and researches should be developped in these patients.
Our study contains limitations for the interpretation of the results. The single‐center cohort is associated with selection bias in patients populations and potentially outcomes of patients populations. We retrospectively analyzed the data of patients included in clinical trials, having received at least one dose of IP, and this only reflects part of the estimation of the medical need of patients, as we did not consider patients who were not eligible, or who were excluded after screening period. The present study is descriptive in nature and although we performed multivariate analysis to adjust for some baseline patient characteristics, there may be other unobserved confounding factors that require further sensitivity analyses. Therefore, factors associated with survival observed in this study should be interpreted with caution. This study nevertheless brings together, to our knowledge, the broadest experience of clinical trials for hematological cancers, in large population, and summarizes a substantial data set.
In conclusion, patients with hematologic malignancies who have participated in early‐phases clinical trials over the past 15 years have achieved variable therapeutic response rates and potentially significant therapeutic advantages with acceptable benefit/risk ratio. This study provides a comprehensive data framework for hematologists to further discuss participation in a clinical trial with their patients.
Author Contributions
Substantial contributions to the conception or design of the work: V.R., J.‐M.M., M.G. Acquisition of data: M.G., E.A., J.‐M.M. Analysis of data: M.G., J.‐M.M., A.D., T.H. Interpretation of data M.G., J.‐M.M., V.R., S.D.B., K.O., A.H., R.B., A.G., C.B., S.P.‐V., J.‐B.M., C.M., T.H. Drafting the work or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Conflicts of Interest
Jean‐Marie Michot reports outside of the submitted work, research funding for Institution as Principal/sub‐Investigator of Clinical Trials for Abbvie, Adaptimmune, Adlai Nortye USA Inc., Aduro Biotech, Agios Pharmaceuticals, Amgen, Astex Pharmaceuticals, Astra Zeneca Ab, Aveo, Basilea Pharmaceutica International Ltd., Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, BicycleTx Ltd., Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Ca, Casi Pharmaceuticals Inc., Celgene Corporation, Cellcentric, Chugai Pharmaceutical Co., Cullinan‐Apollo, Curevarc, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Faron Pharmaceuticals Ltd., Forma Tharapeutics, Gamamabs, Genentech, Glaxosmithkline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Incyte Corporation, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Iteos Belgium SA, Janssen Cilag, Janssen Research Foundation, Janssen R&D LLC, Kura Oncology, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Genomics, Ose Pharma, Pfizer, Pharma Mar, Pierre Fabre Medicament, Relay Therapeutics Inc., Roche, Sanofi Aventis, Seattle Genetics, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Transgene S.A, Turning Point Therapeutics, Xencor. In the last 2 years, Jean‐Marie Michot reports personal fees (Monies paid to you for services rendered, generally honoraria, for consulting fees, lectures, speakers bureaus, expert testimony, advisory boards, steering committee) for Ideogen, Glaxosmithkline, MSD, Therakos/Mallinckrodt, Regeneron, Gilead, and Ed‐Gather company. The other authors report no conflicts of Interest.
Supporting information
Data S1.
Acknowledgments
The authors thank Tina Zaarour for copyediting the manuscript and her medical editorial assistance.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The authors declare that the data presented in this article are available as raw data upon request. This supplementary raw data material of this study is available from the corresponding author upon reasonable request.
References
- 1. Italiano A., “Participation in Phase 1 Trials for Patients With Cancer,” Lancet 400, no. 10351 (2022): 473–475. [DOI] [PubMed] [Google Scholar]
- 2. Chakiba C., Grellety T., Bellera C., and Italiano A., “Encouraging Trends in Modern Phase 1 Oncology Trials,” New England Journal of Medicine 378, no. 23 (2018): 2242–2243. [DOI] [PubMed] [Google Scholar]
- 3. Horstmann E., McCabe M. S., Grochow L., et al., “Risks and Benefits of Phase 1 Oncology Trials, 1991 Through 2002,” New England Journal of Medicine 352, no. 9 (2005): 895–904. [DOI] [PubMed] [Google Scholar]
- 4. Adashek J. J., LoRusso P. M., Hong D. S., and Kurzrock R., “Phase I Trials as Valid Therapeutic Options for Patients With Cancer,” Nature Reviews. Clinical Oncology 16, no. 12 (2019): 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamaker M. E., Stauder R., and van Munster B. C., “Exclusion of Older Patients From Ongoing Clinical Trials for Hematological Malignancies: An Evaluation of the National Institutes of Health Clinical Trial Registry,” Oncologist 19, no. 10 (2014): 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahipal A. and Nguyen D., “Risks and Benefits of Phase 1 Clinical Trial Participation,” Cancer Control 21, no. 3 (2014): 193–199. [DOI] [PubMed] [Google Scholar]
- 7. Berry D. A., “Adaptive Clinical Trials in Oncology,” Nature Reviews. Clinical Oncology 9, no. 4 (2011): 199–207. [DOI] [PubMed] [Google Scholar]
- 8. Koyfman S. A., Agrawal M., Garrett‐Mayer E., et al., “Risks and Benefits Associated With Novel Phase 1 Oncology Trial Designs,” Cancer 110, no. 5 (2007): 1115–1124. [DOI] [PubMed] [Google Scholar]
- 9. T. G. Roberts, Jr. , Goulart B. H., Squitieri L., et al., “Trends in the Risks and Benefits to Patients With Cancer Participating in Phase 1 Clinical Trials,” Journal of the American Medical Association 292, no. 17 (2004): 2130–2140. [DOI] [PubMed] [Google Scholar]
- 10. Chen E. X. and Tannock I. F., “Risks and Benefits of Phase 1 Clinical Trials Evaluating New Anticancer Agents: A Case for More Innovation,” Journal of the American Medical Association 292, no. 17 (2004): 2150–2151. [DOI] [PubMed] [Google Scholar]
- 11. Agrawal M. and Emanuel E. J., “Ethics of Phase 1 Oncology Studies: Reexamining the Arguments and Data,” Journal of the American Medical Association 290, no. 8 (2003): 1075–1082. [DOI] [PubMed] [Google Scholar]
- 12. Alouani E., Gazzah A., Mercier S., et al., “Profile and Outcome of Cancer Patients Enrolled in Contemporary Phase I Trials,” European Journal of Cancer 188 (2023): 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Arkenau H. T., Olmos D., Ang J. E., de Bono J., Judson I., and Kaye S., “Clinical Outcome and Prognostic Factors for Patients Treated Within the Context of a Phase I Study: The Royal Marsden Hospital Experience,” British Journal of Cancer 98, no. 6 (2008): 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bachelot T., Ray‐Coquard I., Catimel G., et al., “Multivariable Analysis of Prognostic Factors for Toxicity and Survival for Patients Enrolled in Phase I Clinical Trials,” Annals of Oncology 11, no. 2 (2000): 151–156. [DOI] [PubMed] [Google Scholar]
- 15. Chihara D., Lin R., Flowers C. R., et al., “Early Drug Development in Solid Tumours: Analysis of National Cancer Institute‐Sponsored Phase 1 Trials,” Lancet 400, no. 10351 (2022): 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Italiano A., Massard C., Bahleda R., et al., “Treatment Outcome and Survival in Participants of Phase I Oncology Trials Carried Out From 2003 to 2006 at Institut Gustave Roussy,” Annals of Oncology 19, no. 4 (2008): 787–792. [DOI] [PubMed] [Google Scholar]
- 17. Paluri R. K., Li P., Anderson A., et al., “First‐In‐Human Phase 1 Clinical Trials—A Single‐Center Experience in the Era of Modern Oncotherapeutics,” Scientific Reports 10, no. 1 (2020): 7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benajiba L., Michot J. M., Baldini C., et al., “Prognostic Factors and Outcome of Patients With Hematological Malignancies in Phase I Trials: The Gustave Roussy Scoring System,” Anti‐Cancer Drugs 28, no. 5 (2017): 540–545. [DOI] [PubMed] [Google Scholar]
- 19. Michot J. M., Benajiba L., Faivre L., et al., “Outcomes and Prognostic Factors for Relapsed or Refractory Lymphoma Patients in Phase I Clinical Trials,” Investigational New Drugs 36, no. 1 (2018): 62–74. [DOI] [PubMed] [Google Scholar]
- 20. Barrenho E., Halmai R., Miraldo M., et al., “Inequities in Cancer Drug Development in Terms of Unmet Medical Need,” Social Science & Medicine 302 (2022): 114953. [DOI] [PubMed] [Google Scholar]
- 21. Boyd N., Dancey J. E., Gilks C. B., and Huntsman D. G., “Rare Cancers: A Sea of Opportunity,” Lancet Oncology 17, no. 2 (2016): e52–e61. [DOI] [PubMed] [Google Scholar]
- 22. Casali P. G., Licitra L., Frezza A. M., and Trama A., “‘Rare cancers’: Not all Together in Clinical Studies!,” Annals of Oncology 33, no. 5 (2022): 463–465. [DOI] [PubMed] [Google Scholar]
- 23. Nicotera G., Sferrazza G., Serafino A., and Pierimarchi P., “The Iterative Development of Medicines Through the European Medicine Agency's Adaptive Pathway Approach,” Frontiers in Medicine (Lausanne) 6 (2019): 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castillo J. J., Vincent M., and Justice E., “Diagnosis and Management of Hyponatremia in Cancer Patients,” Oncologist 17, no. 6 (2012): 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterns R. H., “Disorders of Plasma Sodium—Causes, Consequences, and Correction,” New England Journal of Medicine 372, no. 1 (2015): 55–65. [DOI] [PubMed] [Google Scholar]
- 26. Stein E. M., DiNardo C. D., Pollyea D. A., et al., “Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia,” Blood 130, no. 6 (2017): 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiNardo C. D., Stein E. M., de Botton S., et al., “Durable Remissions With Ivosidenib in IDH1‐Mutated Relapsed or Refractory AML,” New England Journal of Medicine 378, no. 25 (2018): 2386–2398. [DOI] [PubMed] [Google Scholar]
- 28. Levy V., “Of Some Innovations in Clinical Trial Design in Hematology and Oncology,” Thérapie 77, no. 2 (2022): 191–195. [DOI] [PubMed] [Google Scholar]
- 29. Escritt K., Mann M., Nelson A., and Harrop E., “Hope and Meaning‐Making in Phase 1 Oncology Trials: A Systematic Review and Thematic Synthesis of Qualitative Evidence on Patient‐Participant Experiences,” Trials 23, no. 1 (2022): 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackley M. P., Fernandez N. R., Fletcher B., Woolcott C. G., and Fernandez C. V., “Revisiting Risk and Benefit in Early Oncology Trials in the Era of Precision Medicine: A Systematic Review and Meta‐Analysis of Phase I Trials of Targeted Single‐Agent Anticancer Therapies,” JCO Precision Oncology 5 (2021): 17–26. [DOI] [PubMed] [Google Scholar]
- 31. Martino O. I., Ward D. J., Packer C., Simpson S., and Stevens A., “Innovation and the Burden of Disease: Retrospective Observational Study of New and Emerging Health Technologies Reported by the EuroScan Network From 2000 to 2009,” Value in Health 15, no. 2 (2012): 376–380. [DOI] [PubMed] [Google Scholar]
- 32. Collaborators G. B. D. A., “The Burden and Trend of Diseases and Their Risk Factors in Australia, 1990‐2019: A Systematic Analysis for the Global Burden of Disease Study 2019,” Lancet Public Health 8, no. 8 (2023): e585–e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Izutsu K., Makita S., Nosaka K., et al., “An Open‐Label, Single‐Arm Phase 2 Trial of Valemetostat for Relapsed or Refractory Adult T‐Cell Leukemia/Lymphoma,” Blood 141, no. 10 (2023): 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Connor O. A., Pro B., Pinter‐Brown L., et al., “Pralatrexate in Patients With Relapsed or Refractory Peripheral T‐Cell Lymphoma: Results From the Pivotal PROPEL Study,” Journal of Clinical Oncology 29, no. 9 (2011): 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coiffier B., Pro B., Prince H. M., et al., “Results From a Pivotal, Open‐Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T‐Cell Lymphoma After Prior Systemic Therapy,” Journal of Clinical Oncology 30, no. 6 (2012): 631–636. [DOI] [PubMed] [Google Scholar]
- 36. O'Connor O. A., Horwitz S., Masszi T., et al., “Belinostat in Patients With Relapsed or Refractory Peripheral T‐Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN‐19) Study,” Journal of Clinical Oncology 33, no. 23 (2015): 2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The authors declare that the data presented in this article are available as raw data upon request. This supplementary raw data material of this study is available from the corresponding author upon reasonable request.


