Abstract
Frequent mutations in hypervariable region 1 (HVR1) of the main envelope protein of hepatitis C virus (HCV) is a major mechanism of persistence by escaping the host immune recognition. HVR1 contains an epitope eliciting neutralizing antibodies. This study was aimed to prepare broadly cross-reacting, high-affinity, monoclonal antibodies (MAb) to the HVR1 C terminus of HCV with potential therapeutic neutralizing capacity. A conserved amino residue group of glycine (G) at position 23 and glutamic acid (Q) at position 26 in HVR1 was confirmed as a key epitope against which two MAbs were selected and characterized. MAbs 2P24 and 15H4 were immunoglobulin G1 kappa chain [IgG1(κ)], cross-reacted with 32 and 30 of 39 random C-terminal HVR1 peptides, respectively, and did not react with other HCV peptides. The VH of 2P24 and 15H4 heavy chains originated from Igh germ line v gene family 1 and 8, respectively. In contrast, the VL κ sequences were highly homologous. The affinity (Kd) of 2P24 and 15H4 (10−9 or 10−8 M with two immunizing peptides and 10−8 M with two nonimmunizing HVR1 peptides) paralleled the reactivity obtained with peptide enzyme immunoassay. MAbs 2P24 and 15H4 captured 25 of 31 (81%) HCV in unselected patients' plasmas. These antibodies also blocked HCV binding to Molt-4 cells in a dose-dependent fashion. The data presented suggest that broadly cross-reactive MAbs to a conserved epitope within HCV HVR1 can be produced. Clinical application for passive immunization in HCV-related chronic liver disease and after liver transplantation is considered.
Hepatitis C virus (HCV) is a major etiological agent of transfusion-associated hepatitis and chronic liver disease worldwide (1, 6). Approximately 200 million people worldwide who are infected with HCV are susceptible to develop cirrhosis, liver failure, or hepatocellular carcinoma and might need a liver transplantation (30, 46). In organ transplant recipients, particularly in cases of liver and kidney transplantation, HCV reinfection of the transplanted organ is a major cause of morbidity and mortality (4, 12, 15, 35, 43, 47). HCV-related graft disease develops in a majority of patients followed for at least 5 years after transplantation (16, 32). Presently, such an outcome cannot be prevented by an effective prophylactic treatment, and current antiviral treatments of posttransplant HCV disease are of limited efficacy (14, 24, 25, 26, 31, 37, 40).
Hypervariable region 1 (HVR1) of the main E2 envelope protein of HCV is the target of neutralizing antibodies (10, 11, 34, 42). In infected patients, replacement mutations in HVR1 appear to be a major mechanism of HCV persistence by escaping the host immune recognition (11, 19, 21, 22, 38, 42, 45). It is hypothesized that neutralizing antibodies cross-reacting to most or all HVR1 variants should be of substantial help for the prevention and the treatment of chronic HCV infection.
In recent years a number of investigators have observed that antibodies from HCV-infected patients cross-reacted with a wide range of HVR1 peptides (2, 18, 27, 44) and blocked viral attachment to HCV-susceptible cells (39, 51). In addition, HVR1 proteins injected into rabbits and mice elicited antibodies that cross-reacted with a variety of HVR1 peptides and HCV isolates and were able to capture HCV and neutralize viral binding to human cells in vitro (9, 33, 36, 44, 52). Some antibodies to C-terminus HVR1 peptides appeared to be broadly cross-reactive and having a high capacity to capture HCV variants (36, 44, 49), suggesting the recognition of a conserved, partially conformational, epitope.
We sequentially immunized BALB/c mice with multiple HVR1 peptides to prepare MAbs to the putative C-terminus conserved epitope. The MAbs obtained were structurally and functionally characterized in vitro.
MATERIALS AND METHODS
HCV samples.
HCV-containing plasmas or sera were obtained from blood donors with chronic hepatitis C at the East Anglia Blood Centre, from patients at the Addenbrooke's Hospital, Cambridge, United Kingdom, or from blood donors at the Komfo Anokye Teaching Hospital blood bank, Kumasi, Ghana. All samples contained antibodies to HCV and HCV RNA detected by reverse transcription-PCR (RT-PCR) as described previously (29). EH virus (genotype 1a) was obtained from an HCV-infected patient with congenital agammaglobulinemia from T. Wallington, Bristol Blood Centre, Bristol, United Kingdom. This plasma contains three variants differing by one amino acid among 11 sequences. The variant distribution was 5-5-1, and the viral load was 1.7 × 106 IU of free (uncomplexed) HCV/ml.
Real-time quantitative RT-PCR analysis of HCV RNA.
Viral RNA was extracted from patient's plasma with the QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany). HCV RNA was measured by real-time quantitative RT-PCR with a PE Applied Biosystems Prism model 7700 sequence detection instrument. The forward and reverse primers for the noncoding region of HCV RNA were 5′-TCTGCGGAACCGGTGAGTA-3′ and 3′-CGGGTTGATCCAAGAAAGGA-5′, respectively. The TaqMan fluorogenic probe used for quantification of HCV RNA was 5′-6FAM-CCGGAATTGCCAGGACGACCG-3′. The Ct value, which correlates inversely with the concentration of target RNA, was determined as the cycle number at which the fluorescence emission of the reporter probe increases above a threshold level. A reference HCV plasma containing 1.6 × 106 IU/ml was used for the standard curve for quantification of viral load of HCV patient's plasma samples. Each sample was analyzed in triplicate, and the results were averaged.
Peptides and peptide-keyhole limpet hemocyanin (KLH) or peptide-bovine serum albumin (BSA) conjugates.
A series of 16 to 19 residue synthetic peptides (total 53) corresponding to C-terminus sequences of HVR1 and other HCV regions (core, E1, E2, NS3, and NS4) were obtained from Severn Biotech, Ltd. (Kidderminster, Worcestershire, United Kingdom), or Cambridge Research Biochemicals, Ltd. (Cambridge, United Kingdom).
HVR1 peptides MH1, MH2, MH3, W1, L1.1, and EH were conjugated to KLH (Sigma) for mouse immunization. Peptides MH2, EH2, S67, S85, and L1.1 were coupled to BSA when testing MAb affinity. The protocol to couple peptides to KLH or BSA was as described previously (18). The effect of coupling was determined by coating the conjugates to microtiter plates and detecting the capture of immunoglobulin G (IgG) anti-HCV from infected patient plasma (UKS9) by enzyme immunoassay (EIA).
Mouse immunization.
Two groups of six 8-week old BALB/c mice, designated blocks 1 and 2, were immunized with six HVR1 peptides as described previously (36). Block 1 was immunized with four injections at 2-week intervals. The first subcutaneous injection consisted of 40 μg of conjugated MH2 and L1.1 in 100 μl of complete Freund adjuvant. The next two subcutaneous injections were 20 μg of MH1 and EH and 20 μg of MH3 and W1 conjugates/100 μl in incomplete Freund adjuvant. The final dose of 20 μg/100 μl of MH2 conjugate without adjuvant was injected intravenously. For immunizing Block 2, seven injections were performed, with six different peptide conjugates at two week interval. The first injection was 40 μg/100 μl of MH2 conjugate in complete Freund's adjuvant, followed by subcutaneous injections of 20 μg/100 μl of EH, MH1, W1, MH3 and L1.1, respectively. The final boost was an intravenous injection of 20 μg of MH2 conjugate/100 μl. On the third day after the final boost, the best anti-HVR1-responding mice were sacrificed. Spleens were aseptically removed for hybridoma preparation. Sera were also collected (pre- and postimmunization) and used as negative and positive controls for MAb screening.
Preparation of MAbs.
In a ratio of 5:1, the immunized spleen cells were fused with SP2/0 myeloma cells using polyethylene glycol 1500 (Boehringer, Mannheim, Germany). The positive wells were primarily screened by peptide EIA by using a mixture of MH2, EH2, S90, and LB2 peptides as capture antigens. Cloning of positive wells was performed by the limited dilution method in 5% BM-Condimed H1 (Boehringer) and 15% fetal calf serum–RPMI 1640 without feed cells. Clones with broad cross-reactivity were recloned, and MAbs were purified from the culture supernatants in 15% fetal calf serum–RPMI 1640 medium by using a protein G column (Amersham Pharmacia Biotech) and anti-mouse IgG agarose (Sigma).
Isotyping of MAbs was performed with IsoStrip of the Mouse Monoclonal Antibody Isotyping Kit (Roche).
Peptide EIA.
The screening of MAbs and the testing of reactivity of anti-HVR1 MAbs with various peptides were performed by peptide EIA in two kinds of microtiter plates, i.e., Nunc-Immuno plate (Maxisorp; Nalge Nunc International) and the CovABtest plate (CovaLAB, Lyon, France). The latter cross-linked the cysteine residue added at the N terminus of each peptide. A total of 100 μl of a 10-μg/ml dilution of peptides in sodium carbonate (pH 9.6) was added to the Nunc-Immuno plate and incubated overnight at 4°C. The same peptides diluted in phosphate-buffered saline (PBS) were added to the CovaLAB plate and incubated for 1 h at room temperature. The peptide EIA procedure was then carried out as described previously (36). Levels of MAb reactivity as determined by EIA were presented as a sample/cutoff ratio (S/CO). The cutoff was calculated as the mean of the absorbance values of non-HVR1 peptides + six standard deviations.
MAbs were screened, selected, and tested by peptide EIA. Hybridomas were primarily screened with a mixture of four HVR1 peptides (see above). Cells from reactive wells were cloned. Strongly reactive clone supernatants were tested for cross-reactivity with a panel of 12 peptides (MH2, L1.1, MH1, EH, MH3, W1, LB1, LV, MH5, S90, SMH2-2, and S8). Broadly cross-reactive clones were selected, and MAbs were purified from the supernatants for further assessment of cross-reactivity and specificity by using a panel of 53 peptides (Table 1).
TABLE 1.
HCV peptide sequences and reactivity with MAbs
| Peptidea | Sequenceb (positions 11 to 28)
|
Region | EIA (S/CO)c
|
|
|---|---|---|---|---|
| 14 19 23 27 | 2P24 | 15H4 | ||
| Group A | ||||
| MH2 | C K G L N G L F D L G P K Q K I | HVR1 | 24.9 | 18.1 |
| L1.1 | C T T S - - A S - - N S - A R - H | 1.1 | – | |
| MH1 | - N R - G - - - N F - - - - - - | 15.1 | 18.7 | |
| EH | - S - - V - - - T P - A - - N - | 23.3 | 17.3 | |
| MH3 | - Y - - T T - - - P - - R - Q - | 23.8 | 24.7 | |
| W1 | - D T A - - A - - - N - - - - - T | 24.6 | 21.8 | |
| Group B | ||||
| DH1 | - G - V A - - - K M - S Q - - L | 4.6 | 1.3 | |
| DH2 | - G M F T - - - N Q - A Q - - L | 18.6 | 12.9 | |
| FR1 | - N T Y - - T A - L S P - - S - Q | – | – | |
| FR2 | - I V N R R T S F - N - - - S - R | 24.2 | 1.7 | |
| G3959 | C T Y S F V S - L S P - A - - N | 25.3 | 4.1 | |
| G4392 | C T S S - T S I - T Q - A - - N | 25.4 | 7.9 | |
| G3836 | C A S - - T S - - S P - S - - H | 24.6 | 11 | |
| G3895 | C T G - - V S - - N P - - S - D | – | – | |
| G4309 | C T A R V A - - - N P - S Q - R | 1.1 | – | |
| G4720 | C T N - - T A - - T P - A - - N | 24 | 14.2 | |
| G878 | C A R S - A T - - S P - A R - Q | 6.9 | 1.5 | |
| G1192 | C T S - F T - - - S S - A - - T | 25.2 | 2.5 | |
| G1224 | C T Q S - A - - - R P - A S - N | – | – | |
| G4630 | C T A R V A - - - S P - S Q - R | 1.9 | – | |
| G988 | C A L - - T S - - S P - - I - H | 1.7 | – | |
| G1245 | C T S R F A S - - N - - - Q - Q | 25.9 | 28.4 | |
| G886 | C A R S - V N - - T P - A R - N | 5.1 | 1.4 | |
| L1.2 | - - T R - - A S - - T S - A R - H | – | – | |
| LB1 | - - T S - F A S - - K F - - S - - | – | – | |
| LB2 | - - T S - F A S - - R - - - S - - | 25.7 | 14.8 | |
| LV | - N T H - I A S - - A F - - A - - | 1.2 | 1.3 | |
| MH4 | - S - - S S - - T P - - - - Q - | 25 | 21.8 | |
| MH5 | - N R F A - M - S - - A R - - - | 24.5 | 24.4 | |
| RE2 | - S - F V S - L A P - A - - N | 26.7 | 6.2 | |
| S65 | - R R V A S F L I R - S A - - - | 8.6 | 23.9 | |
| S66 | - L - I A S F L I R - - - - N - | 12.7 | 24.8 | |
| S67 | - R T - T - M - S - - A R - - - | 24.7 | 25.1 | |
| S82 | - T - F S N - - N - - S Q - - V | 16.1 | 3.3 | |
| S83 | - G - - T N - - S - - S Q - - V | 11.5 | 2.3 | |
| S85 | - A A - A S - - L S - - Q - - | 25.6 | 24.4 | |
| S87 | - T - F S K H N N M - S Q - - V | – | 8.9 | |
| S90 | - A A - A N S L S - - - Q - - V | 25 | 23.6 | |
| US1 | - S - F V - - - S P - - S - R - | – | 21 | |
| Group C | ||||
| SMH2-1 | - - - - - - - - - - V - - - - - | – | 21.9 | |
| SMH2-2 | - - - - - - - - - - V - - L - - | – | 20.7 | |
| SMH2-3 | - - - - - - - - - - - - - L - - | 18.3 | 18.4 | |
| SMH2-4 | - - - - - - - - - S - - S - - - | – | 5.4 | |
| SMH2-5 | - - - - - - - - - - - - S - - - | 17.2 | 4.2 | |
| SMH2-6 | - - - - - S - - - - - - - - - - | 22.1 | 26.8 | |
| Group D | – | – | ||
| S5 | A T R - T S E R S Q P R - R R - P I C | Core | – | – |
| S6 | P K A R Q P E G R A W A - P G - | – | – | |
| S8 | S R P S W G P T D P R R R S R N L G - | – | – | |
| S19 | S - H R M A W D M M M N W S P - | E1 | – | – |
| S33 | L Q V C - P V Y C - T P S - V V V G - | E2 | – | – |
| S34 | D C F R K H P E A T Y T K C G S G P - | – | – | |
| S41 | T Q R L R R L H Q W I N E D C S T P - | NS4 | – | – |
| S45 | G R H L I F C H S K K K C D E L A A - | NS3 | – | – |
Four groups of HCV peptides are listed: (A) HVR1 peptides for immunization and screening primary by hybridomas, (B) nonimmunizing HVR1 peptides used to assess the cross-reactivity of MAbs, (C) mutated MH2 HVR1 peptides used for mapping the epitope recognized by the MAbs, and (D) HCV non-HVR1 peptides used to assess the specificity of the MAbs.
Peptide sequences are indicated by one-letter code. For clarity, only positions 14, 19, 23, and 27 are indicated by number.
Levels of MAb reactivity as determined by EIA are presented as the S/CO. Cutoff = mean of the absorbance values of non-HVR1 peptides + six standard deviations. –, Absorbance value below the cutoff value. The cutoff of MAb 2P24 was 0.150; the cutoff of MAb 15H4 was 0.125.
Selected MAbs were biotinylated by using a Micro-Biotinylation Kit (Sigma). The biotinylated and nonbiotinylated MAbs were competitively presented to MH2 in an EIA to identify the epitope of HVR1 recognized.
MAb affinity.
The affinity of the MAbs was determined against five selected HVR1 peptides (MH2, EH, S67, S85, and L1.1) with an IAsys optical biosensor (Affinity Sensor, Cambridge, United Kingdom) as described previously (48). BSA-HVR1 peptides were dialyzed, diluted to a concentration of 200 μg/ml in PBS (pH 7.2), and immobilized on the activated surface of carboxymethyl dextran cuvettes in 10 mM of sodium acetate buffer at pH 3.8 or 4.0 according to the manufacturer's instructions. Serial dilutions of MAbs in PBS were added to the peptide-coated cuvettes (final volume, 50 μl). The association and dissociation of MAbs with HVR1 peptides were measured for 10 and 5 min, respectively. Affinity constants (Kd) were calculated from these measurements as Kdiss/Kass by using the FASTFIT program.
Cloning and sequencing of variable region genes.
Single-stranded cDNA of anti-HVR1 MAbs were obtained from cloned hybridoma cells with the First-Strand cDNA Synthesis Kit (Boehringer), the Oligotex mRNA Midi Kit (Qiagen), and the RNeasy Maxi Kit (Qiagen).
The variable-region genes of heavy and light immunoglobulin chains were amplified with mouse VH or Vκ and constant region primers obtained from T. Grunwald (Medical Research Council, Cambridge, United Kingdom). The PCR products were cloned into pPCR-Script Amp SK(+) cloning vector with the PCR-Script Amp Cloning Kit (Stratagene) and then sequenced with M13 forward and reverse primers by using a Thermo Sequenase Dye Terminator Cycle Sequencing Premix kit (Amersham Life Science), a DNA Sequencing Kit (Applied Biosystems, Warrington, United Kingdom), and a 373DNA Sequencer (Applied Biosystems). The deduced amino acid sequences were analyzed and compared with immunoglobulin germ line and reference MAb sequences.
HCV genotyping by HVR1 sequencing.
HCV RNA was extracted from patient or donor plasmas by using the QIAamp Viral RNA Mini kit (Qiagen) according to the manufacturer's instruction. The E1/E2 region of the HCV genome was amplified by using RT-PCR and nested primers as previously described (23). A 513-nucleotide sequence encompassing the 3′ end of E1 and HVR1 was obtained from the amplicons, and phylogenetic analysis was done by using the PHYLIP software package as described previously (3, 8). HVR1 amino acid sequences were deduced from the nucleotide sequences obtained.
HCV capture.
Unselected plasmas from chronically HCV-infected patients were centrifuged for 2 min at room temperature. Next, 50 μl of plasma supernatant or dilution (1:5 in PBS) was added to 50 μl of 20 μg of MAb/ml in PBS containing 0.1% Tween 20 and 4% BSA and preincubated at 37°C for 2 h and then overnight at 4°C. A 100-μl portion of a 10-μg/ml mixture of goat IgG F(ab′)2 fragments of anti-mouse IgG (Fc specific; Sigma) in sodium bicarbonate (pH 9.6) was used to coat the wells of a microtiter plate overnight at 4°C. After saturation of the well surface with 4% BSA, the antibody-HCV mixture was added to the anti-mouse IgG-coated well and incubated for 1.5 h at room temperature. Normal mouse myeloma IgG1 (Sigma) was used as negative control in each capture assay. After four washes with PBS containing 0.1% Tween 20 (no free virus was detectable in the fifth wash buffer), RNA was extracted from each well by using the QIAamp Viral RNA Mini kit and detected by RT-nested PCR as described previously (29). The sensitivity of the RT-PCR was <100 copies of HCV RNA/ml.
Next, 10 μg of MAb/ml was preincubated with 50 μg of HVR1 peptides (G4720, G878, G1245, and G1224), mutated HVR1 peptide (SMH2-2), or core region peptide (S5)/ml for 1 h at 37°C mixed with UKS3 HCV-diluted plasma (1:10). HCV-MAb complexes were captured and detected as described above.
Blocking of HCV binding to target cells.
The MAbs' ability to block HCV binding to Molt-4 cells was investigated. Several dilutions of MAb and normal mouse myeloma IgG1 were preincubated with 100 μl of 1:10-diluted HCV-containing plasma at 4°C overnight. The mixture was added to 2 × 105 cells in a 200-μl final volume and incubated at room temperature for 1 h. The viral and cellular RNA was extracted from cells, which had been washed four times, by using RNeasy Mini kit (Qiagen) and then tested for the presence of HCV RNA by RT-nested PCR (the last wash supernatant did not contain detectable free virus). Under identical conditions, 100 μl of 1:10-diluted HCV-containing plasma without MAbs preincubation and normal plasma were added to cells as positive and negative controls, respectively.
RESULTS
Reactivity of MAbs to HVR1 peptides.
Sixteen MAbs strongly reactive to a mixture of four HVR1 peptides were obtained by screening the supernatants of primary clones from block 1 and 2 mice. Of the 16 clones highly reactive with the screening mixture of HVR1 peptides, 12 were IgG [11 IgG1(κ) and 1 IgG2a(κ)] and 4 were IgM(κ). The 12 IgG-containing hybridoma supernatants were further tested for cross-reactivity with 10 HVR1 peptides and for specificity with two control peptides: SMH2-2 and S8.
One clone (15H4) reacted with five of six immunizing and three of five nonimmunizing peptides, including SMH2-2. Three clones including 2P24 reacted with the same peptides but not SMH2-2. MAbs 15H4 and 2P24 were selected for further characterization.
Protein G-purified MAbs 2P24 and 15H4 cross-reactivity were assessed with a panel of 53 HCV peptides. As shown in Table 1, 2P24 and 15H4 reacted with 32 (82%) and 30 (77%) patient-derived HVR1 peptides, respectively. A mixture of 2P24 and 15H4 reacted with 34 of 39 (87%) patient-derived HVR1 peptides but did not react with HCV core, E1, E2, NS3, and NS4 peptides. An unrelated IgG1 mouse myeloma used as a control did not react with patient-derived peptides.
Epitopes recognized by MAbs to HVR1.
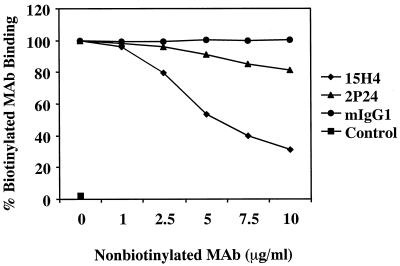
Figure 1 shows the result of competitive binding experiments between 2P24 and 15H4 for the HVR1 MH2 peptide. MAb 15H4 competitive displacement of MAb2P24 was considerably greater than the reverse. This result suggests that 2P24 and15H4 recognize an overlapping region of HVR1, with 15H4 probably recognizing a broader epitope than 2P24.
FIG. 1.
Competitive binding of 15H4 and 2P24 to MH2. A total of 0.25 μg of biotinylated 2P24 or 15H4/ml was mixed with nonbiotinylated 15H4 or 2P24 at concentrations of 0, 1, 2.5, 5, 7.5, and 10 μg/ml. Then, 100 μl of the mixture was added to MH2 HVR1 peptide-coated well. Mouse myeloma IgG1 (mIgG1) and HCV core region peptide S5 (Control) were used as MAb and peptide controls, respectively. Bound biotinylated MAb was detected by avidin-peroxidase with ortho-phenylenediamine substrate.
Table 1 shows the reactivity of 2P24 and 15H4 with patient-derived (peptide group B) or mutated (peptide group C) C-terminus HVR1 peptides assessed by EIA. Table 1 indicates that viral sequences of the last seven amino acids of HVR1 are subject to considerable constraints. Amino acids in positions 23 and 26 are totally conserved, while the amino acid in position 24 has only three options (P, A, and S). In contrast, amino acids in positions 21, 22, and 27 have eight or nine options. HVR1 peptide reactivity with MAbs was independent of the complete peptide sequences. When phylogenetically analyzed, these peptides grouped into four main branches, each containing nonreactive or poorly reactive peptides (data not shown). In contrast, when the analysis was restricted to the five C-terminus amino acids, some features emerged. Both MAbs appear to recognize a limited repertoire of sequences in positions 23 to 26 containing G–P/A–R/Q–Q in positions 23, 24, 25, and 26, respectively. All peptides including these sequences strongly reacted with both MAbs, suggesting the recognition of a common epitope. In contrast, the relatively frequent sequence GPSQ is poorly or not recognized by the antibodies. The importance of the conserved G (position 23) and Q (position 26) amino acids in the structure of the common epitope is critical for 2P24 but of less importance for 15H4 as shown in Table 1. Both MAbs recognized the conserved motif G - - Q, although only 2P24 was sensitive to the replacement of the conserved G (position 23) or Q (position 26) amino acid. Low reactivity with both or one of the two MAbs appeared to be mostly, though not exclusively, related to the presence of a serine at position 25 or 24. This observation was confirmed by mutations of peptide MH2 (Table 1). No other amino acid sequence patterns seemed to affect MAb binding to HVR1 peptides.
To confirm the antigenic importance of the conserved G - -Q group, three mutated MH2 peptides (SMH2-1, SMH2-2, and SMH2-3) were tested for reactivity with previously described rabbit anti-HVR1 antibodies (36). The result shows that SMH2-2 (with V [valine] and L [leucine] replacing G and Q at positions 23 and 26 of the MH2 sequence, respectively) completely lost antigenic reactivity with the HVR1 antibodies. The substitution of either G or Q markedly decreased peptide reactivity with rabbit anti-HVR1. These results confirmed that the G - -Q group in HVR1 was a key epitope.
Sequencing the variable regions of MAbs.
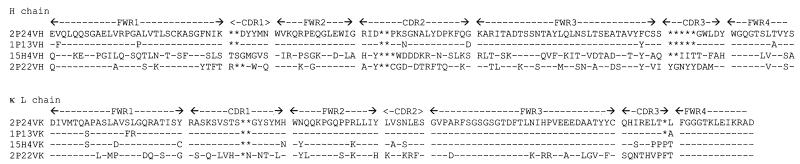
Four IgG1(κ) MAbs with different levels of cross-reactivity to HVR1 peptides were selected for sequencing of the IgG variable regions. Deduced amino acid sequences of heavy- and light-chain V regions were aligned and compared to mouse Igh and Igκ germ line V genes (Fig. 2). The VH of 2P24 and 15H4 were the closest to Igh germ line V genes J558 (V130) and 3609 (CB17H-1), which belong to VH families 1 and 8, respectively. The main feature of the VH sequences was the presence of complementarity-determining regions (CDRs) two to five amino residues shorter than the germ line antibodies. The VH sequences of 2P24 and 1P13 were nearly identical but differed from 15H4 and 2P22, particularly in CDR3 where the former was two and five amino residues shorter than the latter, respectively. The light-chain sequences of all anti-HVR1 MAbs were highly homologous, although CDR3 showed some diversity. Compared to the closest reference sequences, the VH sequences of 2P24 and 15H4 were 80 and 86% identical to X00894–1 and X75098–1, respectively. The Vκ sequences of 2P24 and 15H4 were 96 and 95% homologous to Y16454–1 and X90897–1, respectively.
FIG. 2.
Amino acid sequences of the HVR1 MAbs. Amino acid sequences are presented with a single-letter code. Dashes indicate identity with the lead sequence. Asterisks indicate gaps compared with the reference antibody sequence. FWR1, -2, -3, and -4 correspond to framework regions 1, 2, 3, and 4, resepctively. CDR1, -2 and -3 correspond to complementarity-determining regions 1, 2, and 3, respectively.
Affinity of MAb binding to HVR1 peptides.
BSA-conjugated HVR1 peptides were used to determine the MAb affinity. MH2 and EH were immunizing peptides; S67 and S85 were nonimmunizing peptides. All four peptides were reactive with 2P24 and 15H4 by EIA. For comparison, the affinity of the immunizing peptide L1.1 that had low EIA reactivity with 2P24 and no detectable reactivity with 15H4 was also tested. The affinity constants (Kd) are presented in Table 2. The affinities of 2P24 and 15H4 were 10−9 and 10−8 M, respectively. Low affinity and no detectable EIA reactivity were observed with control peptide or control MAbs. The affinity levels paralleled the peptide reactivity estimated by EIA. An affinity of 10−7 M seemed to be the limit of affinity quantification and corresponded to the EIA limit of reactivity.
TABLE 2.
Affinity constants and EIA reactivitiesa of MAbs to HVR1 peptides
| MAb | MH2
|
EH
|
S67
|
S85
|
L1.1
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kd (M) | EIA | Kd (M) | EIA | Kd (M) | EIA | Kd (M) | EIA | Kd (M) | EIA | |
| 2P24 | 5.85 × 10−9 | 24.9 | 6.52 × 10−9 | 23.3 | 1.81 × 10−8 | 24.7 | 2.43 × 10−8 | 25.6 | 1.44 × 10−7 | 1.1 |
| 15H4 | 2.15 × 10−8 | 13.6 | 3.9 × 10−8 | 17.3 | 1.1 × 10−7 | 25.1 | 5.85 × 10−8 | 24.4 | – | – |
| 1P13 | 1.01 × 10−7 | 8.9 | – | – | 1.31 × 10−7 | 7.4 | 8.99 × 10−8 | 11.2 | – | – |
| 2P22 | 2.82 × 10−8 | 10.5 | – | – | – | – | – | – | – | – |
EIA results are presented as S/CO ratios determined according to the method shown in Table 1. –, No reaction in the affinity assay as determined by biosensor IAsys or peptide EIA.
HCV capture with MAbs to HVR1.
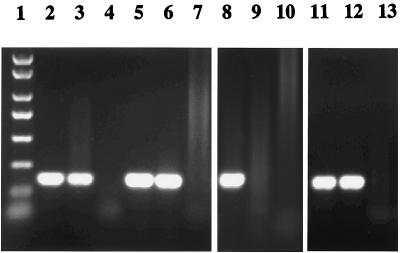
Thirty-one HCV RNA-positive patient plasmas were used to assess the capacity of MAbs 2P24 and 15H4 to capture HCV. HCV genotypes 1a, 1b, 1c, 2, 2a, 2b, 2c, 3a, and 4a were represented in the panel. The results of viral capture from patients' plasmas and corresponding consensus HVR1 sequences are presented in Table 3. In 25 of 31(81%) plasmas tested, HCV was captured by MAb 2P24, MAb 15H4, or both. Capture was not related to genotype, but a noncapture result was partially related to the low viral load of patient's plasma. Four of six HCV strains not captured by either MAb were at a concentration of viral RNA of <104 IU/ml. When a mixture of the two MAbs was used for capture, HCV from the same 25 samples was captured (Table 3). Figure 3 shows representative results of HCV capture from four representative patient's plasmas tested by RT-nested PCR. To confirm that the captured virus consisted essentially of free (uncomplexed) virus, similar amounts of HCV were captured in native or IgG-depleted HCV plasmas S2 and S7 by both MAbs, suggesting that free HCV in the patients' plasma samples was preferentially captured (data not shown).
TABLE 3.
HCV captures by MAbs to HVR1a
| HCV plasma sample | HVR1 sequence | Genotype | Viral load (IU/ml) | Presence (+) or absence (−) of
|
|
|---|---|---|---|---|---|
| 2P24 | 15H4 | ||||
| UK12700 | KTLTTGGSAARGAYGIASLFNPGAKQN | 1a | 5.1 × 106 | + | − |
| UK28098 | N-H----TV--TTQRFV---S--P--- | 1a | 2.9 × 106 | + | + |
| UK23363 | E-HV----L-Q--S-F-N-LT---R-- | 1a | 1.2 × 106 | + | + |
| UK11373 | G-YI-------TTL-F---LS---R-K | 1a | 3.6 × 103 | − | − |
| UK1544 | D-HI------KDIS-LTT-----PS-- | 1a | 6.4 × 106 | + | + |
| UK36854 | THPHD--T-GHT-S-LV---SP-PQ-K | 1b | 6 × 105 | + | + |
| UK25678 | E-H----A---N-W-LT-FLTR-PS-T | 1b | 9.2 × 103 | + | + |
| UK12927 | V-QV---AS--TTHSFVR-LDI-PR-- | 1c | 1.1 × 104 | − | + |
| UK11511 | N-R---SA-GQATS-L----TT-PT-K | 2b | <0.1 × 103 | − | + |
| UK22731 | H-YLI--KT-KQ-RMVT-F-SL--S-- | 3a | <0.1 × 103 | − | − |
| UK12726 | T-Y----NI---TRTL-G--TV-PS-D | 3a | 1.9 × 104 | − | − |
| UK35012 | S-Y-S-----QNTHS-T---SY-PK-- | 4a | 3.6 × 103 | + | − |
| UK31269 | E-HLAAS---QQTGRF-NM-ST-SQ-T | 4a | 1.6 × 104 | − | − |
| UKS2 | E-Y--------TMS-L-G--S---R-- | 1a | 5.5 × 106 | + | + |
| UKS3 | N-RTIGGTVS---SALT--LTK-PS-- | 3a | 4.8 × 103 | + | + |
| UKS7 | A-YV-------SVH-FT---SR-PS-- | 3a | 1.6 × 106 | + | + |
| UKS8 | N-HV---T--SN--------SQ-SR-- | 3a | 8.6 × 103 | + | − |
| UKS10 | N-MV---A-G-T-F-LT---TS--R-- | 2a | 1.1 × 104 | + | + |
| UKS12 | E-H-------HS----T---S---N-K | 3a | <0.1 × 103 | − | − |
| EH | E-RV---V-G-AMS-LVG--T------ | 1a | 1.7 × 106 | + | + |
| G4720 | S-TL--T--G-NTN-LTA--T------ | 2 | 0.8 × 103 | + | + |
| G4309 | H-YSV--AP--TTARV-G-----SQ-R | 2 | <0.1 × 103 | + | + |
| G3923 | H-R-I-A-T-Q--K-LT-F-A---Q-H | 2c | 0.9 × 103 | + | + |
| G3763 | S-T-V--RV-QATK-FT-F--S-PS-R | 2c | 1.2 × 104 | + | − |
| G3895 | S-Y-S--A-G-TTG-LV------PS-D | 2 | 2.8 × 104 | + | + |
| G455 | D-RV---T--FNTR-L-N--A---R-K | 2a | 0.13 × 103 | + | + |
| G878 | H-PF--STTAQG-RSL-T--S---R-Q | 2 | 2.1 × 103 | − | − |
| G886 | D-YA--A---Q--RSLVN--T---R-- | 2 | 1.9 × 104 | + | − |
| G988 | H-F----T-GHT-L-LT---S--PI-H | 2 | 5.8 × 103 | − | + |
| G3959 | T-Y-S--V---TT-SFVS-LS------ | 2b | 0.11 × 103 | + | − |
| G4630 | H-YSV--AP--TTARV-G--S--SQ-R | 2 | 0.15 × 103 | + | − |
Samples were obtained from blood donors from the United Kingdom (UK) or Ghana (G) as indicated. Sample EH virus was obtained from a patient with congenital agammaglobulinemia. The viral loads were determined by real-time quantitative RT-PCR. Plasma samples were incubated with MAbs, and complexes were captured by a goat IgG F(ab′)2 from anti-mouse IgG Fc specific in microtiter plates. Captured virus was detected by RT-nested PCR.
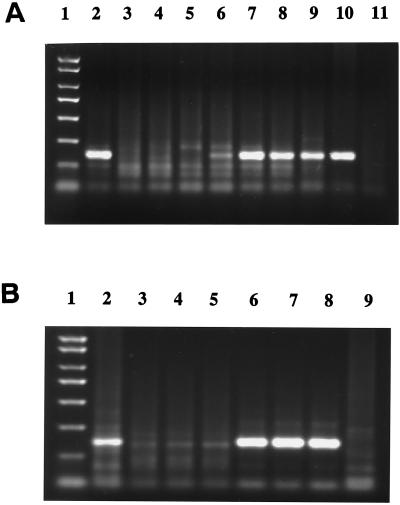
FIG. 3.
Capture of HCV from plasma samples of representative patients by HVR1 MAbs 2P24 and 15H4. Captured HCV was detected by RT-nested PCR. A mouse myeloma IgG1 was used as a negative control. Lanes: 1, PCR molecular weight markers; 2 to 4, EH with antibodies 2P24, 15H4, and IgG1, respectively; 5 to 7, UKS3 with antibodies 2P24, 15H4, and IgG1, respectively; 8 to 10, UK12700 with antibodies 2P24, 15H4, and IgG1, respectively; 11 to 13, G4720 with antibodies 2P24, 15H4, and IgG1, respectively.
Recognition of HVR1 as a whole virus or peptide by MAbs.
In nine cases, MAbs binding to different viral strains and to the corresponding consensus sequence-derived HVR1 peptides were available. In this situation, MAb recognition of HVR1 C-terminus epitope in its natural presentation and as an immobilized 15-mer peptide could be compared (Table 4). In six of nine cases, a concordant capture and EIA reactivity by 2P24 was found, suggesting that HVR1 peptides reliably mimicked the viral epitope.
TABLE 4.
Comparison of MAbs bound to HCV and HVR1 variants
| HVR1 or HCV sample | HVR1 peptide EIA (S/CO)
|
HCV capture
|
||
|---|---|---|---|---|
| 2P24 | 15H4 | 2P24 | 15H4 | |
| G4720 | 24 | 14.2 | + | + |
| G4309 | 1.1 | + | + | |
| G3895 | + | + | ||
| G878 | 6.9 | 1.5 | − | − |
| G886 | 5.1 | 1.4 | + | − |
| G3959 | 23.3 | 4.1 | + | − |
| G4630 | 1.9 | + | − | |
| G988 | 1.7 | − | + | |
| EH | 23.3 | 17.3 | + | + |
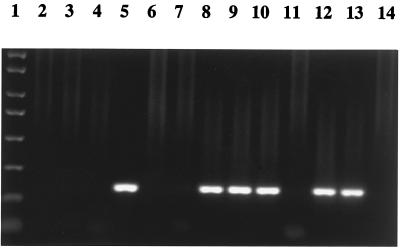
To further expand on this preliminary conclusion, experiments were designed to test the ability of HVR1 peptides to block viral capture by MAbs. Four HVR1 peptides derived from Ghanaian patients (G4720, G878, G1245, and G1224), one mutated HVR1 peptide (SMH2-2), and one HCV core region control peptide (S5) were selected to inhibit HCV UKS3 capture by MAbs to HVR1. Figure 4 shows that HVR1 peptides reacting with MAbs by EIA inhibited HCV capture and that peptides not reacting with MAbs by EIA did not inhibit HCV capture. These results strongly suggest that 2P24 and 15H4 capture HCV through specific binding to an HVR1 C-terminal epitope.
FIG. 4.
Inhibition of MAb HCV capture by HVR1 peptides. G4720 and G1245 (reactive with 2P24 and 15H4 by EIA) and G878 and G1224 (reactive at a low level or not reactive with 2P24 and 15H4 by EIA) are patient-derived HVR1 peptides. SMH2-2 (reactive with 15H4 but not with 2P24 by EIA) is a mutated HVR1 peptide. S5 is an HCV core region peptide used as control. A mouse myeloma IgG1 was used as a negative antibody control. Peptides (50 μg/ml) were preincubated with 2P24 and 15H4 or control IgG1 (10 μg/ml) and then incubated with HCV UKS3 plasma (1:10 diluted). Captured HCV was detected by RT-nested PCR. Lanes: 1, PCR molecular weight markers; 2 and 3, peptide G4720 with MAbs 2P24 and 15H4, respectively; 4 and 5, peptide G878 with MAbs 2P24 and 15H4, respectively; 6 and 7, peptide G1245 with MAbs 2P24 and 15H4, respectively; 8 and 9, peptide G1224 with MAbs 2P24 and 15H4, respectively; 10 and 11, peptide SMH2-2 with MAbs 2P24 and 15H4, respectively; and 12 to 14, peptide S5 with MAbs 2P24, 15H4, and IgG1, respectively.
Ability of MAbs to block HCV binding to target cells.
The EH (HCV without anti-HCV) and UKS3 (HCV with anti-HCV) viruses were preincubated with a mixture of 2P24 and 15H4 MAbs or separately with 2P24 or 15H4 MAb or with control MAb and then added to Molt-4 cells. HCV binding was detected by RT-nested PCR. As shown in Fig. 5, both sources of HCV bound to human T cells, and this binding was blocked by either the mixture of two MAbs or a single MAb to HVR1 in a dose-dependent fashion.
FIG. 5.
Blocking of HCV binding to Molt-4 with MAbs 2P24 and 15H4. (A) EH virus. A total of 1.7 × 104 IU of EH virus was preincubated with different concentrations of a 1:1 mixture of 2P24 and 15H4 and added to 2 × 105 Molt-4 cells. Lanes: 1, PCR molecular weight markers; 2, PC; 3 to 6, MAbs 2P24 and 15H4 at 50, 25, 2.5, and 0.25 μg/ml, respectively; 7 to 10, MAb control at 50, 25, 2.5, and 0.25 μg/ml, respectively; 11, NC. (B) UKS3 virus. A total of 100 μl of 1:10 diluted UKS3 viruses were separately preincubated with 2P24 or 15H4 and then added to Molt-4 cells. Lanes: 1, PCR molecular weight markers; 2, PC; 3 and 4, MAb 2P24 at 25 and 2.5 μg/ml, respectively; 5 and 6, MAb 15H4 at 25 and 2.5 mg/ml, respectively; 7 and 8, MAb control at 25 and 2.5 μg/ml, respectively; 9, NC. In both panels, bound HCV was detected by RT-nested PCR; PC indicates positive control (HCV binding without MAb preincubation) and NC indicates negative control (normal plasma). MAb negative control is a mouse myeloma IgG1.
DISCUSSION
It was previously shown that the C-terminus part of HVR1 widely cross-reacted with HCV patient's plasma and antibodies raised to C-terminus HVR1 peptides (2, 9, 18, 27, 33, 44, 49), suggesting the existence of common and conserved antigenic epitope(s) in HVR1. Data obtained in rabbits immunized with HVR1 peptides according to a protocol similar to the one used in mice in the present study further suggested the presence of a conserved, partially conformational, epitope located at the C terminus of HVR1 and including the conserved G and Q amino acids in positions 23 and 26 of HVR1, respectively (36). Our results confirmed that anti-HVR1 cross-reactivity of a rabbit polyclonal antibody and one mouse MAb was highly dependent on the presence of these two conserved amino acids in their respective positions. The replacement of either or both by leucine or valine amino acids resulted in a significant loss or a complete disappearance of antigenic recognition by cross-reactive HVR1 antibodies. These data further confirm the critical importance of these two conserved amino acids in the recognition of HVR1 epitope.
The characteristics of the two MAbs (2P24 and 15H4) selected for their broad cross-reactivity with HVR1 variant peptides were similar to the one of rabbit anti-HVR1 polyclonal antibodies previously studied in this laboratory (36). Compared with other HVR1 antibodies, either monoclonal or polyclonal (9, 49, 50), the broad cross-reactivity, high affinity, and specificity of MAbs 2P24 and 15H4 appeared to be related to two main factors. (i) Multiple HVR1 peptides with considerable sequence difference rather than a single peptide were used in the immunizing protocol. Antibodies to a common epitope of HVR1 were elicited and boosted by the multiple injections of different HVR1 peptides containing the conserved epitope. A large number of MAbs to the C terminus of HVR1 were easily obtained, while others had considerable difficulty obtaining HVR1 MAbs with a single immunizing peptide (50). (ii) MAbs 2P24 and 15H4 reacted with the conserved C-terminus epitope of HVR1 and so appeared to be broadly cross-reacting with HVR1 variants. Poorly cross-reacting MAbs to HVR1 mostly recognized epitopes in the N terminus of HVR1 (positions 1 to 20), where only a threonine in position 2 is relatively conserved (50).
The epitope recognized by the described MAbs appears to be essentially the same and limited to amino acids 23 to 26 of HVR1 on the basis of multiple evidence. (i) Among the 39 patient-derived peptides presented in Table 1, 30 were similarly recognized by both MAbs (16 strongly, 14 poorly or not at all). Only nine presented discrepant reactivity. (ii) High reactivity of both MAbs was associated with only five amino acid sequences including GPKQ, GPQQ, GAKQ, GAQQ, or GPRQ. The presence of a serine in position 25 or 24 was associated with poor or no reactivity. (iii) Among the patient-derived peptide sequences, only amino acids in positions 23, 24, and 26 were conserved or highly restricted. Amino acids on either side of this central element were highly variable with at least eight possible substitutions. However, the epitope recognition was not entirely related to the linear sequence. While 2P24 was very sensitive to the substitution of either G or Q in their respective positions, 15H4 was not (Table 1). In contrast, the sensitivity of 15H4 to the substitution of the lysine in position 25 by a serine substantially decreased HVR1 motif recognition. These data suggest that MAb 15H4 and 2P24 recognize slightly different conformations of the HVR1 C-terminus epitope. It also suggests that within the conserved frame of the G - - Q sequence, other amino acids can modify the epitope conformation. This interpretation of our results is further supported by the competition experiments between the two MAbs (Fig. 1). In addition to a more flexible recognition of the common epitope by MAb 15H4, competition results might be explained by a high affinity or the recognition of different epitopes. The higher affinity of 2P24 (Table 2) and the parallel sensitivity of the two MAbs to amino acids in position 23 to 26 variations do not support these hypotheses.
In our study, most IgG MAbs to the C-terminus HVR1 produced from BALB/c mice are IgG1(κ) (11 of 12 identified). This result is consistent with findings of other investigators, suggesting that the antibody response to HVR1 is mostly of the IgG1 isotype (27, 50).
MAbs 2P24 and 15H4 had high affinity with both immunizing and nonimmunizing HVR1 peptides. The correlation observed between antibody affinity for the target peptides and the detectability of reactivity by EIA suggests that below an affinity level of Kd = 10−7 M, anti-HVR1 peptide reactivity is no longer detectable (Table 2).
Analysis of the MAb sequences (Fig. 2) revealed that, although the VH sequences of MAbs 2P24 and 15H4 were very different, they had in common short and heavily mutated CDRs. Although the sequence of 1P13 differs from 2P24 by only six amino acids in the VH and four amino acids in the VL sequences, the cross-reactivity and affinity for all five HVR1 peptides tested were consistently lower (except for peptide S85). These data suggest that the development of an effective anti-HVR1 requires fine-tuning that exposure to uniquely different sequences can provide but that exposure to related variants in patient quasispecies cannot. A previously described human recombinant single-chain antibody fragment specific to an HVR1 peptide (S52/20) that reacted exclusively with S52 HVR1 peptide (48) presented CDR sequences that were longer by two to five amino acids compared to 2P24 and 15H4. This suggests that mutations in CDRs are critical for antibody cross-reactivity with HVR1 variants.
Cross-reactive MAb 2P24 and 15H4 high affinity for HVR1 variants translated functionally into a high capacity to capture random HCV isolates (Table 3 and Fig. 3). The high percentage (81%) of HCV strain capture confirmed that the common epitope presented by HVR1 C-terminus peptides was also present and cross-reactive at the HCV surface (20, 41). Several hypotheses can be offered to explain the small percentage of viral strains (and HVR1 peptides) not captured (reactive) with MAbs 2P24 and 15H4. (i) Some differences in linear amino acid sequences might sufficiently modify the epitope conformation to react poorly (low affinity) with the antibodies. This hypothesis is not supported by the considerable sequence variability of amino acids 20 to 27 of the noncaptured strains (Table 3). (ii) MAbs might preferentially or specifically capture free (uncomplexed) viruses and, in some patients, this population of virus might be too small to be detectable by the method used. Previous studies (S. Hamaia, unpublished data) suggest that only 5% of total HCV circulate as free virus. Capture in samples with low viral load or with <5% free virus might be undetectable with our system. This hypothesis is supported by the observation that 10 of 16 plasma samples containing <104 HCV RNA IU/ml were captured by none or one MAbs compared to 6 of 15 samples containing >104 (Table 3). (iii) MAbs might capture complexed HCV by competing with patient anti-HVR1. High-affinity patient polyclonal antibodies might not allow displacement by the MAbs. In this case, differences in affinity for a specific HVR1 epitope might explain discrepancies between MAbs for viral capture.
Having obtained the consensus HVR1 sequence of HCV from some patients, HVR1 reactivity with, and viral capture by, MAbs 2P24 and 15H4 could be compared (Table 4). In most cases, a gross parallelism of reactivity and capture was observed, reinforcing the conclusion presented above that HVR1 peptides were representative of live virus HVR1. In two cases, however, major discrepancies were seen. This might be due to the fact that the consensus HVR1 sequence obtained did not coincide with the sequence of the viral subpopulation captured by the MAbs.
Like other HVR1 polyclonal antibodies and MAbs (9, 36, 39, 50), MAbs 2P24 and 15H4 had a high capacity of blocking HCV isolate binding to target cells (Molt-4) in an antibody dose-dependent fashion (Fig. 5). Complete blocking was observed with 0.5 μg of 2P24 and 15H4 MAbs added to 1.7 × 104 IU of HCV presented to 2 × 105 Molt-4 cells. These data confirm that HVR1 is the target of blocking antibodies and probably an important ligand of HCV to T cells (21, 34, 51). The ability of peptides to mimic live virus interaction was confirmed by the ability of EIA-reactive HVR1 peptides to block viral capture by MAbs (Fig. 4).
Multiple approaches have been chosen to obtain HCV preventive or therapeutic vaccines (7, 10, 13, 17, 28, 33, 52). We have shown that rabbit or mouse immunization with multiple HVR1 peptides elicited high-level, cross-reactive antibodies with clear functional blocking activity. The development of potent antivirals (drugs or antibodies) to be given either before or after liver transplantation will change the course of posttransplant disease (5). Such an approach might be particularly useful in preventing liver reinfection after transplantation. HVR1 peptide vaccination may also be a promising approach to therapeutic vaccination of patients chronically infected with HCV.
ACKNOWLEDGMENTS
This work was supported by the National Blood Service and the University of Cambridge, Cambridge, England.
We thank T. Wallington for providing plasma from patient EH and T. Grunwald, who provided the primers for IgG sequencing. We also thank P. Smethurst, S. Hamaia, and N. Watkins for technical help.
REFERENCES
- 1.Alberti A, Chemello L, Benvegnu L. Natural history of hepatitis C. J Hepatol. 1999;31(Suppl. 1):17–24. doi: 10.1016/s0168-8278(99)80369-9. [DOI] [PubMed] [Google Scholar]
- 2.Allain J P, Zhai W, Shang D, Timmers E, Alexander G J M. Hypervariable region diversity of hepatitis C virus and humoral response: comparison between patients with or without cirrhosis. J Med Virol. 1999;59:25–31. doi: 10.1002/(sici)1096-9071(199909)59:1<25::aid-jmv5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Allain J P, Dong Y, Vandamme A M, Moulton V, Salemi M. Evolutionary rate and genetic drift of hepatitis C virus are not correlated with the host immune response: studies of infected donor-recipient clusters. J Virol. 2000;74:2541–2549. doi: 10.1128/jvi.74.6.2541-2549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aswad S, Mendez R, Weingart R G, Mendez R. Expanding organ availability by using hepatitis C antibody-positive donors. Transplant Proc. 1993;25:2270–2271. [PubMed] [Google Scholar]
- 5.Berenguer M, Wright T L. Hepatitis C and liver transplantation. Gut. 1999;45:159–163. doi: 10.1136/gut.45.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 7.Choo Q L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, Kansopon J, McFarland J, Tabrizi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies F J, d'Almeida O, Timmers E, d'Almeida J, Fasken M, Bassabi K, Lee H, Allain J P. Molecular genotyping of HIV-1 in 61 patients with AIDS from Lome, Togo. J Med Virol. 1999;57:25–30. [PubMed] [Google Scholar]
- 9.Esumi M, Ahmed M, Zhou Y H, Takahashi H, Shikata T. Murine antibodies against E2 and hypervariable region 1 cross-reactivity capture hepatitis C virus. Virology. 1998;251:158–164. doi: 10.1006/viro.1998.9393. [DOI] [PubMed] [Google Scholar]
- 10.Esumi M, Rikihisa T, Nishimura S, Goto J, Mizuno K, Zhou Y H, Shikata T. Experimental vaccine activities of recombinant E1 and E2 glycoproteins and hypervariable region 1 peptides of hepatitis C virus in chimpanzees. Arch Virol. 1999;144:973–980. doi: 10.1007/s007050050559. [DOI] [PubMed] [Google Scholar]
- 11.Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter H J, Purcell R. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope E2 protein. Proc Natl Acad Sci USA. 1996;96:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feray C, Caccamo L, Alexander G J, Ducot B, Gugenheim J, Casanovas T, Loinaz C, Giegou M, Burra P, Barkholt L, Esteban R, Bizollon T, Lerut J, Minello-Franza A, Bernard P H, Nachbaur K, Botta-Fridlund D, Bismuth H, Schalm S W, Samuel D. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. European Concerted Action on Viral Hepatitis Group (EURHEP) Gastroenterology. 1999;117:619–625. doi: 10.1016/s0016-5085(99)70454-3. [DOI] [PubMed] [Google Scholar]
- 13.Forns X, Payette P J, Ma X, Satterfield W, Eder G, Mushahwar I K, Govindarajan S, Davis H L, Emerson S U, Purcell R H, Bukh J. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618–625. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 14.Fried M W, Hoofnagle J H. Therapy of hepatitis C. Semin Liver Dis. 1995;15:82–91. doi: 10.1055/s-2007-1007265. [DOI] [PubMed] [Google Scholar]
- 15.Fritsche C, Brandes J C, Delaney S R, Gallagher-Lepak S, Menitove J E, Rich L, Scannell C, Swanson P, Lee H H. Hepatitis C is a poor prognostic indicator in black kidney transplant recipients. Transplantation. 1993;55:1283–1287. doi: 10.1097/00007890-199306000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Gane E J, Portmann B C, Naoumov N V, Smith H M, Underhill J A, Donaldson P T, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 17.Heile J M, Fong Y L, Rosa D, Berger K, Saletti G, Campagnoli S, Bensi G, Capo S, Coates S, Crawford K, Dong C, Wininger M, Baker G, Cousens L, Chien D, Ng P, Archangel P, Grandi G, Houghton M, Abrignani S. Evaluation of hepatitis C virus glycoprotein E2 for vaccine design: an endoplasmic reticulum-retained recombinant protein is superior to secreted recombinant protein and DNA-based vaccine candidates. J Virol. 2000;74:6885–6892. doi: 10.1128/jvi.74.15.6885-6892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson P, Petrik J, Alexander G J M, Pearson G, Allain J P. Reactivity of synthetic peptides representing selected sections of hepatitis C virus core and envelope proteins with a panel of hepatitis C virus-seropositive human plasma. J Med Virol. 1997;51:67–79. doi: 10.1002/(sici)1096-9071(199701)51:1<67::aid-jmv11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimothono K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato N, Ootsuyama Y, Sekiya S, Ohkoshi S, Nakazawa T, Hijikata M, Shimothono K. Genetic drift in hypervariable region 1 of viral genome in persistent hepatitis C infection. J Virol. 1994;68:4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima M, Osuga T, Tsuda F, Tanaka T, Okamoto H. Influence of antibodies to the hypervariable region of E2/NS1 glycoprotein on the selective replication of hepatitis C virus in chimpanzees. Virology. 1994;204:665–672. doi: 10.1006/viro.1994.1582. [DOI] [PubMed] [Google Scholar]
- 22.Kumar U, Monjardino J, Thomas H C. Hypervariable region of hepatitis C virus envelope glycoprotein (E2/NS1) in agammaglobulinemic patient. Gastroenterology. 1994;106:1072–1075. doi: 10.1016/0016-5085(94)90770-6. [DOI] [PubMed] [Google Scholar]
- 23.Lawal Z, Petrik J, Wong V S, Alexander G J, Allain J P. Hepatitis C virus genomic variability in untreated and immunosuppressed patients. Virology. 1997;228:107–111. doi: 10.1006/viro.1996.8359. [DOI] [PubMed] [Google Scholar]
- 24.Marzano A, Salizzoni M, Rizzetto M. Liver transplantation in viral hepatitis. New insights. Acta Gastroenterol Belg. 1999;62:342–347. [PubMed] [Google Scholar]
- 25.Mazzaferro V, Regalia E, Pulvirenti A, Tagger A, Andreola S, Pasquali M, Baratti D, Romano F, Palazzo U, Zuin M, Bonino F, Ribero M L, Gennari L. Prophylaxis against HCV recurrence after liver transplantation: effect of interferon and ribavirin combination. Transplant Proc. 1997;29:519–521. doi: 10.1016/s0041-1345(96)00248-5. [DOI] [PubMed] [Google Scholar]
- 26.McHutchison J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alpha-2 alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 27.Mondelli M U, Cerino A, Lisa A, Brambilla S, Segagni L, Cividini A, Bissolati M, Missale G, Bellati G, Meola A, Bruniercole B, Nicosia A, Galfre G, Silini E. Antibody responses to hepatitis C virus hypervariable region 1: evidence for cross-reactivity and immune-mediate sequence variation. Hepatology. 1999;30:537–545. doi: 10.1002/hep.510300233. [DOI] [PubMed] [Google Scholar]
- 28.Netter H J, Macnaughton T B, Woo W P, Tindle R, Gowans E J. Antigenicity and immunogenicity of novel chimeric hepatitis B surface antigen particles with exposed hepatitis C virus epitopes. J Virol. 2001;75:2130–2141. doi: 10.1128/JVI.75.5.2130-2141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrik J, Pearson G J M, Allain J P. High throughput PCR detection of HCV based on semiautomated multisample RNA capture. J Virol Methods. 1997;64:147–159. doi: 10.1016/s0166-0934(96)02153-2. [DOI] [PubMed] [Google Scholar]
- 30.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 31.Poynard T, Marcellin P, Lee S S, Niederau C, Minuk G S, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon α2b plus ribavirin for 48 weeks or 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 32.Prieto M, Berenguer M, Rayon J M, Cordoba J, Arguello L, Carrasco D, Garcia-Herola A, Olaso V, De Juan M, Gobernado M, Mir J, Berenguer J. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29:250–256. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 33.Puntoriero G, Meola A, Lahm A, Zucchelli S, Ercole B B, Tafi R, Pezzanera M, Mondelli M U, Cortese R, Tramontano A, Galfre G, Nicosia A. Towards a solution for hepatitis C virus hypervariability: mimotopes of the hypervariable region 1 can induce antibodies cross-reacting with a large number of viral variants. EMBO J. 1998;17:3521–3533. doi: 10.1093/emboj/17.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa D, Campagnoli S, Morretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner A J, Lau J Y N, Choo Q L, Chien D, Pileri P, Houghton M, Abrignani S. A quantitative test to estimate neutralizing antibodies to the glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth D, Zucker K, Cirocco R, DeMattos A, Burke G W, Nery J, Esquenazi V, Babischkin S, Miller J. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45:238–244. doi: 10.1038/ki.1994.29. [DOI] [PubMed] [Google Scholar]
- 36.Shang D, Zhai W, Allain J P. Broadly cross-reactive, high-affinity antibody to hypervariable region 1 of the hepatitis C virus in rabbits. Virology. 1999;258:396–405. doi: 10.1006/viro.1999.9730. [DOI] [PubMed] [Google Scholar]
- 37.Sheiner P A, Boros P, Klion F M, Thung S N, Schluger L K, Lau J Y, Mor E, Bodian C, Guy S R, Schwartz M E, Emre S, Bodenheimer H C J, Miller C M. The efficacy of prophylactic interferon alpha-2b in preventing recurrent hepatitis C after liver transplantation. Hepatology. 1998;28:831–838. doi: 10.1002/hep.510280334. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y K, Hijikata M, Iwamoto A, Alter H J, Purcell R H, Yoshikura H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;65:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu Y K, Igarashi H, Kiyohara T, Cabezon T, Farci P, Purcell R H, Yoshikura H. A hyperimmune serum against a synthetic peptide corresponding to the hypervariable region 1 of hepatitis C virus can prevent viral infection in cell cultures. Virology. 1996;223:409–412. doi: 10.1006/viro.1996.0497. [DOI] [PubMed] [Google Scholar]
- 40.Singh N, Gayowski T, Wannstedt C F, Shakil A O, Wagener M M, Fung J J, Marino I R. Interferon-α for prophylaxis of recurrent viral hepatitis C in liver transplant recipients: a prospective, randomized, controlled trial. Transplantation. 1998;65:82–86. doi: 10.1097/00007890-199801150-00016. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi S, Okamoto H, Sakamoto M, Kojima M, Tsuda F, Tanaka T, Munekata E, Muchmore E E, Peterson D A, Mishiro S. A structurally flexible and antigenically variable N-terminal domain of the hepatitis C virus E2/NS1 protein: implication for an escape from antibody. Virology. 1993;195:297–301. doi: 10.1006/viro.1993.1378. [DOI] [PubMed] [Google Scholar]
- 42.Van Doorn L J V, Capriles I, Maertens G, Deleys R, Murray K, Kos T, Schellekens H, Quint W. Sequence evolution of the hypervariable region in putative envelope region E2/NS1 of hepatitis C virus is correlated with specific humoral responses. J Virol. 1995;69:773–778. doi: 10.1128/jvi.69.2.773-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vierling J M, Villamil F G, Rojter S E, Camacho K B, Goldman D E. Morbidity and mortality of recurrent hepatitis C infection after orthotopic liver transplantation. J Viral Hepatitis. 1997;4(Suppl. 1):117–124. doi: 10.1111/j.1365-2893.1997.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe K, Yoshioka K, Ito H, Kazumasa Watanabe, Ishigami M, Takagi K, Utsunomiya S, Kobayashi M, Kishimoto H, Yano M, Kakumu S. The hypervariable region 1 protein of hepatitis C virus broadly reactive with sera of patients with chronic hepatitis C has a similar amino acid sequence with the consensus sequence. Virology. 1999;264:153–158. doi: 10.1006/viro.1999.0004. [DOI] [PubMed] [Google Scholar]
- 45.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino H, Crawford K, Marion C D, Crawford K A, Brunetto M, Barr P J, McHutchinson J, Houghton M. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infection. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. Hepatitis C. Wkly Epidemiol Rec. 1997;72:65–69. [Google Scholar]
- 47.Wright T L, Donegan E, Hsu H H, Ferrell L, Lake J R, Kim M, Combs C, Fennessy S, Roberts J P, Ascher N L. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317–322. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 48.Zhai W, Davies J, Shang D Z, Chan S W, Allain J P. Human recombinant single-chain antibody fragments, specific for the hypervariable region 1 of hepatitis C virus, from immune phage-display libraries. J Viral Hepatitis. 1999;6:115–124. doi: 10.1046/j.1365-2893.1999.00146.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y H, Moriyama M, Esumi M. Multiple sequence-reactive antibodies induced by a single peptide immunization with hypervariable region 1 of hepatitis C virus. Virology. 1999;256:360–370. doi: 10.1006/viro.1999.9635. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y H, Shimizu Y K, Esumi M. Monoclonal antibodies to the hypervariable region 1 of hepatitis C virus capture virus and inhibit virus adsorption to susceptible cells in vitro. Virology. 2000;269:276–283. doi: 10.1006/viro.2000.0227. [DOI] [PubMed] [Google Scholar]
- 51.Zibert A, Schreier E, Roggendorf M. Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology. 1995;208:653–661. doi: 10.1006/viro.1995.1196. [DOI] [PubMed] [Google Scholar]
- 52.Zucchelli S, Capone S, Fattori E, Folgori A, Marco A D, Casimiro D, Simon A J, Laufer R, Monica N L, Cortese R, Nicosia A. Enhancing B- and T-cell immune response to hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol. 2000;74:11598–11607. doi: 10.1128/jvi.74.24.11598-11607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]