Abstract
Purpose
There is limited research on the relationship between structural environmental factors and genomics-related knowledge, self-efficacy, perceived importance, and communication. We examined the potential impact of racial segregation on these genomics-related outcomes among medically underserved patients.
Methods
We analyzed data from a sample of 546 patients recruited from a primary care clinic in St. Louis, Missouri. Multivariable models were used to examine associations between racial composition of social environments across the life course and genomics-related knowledge, self-efficacy, perceived importance, and communication.
Results
Non-Hispanic White patients reporting multiple past White social environments had higher genetic knowledge than non-Hispanic White patients reporting one or no past White social environments (P = .021), Black patients reporting 1 or no past White social environments (P = .002), and Black patients reporting multiple past White social environments (P < .001). We also found that among those reporting multiple current White social environments, Black patients were more likely than non-Hispanic White patients to frequently communicate about family history with family (P = .003).
Conclusion
These findings indicate that structural factors may affect understanding of genetic information and communication about family history among medically underserved patients. Targeted interventions may increase the use of genetic services among this population and reduce health inequities.
Keywords: Residential segregation, Family health history, Genetic services, Genetic testing, Racial and ethnic minorities
Introduction
About 2 million individuals in the United States carry genes that predispose them to genetic disorders, such as hereditary breast and ovarian cancer and familial hypercholesterolemia.1 Health care settings address this heightened risk by providing genetic counseling and testing services to their patients.2 These resources aid in identifying suitable therapeutic drugs, guiding clinical management choices, and helping patients make informed decisions about their treatment.3, 4, 5 Technological advances are also making these resources more widely available to improve population health and reduce health disparities.6 However, health disparities may persist or worsen if genetic services are underutilized by vulnerable populations.6
Disparities in genetic service utilization may be attributed to patient-level factors, such as race, ethnicity, age, and socioeconomic status.7 For example, Nikolaidis and colleagues found that lower use of genetic services among young breast cancer survivors was associated with Black race, older age, high out-of-pocket expenses, and longer time since diagnosis.7 Moreover, patients’ lower awareness about genetic testing and familial risk, perceptions of cancer risk factors, and geographic barriers to genetic counselors may also contribute to disparities in genetic service utilization.7, 8, 9 Prior research has yet to examine the structural factors that may improve our understanding of why disparities in genetic service utilization persist.
The theory of fundamental causes suggests investigating structural factors to tackle health inequities.10 Structural factors, including racial discrimination and residential segregation, have been linked to adverse health outcomes.11,12 For example, research shows that individuals living in highly black-segregated areas face an increased risk of breast cancer mortality, delayed diagnosis, and inadequate care compared with those in less segregated areas.13, 14, 15 In addition, many of the residents of these segregated areas are racial and ethnic minorities who are less familiar with genetic services than their White counterparts,16 which may be linked to the lower use of genetic counseling and testing among marginalized populations.
As genetic counseling and testing become more widespread in the United States,17 it is crucial to evaluate whether racial segregation affects how marginalized individuals understand and view genetic information and family history and communicate about family history. In addition, it is also essential to understand whether racial segregation impacts their confidence in communicating genetic topics (eg, their self-efficacy). Such findings would indicate how best to increase genetic service utilization within their households and social network (eg, relatives and friends) and help alleviate health inequities, for example, by improving genetic knowledge, awareness of familial risk, self-efficacy, and willingness to communicate about genetic information and family history.18
This study therefore investigated the relationship between racial segregation and genomics-related knowledge, self-efficacy, perceived importance, and communication. We also tested whether associations of racial segregation differ by race/ethnicity. We used data from a survey of medically underserved patients at a primary care clinic in St. Louis, Missouri, and a pictorial measure of the racial composition of social environments over the life course. Based on prior evidence indicating that neighborhoods have a greater impact earlier in life,19 we hypothesized that (1) individuals reporting multiple past White social environments would have higher genetic knowledge and family history knowledge than those reporting 1 or no past White environments, (2) individuals reporting multiple past White social environments would have higher genetic self-efficacy and family history self-efficacy than those reporting 1 or no past White environments, (3) individuals reporting multiple past White social environments would have more communication about family history and health than those reporting 1 or no past White environments, and (4) the associations between past racial segregation and genomics-related knowledge, self-efficacy, perceived importance, and communication would differ by race/ethnicity.
Materials and Methods
Setting
We used cross-sectional data from a convenience sample at the Barnes-Jewish Hospital Center for Outpatient Health (COH), which is a primary care clinic located within a large academic medical hospital in St. Louis, Missouri. The COH provides primary care services, including nutrition, social work, foot care, and pharmacy, to medically underserved patients from St. Louis and surrounding areas. During 1 year, the COH served over 16,000 unique patients, the majority of whom were African American (64%), female (67%), and aged between 35 and 64 years. Most patients (77%) resided in St. Louis City or County, and 80% were insured by Medicare or Medicaid.
Data collection
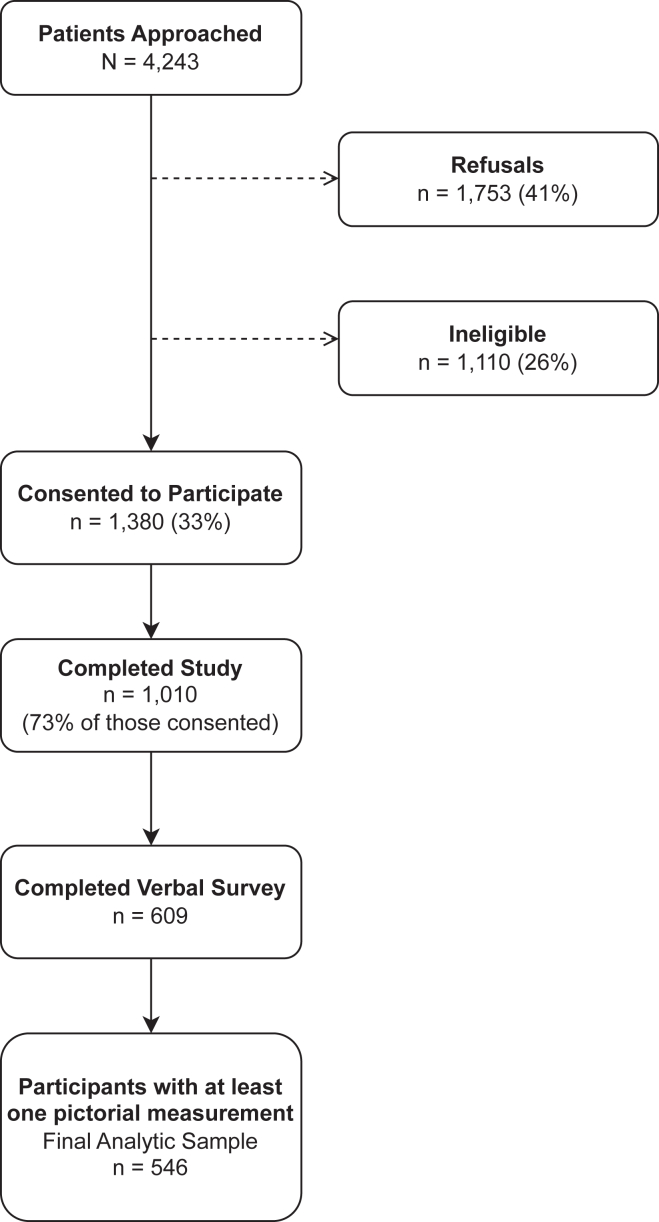
Between July 2013 and April 2014 (Figure 1), trained research assistants approached 4243 individuals in the COH waiting room to complete a survey in English, as described in detail elsewhere.20, 21, 22, 23, 24, 25 The surveys were conducted at different times of the day and on different days of the week. To be eligible for the study, participants had to be at least 18 years old, be a COH patient, and speak English. Research assistants then asked eligible participants to complete a self-administered written questionnaire that assessed genetic and family history knowledge, genetic and family history self-efficacy, perceived importance of genetic information and family history, communication behaviors, and demographic characteristics, followed by a verbally administered survey component that assessed racial composition of past and current environments using measures described below. All participants provided verbal and written consent before completing the survey and received a small incentive for their participation. The Human Research Protection Office at the Washington University School of Medicine approved this study. Secondary data analysis was approved by the New York University Institutional Review Board.
Figure 1.
Recruitment flowchart from a primary care clinic in St. Louis, Missouri, 2013-2014.
Outcome measures
Genetic and family health history knowledge
We used an adapted version of the Genetic Knowledge Index to assess genetic knowledge that we have utilized in prior studies.20,26, 27, 28 This index has been validated in studies of educational attainment and attitudes toward genetic discrimination.29 Participants responded to 5 true/false questions, and their correct responses were summed to create a continuous outcome for analysis. For example, participants indicated whether the following statement is true or false: “Once a genetic marker for a health condition is found in a person, the condition can be prevented or cured.” We assessed knowledge of family history using the following question: “Before today, had you ever heard of a family health history?”30 A binary outcome of “yes” or “no/not sure” responses was created for analysis.
Genetic and family health history self-efficacy
We assessed genetic self-efficacy using 5 questions, each with a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree).31 Participants responded to statements such as “I understand how to assess the role of genes for health.” Responses were summed, averaged, and treated as a continuous outcome in analysis (Cronbach’s alpha = 0.87). We assessed family history self-efficacy with the following question: “How sure are you that you could discuss family health history with members of your family?”30 A binary outcome of “very sure” or “not at all sure/somewhat sure” responses was created for analysis.
Perceived importance of genetic information and family health history
Perceived importance of genetic information was assessed using 1 question with a 5-point Likert scale from not at all important to very important.26 Participants were asked, “How important is it to you to learn more about how your genes, that is the characteristics that are passed from one generation to the next, affect your chance of getting certain health conditions?” A binary outcome of “very important” versus other categories was created for analysis. Perceived importance of family history was assessed using 1 item: “Would you agree or disagree with the following statement: It is important for my own health to know if diseases like cancer, diabetes, stroke, or heart disease run in my family.”32 Possible responses were on a 5-point Likert scale from strongly disagree to strongly agree. A binary outcome of “strongly/somewhat agree” or “neither agree nor disagree/somewhat disagree/strongly disagree” responses was created for analysis.
Communication behaviors
We used 3 items to assess the frequency with which participants discussed their family history and personal health with their friends, family, and doctor.33,34 These measures have been used in a study conducted among 8 community health centers serving medically underserved populations.33 The 3 items used were: “I talk with family members about our family health history,” “I talk with a doctor about my family health history,” and “How often do you talk to friends or family members about health?” The possible responses were “very often,” “somewhat often,” “not very often,” and “not at all.” For each item, we dichotomized the responses into “very often” versus all other categories for analysis.
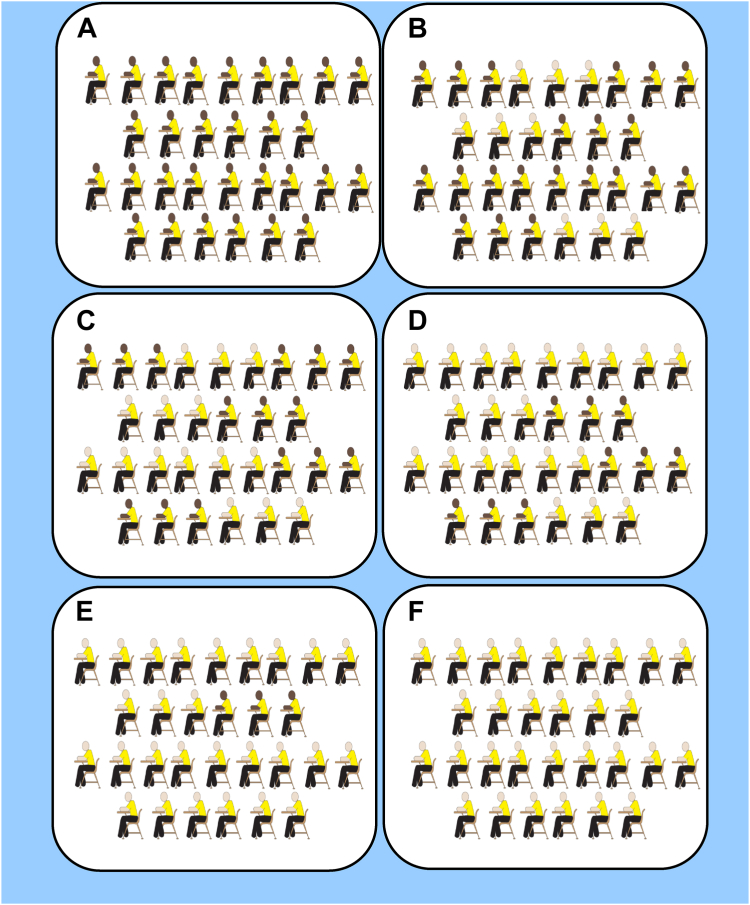
Racial segregation
We used an adapted version of the “neighborhood cards” developed by Krysan and Farley to measure the racial composition of participants’ past and current social environments.35 Figure 2 provides an example of the adapted pictorial measure. During the oral component of the survey, participants were asked to indicate whether the racial composition of each social environment was “100% Black,” “70% Black,” “50% Black,” “30% Black,” “10% Black,” “100% White,” or “Not Applicable.” The 10 social environments included 6 past environments (block, neighborhood, junior high, junior-high classroom, high school (HS), and HS classroom) and 4 current environments (block, neighborhood, place of worship, and workplace). For each environment, we dichotomized the responses into “100% White/10% Black/30% Black” versus “100% Black/70% Black/50% Black” to identify predominantly White social environments. Participants’ responses indicating “Not Applicable” or “Did not go to high school (or junior high school) in the US” were coded as missing. We then summed indicator variables for past and social environments to create final binary measures of “0 or 1” versus “2 or more” predominantly White social environments.
Figure 2.
Pictorial card used to measure the racial composition of the study participant’s junior high school. (A) 100% Black, (B) 70% Black, (C) 50% Black, (D) 30% Black, (E) 10% Black, and (F) 100% White.
Covariates and potential confounders
The following covariates were selected based on their known associations with genomics-related knowledge, self-efficacy, perceived importance, and communication: age, race/ethnicity, educational attainment, income, and health literacy.20,36,37 Age was measured as a continuous variable, whereas sex assigned at birth was categorized as male or female. Race/ethnicity was categorized as non-Hispanic White or Black (including multi-racial). Participants who identified as “other/unknown” race (7%, n = 36) were not included in the analyses. Educational attainment was categorized as <HS education, HS/general equivalency diploma, or ≥some college education. Household income was categorized as <$20,000 or ≥$20,000. To measure health literacy, we used a validated word recognition test, the Rapid Estimate of Adult Literacy in Medicine-Revised.38 Based on standard scoring, participants with a score of 6 to 8 were classified as having adequate health literacy, whereas those with a score below 6 were classified as having limited health literacy. Four additional measures abstracted from medical records that identified participants’ personal history of hypertension, diabetes, cancer, or heart disease were also considered for model inclusion.
Statistical analysis
Descriptive statistics were generated to summarize the demographic and health-related characteristics of the study population overall and then stratified by racial segregation measures. Frequencies for genomics-related knowledge, self-efficacy, importance, and communication behaviors were tabulated. We fit multivariable linear and logistic regression models that included the following potential predictors: racial segregation, age, race/ethnicity, educational attainment, income, and health literacy. To test whether associations of racial segregation differed by race/ethnicity, we included interactions of racial segregation and race/ethnicity in multivariable models. We also estimated marginal means and probabilities of subgroups and performed pairwise contrasts between each subgroup to interpret significant interaction effects. The Holm-Bonferroni correction was used to address the issue of multiple comparisons. Measures characterizing personal history of hypertension, diabetes, cancer, or heart disease were retained in final models if adjusted P values were < .05. We present measures of association with corresponding 95% confidence intervals and P values. Significance levels were set at the 5% level. All analyses were performed using R.39
Results
Analytic sample
Of the 4243 approached individuals, 41% (n = 1753) refused to participate, and 26% (n = 1110) were ineligible based on study inclusion criteria (Figure 1). This resulted in 1380 (44%) participants who provided oral and written consent. Of the consenting participants, 73% (n = 1010) fully completed the written survey or partially completed the written survey with completion of the verbal component. Among the 1010 participants, 609 (60%) completed the verbal component. Of the 609 participants that completed the verbal component, 546 (90%) had at least 1 pictorial measurement and were included in the final analytic sample. Sociodemographic characteristics (eg, age and race/ethnicity) of the COH patient population were similar to those included in the study.
Study population characteristics
Table 1 summarizes the sample demographic characteristics consisting of 546 COH patients. Two-thirds of the sample identified as female (66%) and Black (66%), with an average age of 51 years (SD: 12). Nearly half of the sample had at least some college education (46%) and lived in St. Louis city (45%), whereas 72% had a household income of less than $20,000. According to the Rapid Estimate of Adult Literacy in Medicine-Revised, 45% of the patients had limited health literacy. A relatively small percentage of the study population had a history of cancer (12%) or heart disease (19%). However, we found higher proportions of those with a history of hypertension (66%) or diabetes (38%).
Table 1.
Sample characteristics overall and stratified by racial segregation
| Characteristic | Predominantly White Social Environments |
P Value | |||||
|---|---|---|---|---|---|---|---|
| Overall |
Past |
P Value | Current |
||||
|
n = 546 |
0/1 |
2+ |
0/1 |
2+ |
|||
|
n = 311 |
n = 235 |
n = 372 |
n = 174 |
||||
| Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age (n = 523) | 50.8 (11.5) | 51.4 (11.8) | 50.1 (11.1) | .18 | 51.0 (11.8) | 50.4 (10.7) | .58 |
| n (%) | n (%) | n (%) | |||||
| Sex (n = 531) | .79 | .14 | |||||
| Female | 350 (65.9) | 199 (66.6) | 151 (65.1) | 244 (68.2) | 106 (61.3) | ||
| Male | 181 (34.1) | 100 (33.4) | 81 (34.9) | 114 (31.8) | 67 (38.7) | ||
| Race/ethnicity (n = 510) | < .001a | < .001a | |||||
| non-Hispanic White | 175 (34.3) | 27 (9.4) | 148 (66.1) | 69 (19.9) | 106 (65.0) | ||
| Black (including multi-racial) | 335 (65.7) | 259 (90.6) | 76 (33.9) | 278 (80.1) | 57 (35.0) | ||
| Educational attainment (n = 519) | < .001a | .09 | |||||
| Less than high school | 86 (16.6) | 58 (20.0) | 28 (12.2) | 62 (17.8) | 24 (14.1) | ||
| High school diploma or GED | 194 (37.4) | 123 (42.4) | 71 (31.0) | 138 (39.5) | 56 (32.9) | ||
| Some college or higher | 239 (46.1) | 109 (37.6) | 130 (56.8) | 149 (42.7) | 90 (52.9) | ||
| Household income (n = 482) | .044a | .013a | |||||
| Less than $20,000 | 347 (72.0) | 204 (75.8) | 143 (67.1) | 248 (75.6) | 99 (64.3) | ||
| $20,000 or more | 135 (28.0) | 65 (24.2) | 70 (32.9) | 80 (24.4) | 55 (35.7) | ||
| Urban status (n = 546) | .011a | < .001a | |||||
| St. Louis City | 248 (45.4) | 156 (50.2) | 92 (39.1) | 188 (50.5) | 60 (34.5) | ||
| St. Louis County | 170 (31.1) | 95 (30.5) | 75 (31.9) | 123 (33.1) | 47 (27.0) | ||
| Other | 128 (23.4) | 60 (19.3) | 68 (28.9) | 61 (16.4) | 67 (38.5) | ||
| Limited health literacy (n = 546) | 243 (44.5) | 173 (55.6) | 70 (29.8) | < .001a | 187 (50.3) | 56 (32.2) | < .001a |
| Personal history of | |||||||
| Hypertension (n = 440) | 293 (66.6) | 175 (72.3) | 118 (59.6) | .007a | 207 (70.2) | 86 (59.3) | .031a |
| Diabetes (n = 437) | 166 (38.0) | 103 (42.9) | 63 (32.0) | .025a | 112 (38.2) | 54 (37.5) | 0.97 |
| Cancer (n = 435) | 50 (11.5) | 29 (12.1) | 21 (10.8) | 0.78 | 27 (9.3) | 23 (16.0) | 0.06 |
| Heart disease (n = 437) | 84 (19.2) | 54 (22.5) | 30 (15.2) | 0.07 | 66 (22.5) | 18 (12.5) | .018a |
P value by t test or χ2 test.
GED, general equivalency diploma; SD, standard deviation.
P < .05
Frequencies of genomics-related outcomes
Overall, the study population had moderate-to-high knowledge of genetic information and family history. The mean score on the Adapted Genetic Knowledge Index was 3.5 (SD: 1.1, Range: 0-5), and 87% of participants were aware of family history (Supplemental Table 1). On average, participants demonstrated a moderate level of genetic self-efficacy (M: 3.2, SD: 1.1, Range: 1-5), and about 75% were very sure that they could discuss family history with their family. Nearly 60% of the study population rated genetic information (54%) and family history (57%) as very important. Regarding communication behaviors, a quarter of the sample communicated very often with their family (26%) or doctor (24%) about family history. About half of the sample (48%) communicated very often about health with their friends or family.
Study population characteristics by racial segregation
Bivariate analyses revealed differences between past racial segregation groups across educational attainment, household income, race/ethnicity, health literacy, urban status, and medical history (Table 1). Compared with COH patients reporting 1 or no past White environments, those reporting multiple environments tended to have higher educational attainment (57% vs 38% with some college education, P < .001) and higher household incomes (33% vs 24% with $20,000 or more, P = .044). Participants in the multiple past White environment group also had higher proportions of patients that identified as White (66% vs 9%, P < .001) and patients with adequate health literacy (70% vs 44%, P < .001), but a lower proportion of patients that lived in St. Louis city (39% vs 50%, P = .011). Additionally, patients reporting multiple past White environments had lower proportions of individuals with a history of hypertension (60% vs 72%, P = .007) and diabetes (32% vs 43%, P = .025) than those reporting 1 or no past White environments. We observed no statistically significant differences across age, sex, and personal histories of cancer or heart disease. The bivariate analyses of current racial segregation groups were relatively similar, except that 18 patients (13%) in the multiple current White environment group had a history of heart disease compared with 66 patients (23%) in the 1 or no current White environment group (P = .018).
Past racial segregation and genetic knowledge
Table 2 shows the results from the multivariable model of past racial segregation and genetic knowledge. We found a significant main effect of past White environments (β = 0.65, 95% CI: 0.20 to 1.11) and that this effect differed by race/ethnicity (β = −0.82, 95% CI: −1.36 to −0.28). To interpret these effects, we estimated marginal means across past White social environment groups stratified by race/ethnicity (Table 3). Non-Hispanic White patients reporting 2 or more past White social environments had the highest estimated mean score (est. M = 3.80) on the Adapted Genetic Knowledge Index. This score was higher than scores of non-Hispanic White patients reporting 1 or no past White social environments (est. M = 3.15, difference = 0.65, P = .021), Black patients reporting 1 or no past White social environments (est. M = 3.37, difference = 0.43, P = .002), and Black patients reporting 2 or more past White social environments (est. M = 3.20, difference = 0.60, P < .001). These means were estimated at the mean age of the sample (51 years) and averaged over levels of educational attainment, household income, and health literacy. Independent of the racial segregation effect and its interaction with race/ethnicity, we also found that genetic knowledge was associated with age (β = −0.01, 95% CI: −0.021 to −0.003), some college education (β = 0.33, 95% CI: 0.03 to 0.62), and limited health literacy (β = −0.50, 95% CI: −0.72 to −0.28). The main effects of race/ethnicity (β = 0.22, 95% CI: −0.23 to 0.67) and household income of less than $20,000 (β = −0.07, 95% CI: −0.28 to 0.15) were not statistically significant.
Table 2.
Multivariable models of the relationship between racial segregation and genetic knowledge and communication about family history
| Predictors | Genetic knowledge (n = 409) |
Communication about family history with family (n = 425) |
Communication about family history with a doctor (n = 422) |
|||
|---|---|---|---|---|---|---|
| Past |
Current |
Past |
Current |
Past |
Current |
|
| β (95% CI) | β (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Number of White environments | ||||||
| 0/1 (ref.) | ||||||
| 2+ | 0.65 (0.20, 1.11)a | −0.06 (−0.39, 0.27) | 1.35 (0.41, 6.15) | 0.52 (0.22, 1.22) | 0.54 (0.19, 1.71) | 0.69 (0.29, 1.64) |
| Race | ||||||
| White (ref.) | ||||||
| Black | 0.22 (−0.23, 0.67) | −0.40 (−0.70, −0.11)a | 2.03 (0.64, 8.98) | 1.05 (0.54, 2.15) | 0.61 (0.22, 1.84) | 0.76 (0.37, 1.61) |
| Age | −0.01 (−0.021, −0.003)a | −0.01 (−0.019, −0.002)a | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.03) | 1.01 (0.98, 1.03) |
| Educational attainment | ||||||
| Less than HS (ref.) | ||||||
| HS/GED | −0.01 (−0.30, 0.28) | −0.04 (−0.33, 0.26) | 1.38 (0.71, 2.81) | 1.39 (0.71, 2.83) | 1.13 (0.57, 2.31) | 1.14 (0.58, 2.32) |
| Some college or above | 0.33 (0.03, 0.62)a | 0.31 (0.01, 0.60)a | 1.11 (0.55, 2.29) | 1.16 (0.58, 2.39) | 1.19 (0.59, 2.46) | 1.21 (0.61, 2.50) |
| Household income | ||||||
| $20,000 or more (ref.) | ||||||
| Less than $20,000 | −0.07 (−0.28, 0.15) | −0.06 (−0.27, 0.16) | 1.25 (0.74, 2.14) | 1.26 (0.75, 2.18) | 1.56 (0.90, 2.80) | 1.57 (0.90, 2.84) |
| REALM-R | ||||||
| Adequate health literacy (ref.) | ||||||
| Limited health literacy | −0.50 (−0.72, −0.28)a | −0.51 (−0.74, −0.29)a | 1.25 (0.76, 2.06) | 1.19 (0.73, 1.96) | 2.22 (1.32, 3.76)a | 2.22 (1.32, 3.76)a |
| Multiple White environments × Black | −0.82 (−1.36, −0.28)a | 0.01 (−0.45, 0.48) | 1.12 (0.22, 4.32) | 4.40 (1.49, 13.06)a | 2.71 (0.73, 9.27) | 3.47 (1.15, 10.35)a |
Black, Black including multi-racial; CI, confidence interval; GED, general equivalency diploma; HS, high school; OR, odds ratio; ref., reference group; REALM-R, Rapid Estimate of Adult Literacy in Medicine-Revised; White, non-Hispanic White.
P < .05.
Table 3.
Population marginal means and probabilities stratified by racial segregation and race/ethnicity
| Outcome: Genetic Knowledge |
||||||
|---|---|---|---|---|---|---|
| Primary Exposure: Past Social Environments |
Pairwise Comparison |
|||||
| Population | Number of White Environments | Race/Ethnicity | Est. Meanb | Contrast of Est. Mean | Est. Difference | Adj. P Valuec |
| A | 0/1 | White | 3.15 | A-B | −0.65 | .021a |
| B | 2 or more | White | 3.80 | A-C | −0.22 | .73 |
| C | 0/1 | Black | 3.37 | A-D | −0.05 | .83 |
| D | 2 or more | Black | 3.20 | B-C | 0.43 | .002a |
| B-D | 0.60 | < .001a | ||||
| C-D | 0.17 | .73 | ||||
| Outcome: Communication About Family History With Family |
||||||
|---|---|---|---|---|---|---|
| Primary Exposure: Current Social Environments |
Pairwise Comparison |
|||||
| Population | Number of White environments | Race/ethnicity | Est. Prob. b | Contrast of Odds | Est. OR | Adj. P Valuec |
| A | 0/1 | White | 0.23 | B/A | 0.52 | 0.26 |
| B | 2 or more | White | 0.13 | C/A | 1.05 | 0.89 |
| C | 0/1 | Black | 0.23 | C/B | 2.03 | 0.18 |
| D | 2 or more | Black | 0.41 | D/A | 2.39 | 0.18 |
| D/B | 4.63 | 0.003a | ||||
| D/C | 2.28 | 0.07 | ||||
| Outcome: Communication About Family History With a Doctor |
||||||
|---|---|---|---|---|---|---|
| Primary Exposure: Current Social Environments |
Pairwise Comparison |
|||||
| Population | Number of White Environments | Race/Ethnicity | Est. Prob. b | Contrast of Odds | Est. OR | Adj. P Valuec |
| A | 0/1 | White | 0.23 | B/A | 0.69 | 1.00 |
| B | 2 or more | White | 0.17 | C/A | 0.76 | 1.00 |
| C | 0/1 | Black | 0.19 | C/B | 1.10 | 1.00 |
| D | 2 or more | Black | 0.35 | D/A | 1.83 | 0.74 |
| D/B | 2.64 | 0.13 | ||||
| D/C | 2.40 | 0.08 | ||||
Adj., adjusted; Black, Black including multi-racial; Est., estimated; OR, odds ratio; White, non-Hispanic White.
P < .05.
Estimations are averaged over levels of educational attainment, household income, and health literacy.
Holm-Bonferroni correction used to determine adjusted P values for multiplicity comparisons.
In contrast to the model with past White social environments, the main effect of current White social environments (β = −0.06, 95% CI: −0.39 to 0.27) was not statistically significant, and this effect did not differ by race/ethnicity (β = 0.01, 95% CI: −0.45 to 0.48). However, the main effect of race/ethnicity was statistically significant (β = −0.40, 95% CI: −0.70 to −0.11), indicating that Black patients had lower genetic knowledge scores than their non-Hispanic White counterparts.
Current racial segregation and family health history communication
Table 2 shows statistically significant interactions between current White social environments and race/ethnicity in communication behavior models. Although the main effects of current White social environments (adjusted odds ratio [aOR] = 0.52, 95% CI: 0.22 to 1.22) and race/ethnicity (aOR = 1.05, 95% CI: 0.54 to 2.15) were not statistically significant in the model predicting frequent communication about family history with family, the interaction term (aOR = 4.40, 95% CI: 1.49 to 13.06) was statistically significant. Among those reporting 2 or more current White social environments, we found that Black patients (est. OR = 4.63, P = .003) were 4 times more likely than White patients to communicate about family history with family frequently. These results were based on estimated marginal probabilities for each subgroup at mean age (51 years) and averaged over levels of educational attainment, household income, and health literacy.
Similarly, the interaction term between current White social environments and race/ethnicity (aOR = 3.47, 95% CI: 1.15 to 10.35) was also statistically significant in the model predicting frequent communication about family history with a doctor. Among Black patients, we found a trend suggesting that those reporting 2 or more current White social environments (est. OR = 2.40, P = .08) were 2 times more likely than those reporting 1 or no current White social environments to communicate about family history with a doctor frequently.
The main effects for age, educational attainment, and household income were not statistically significant in either past or current models of each outcome. However, we found that those with limited health literacy (past and current aOR = 2.22, 95% CI: 1.32 to 3.76) were 2 times more likely than those with adequate health literacy to frequently communicate about family history with a doctor in past and current models.
Racial segregation and other genomics-related outcomes
Supplemental Table 2 presents multivariable models of the relationship between racial segregation and knowledge of family history, genetic self-efficacy, and family history self-efficacy. We did not find statistically significant differences in knowledge of family history, genetic self-efficacy, and family history self-efficacy by the number of past and current White social environments. The interaction term between racial segregation and race/ethnicity was also not statistically significant. Supplemental Table 3 shows results from multivariable models of the relationship between racial segregation and the perceived importance of genetics and family history and communication about health. We did not find statistically significant differences in the perceived importance of genetics and family history and communication about health by the number of past and current White social environments. We also did not find that racial segregation associations differed by race/ethnicity.
Discussion
To our knowledge, this is the first study to examine the relationship between racial segregation and genomics-related knowledge, self-efficacy, perceived importance, and communication among medically underserved patients. We found that non-Hispanic White patients reporting multiple past White social environments had higher genetic knowledge than 3 other subgroups: (1) non-Hispanic White patients reporting 1 or no past White social environments, (2) Black patients reporting 1 or no past White social environments, and (3) Black patients reporting multiple past White social environments. We also found that among those reporting multiple current White social environments, Black patients were more likely than non-Hispanic White patients to communicate about family history with family frequently. Taken together, these findings provide novel insights into how the racial composition of past and current social environments play a role in knowledge about genetic information and communication about family history among medically underserved patients.
Although there is limited data on the impact of racial segregation on racial-ethnic disparities in genomics-related outcomes, ample evidence suggests that segregation adversely affects other health outcomes.15,40,41 For example, Haas and colleagues reported that adequate breast cancer care was less likely to be provided to Black and White women living in highly Black-segregated areas.15 Recent research also shows that Black individuals living in more segregated regions face higher breast cancer mortality rates and a lower chance of surviving colorectal cancer.40,41 Our finding that patients reporting predominantly Black social environments have lower genetic knowledge aligns with these studies.
Our secondary finding that Black patients frequently communicate about family history with family is consistent with previous research.42,43 For instance, a qualitative study found that older Black adults prefer open communication about family history, motivated by their desire to break the noncommunication habits of previous generations.42 Another qualitative study identified “Caregiver Responsibilities” and “Improving the Health of Younger Generations” as prevalent communication themes among older Black women.43 These women frequently communicated with younger family members to assist them in monitoring their blood pressure and reducing their risk of hypertension.43
We found that individuals with limited health literacy more frequently discussed family history with their doctors and had lower genetic knowledge than those with adequate health literacy. In our sample, a Pearson’s χ2 test showed a statistically significant association between race and health literacy. The limited health literacy group had a higher proportion of participants who were racialized as Black (83%) than the adequate health literacy group (51%, P < .001). This could account for the increased frequency of discussing family history among those with limited health literacy. It is also plausible that this explains why limited health literacy and Black racialization had relatively similar effects on genetic knowledge. Further research should investigate whether limited health literacy mediates the relationship between structural environmental factors and genomics-related outcomes.
Collectively, the findings from the present study and other studies highlight the need for interventions that target segregated populations. This is important because in 2019, over 80% of the metropolitan areas in the United States exhibited higher levels of segregation compared with 1990.44 One possible step forward is to develop stepped-wedged trials that implement an intervention to increase genetic knowledge and communication about family history across multiple generations impacted by racial residential segregation. This study design is advantageous because it allows for all study participants to receive the intervention, regardless of whether they were in the control or intervention group.45 Adding a qualitative component, such as focus groups and interviews, may also be beneficial because it can provide insights that quantitative data may not capture. Increasing knowledge about genetic information and communication about family history among these populations may increase their use of genetic services.
It is important to acknowledge the limitations of our study. First, the convenience sampling used to collect survey data from COH patients is a non-probability sampling approach susceptible to sampling error and bias. To address this limitation, study recruitment took place at different times of the day and on different days of the week. Second, participant responses to survey measures, such as the racial composition of social environments, were subjective and thus prone to recall bias and misclassification. However, unpublished qualitative data show that recall bias and misclassification were reduced based on results from the construct validation. Additionally, many of the constructs were assessed with brief or single-item measures to reduce burden on respondents. However, these constructs should be explored with more comprehensive measures. Third, although we observed a statistically significant difference in genetic knowledge, there is no agreed upon standard for a clinically meaningful difference in knowledge. It will be important for future research to investigate what change in knowledge is associated with changes in downstream outcomes such as use of genetic services or responses to genetic test results. Fourth, the data used in this study was cross-sectional, which makes it impossible to make causal inferences about the relationship between racial segregation and genomics-related outcomes. Also, there may have been unmeasured confounders that could have influenced the observed associations. Fifth, the findings from this study may not be generalizable because the sample population was drawn from a primary care clinic serving a medically underserved patient population in the Midwest. Sixth, we did not investigate the impact of other structural factors, such as health care access and utilization. Future work should evaluate the life-course impact of these factors on genomics-related knowledge, self-efficacy, perceived importance, and communication. Lastly, we did not include family history measures (eg, cancer or hypertension) because of large amounts of missing data (range of missingness = 58%-67%). Having sufficient family history data would provide better understanding of whether patients with clinically significant family history communicated this information better with family and providers, depending on the racial composition of their past and current social environments.
Despite these limitations, this study has several strengths. We included a novel pictorial measure of the racial composition of social environments. We distinguished between past and current periods, looking at racial segregation over the life course and moving beyond evaluating the potential impacts of racial segregation at a single timepoint. We also tested whether racial segregation differed by race/ethnicity, allowing for us to better characterize potential and existing health inequities. In summary, this study provides valuable insights into how structural factors may affect knowledge about genetic information and communication about family history.
Data Availability
The data supporting the findings of this study are from the Survey of Center for Outpatient Health Patients, and can be obtained by making a request to M.S.G. and K.A.K. through the following link: https://wp.nyu.edu/collegeofglobalpublichealth-goodman_mle_lab/survey-of-center-for-outpatient-health-patients/. Access to the data requires IRB approval because the data contains information (eg, medical records) that could compromise the privacy of study participants.
ORCIDs
Jemar R. Bather: http://orcid.org/0000-0002-0285-3678
Melody S. Goodman: http://orcid.org/0000-0001-8932-624X
Kimberly A. Kaphingst: http://orcid.org/0000-0003-2668-9080
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank the study participants, data collection and entry team, COH staff, administrators, and residents for contributing to this study. The authors are grateful to the editors and reviewers for their helpful comments.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Information
Conceptualization: J.R.B., M.S.G., K.A.K.; Data Curation: M.S.G., K.A.K.; Formal Analysis: J.R.B.; Funding Acquisition: M.S.G., K.A.K.; Investigation: M.S.G., K.A.K.; Methodology: J.R.B., M.S.G.; Project Administration: M.S.G., K.A.K.; Resources: M.S.G., K.A.K.; Supervision: M.S.G., K.A.K.; Writing-original draft: J.R.B.; Writing-review and editing: M.S.G., K.A.K.
Ethics Declaration
All study participants provided oral and written consent and had the option to merge their survey responses with their medical records. The primary data collection was approved by the Human Research Protection Office at the Washington University School of Medicine, and secondary data analysis was approved by the New York University Institutional Review Board.
Footnotes
This article was invited and the Article Publishing Charge (APC) was waived.
Additional Information
The online version of this article (https://doi.org/10.1016/j.gimo.2023.100844) contains supplemental material, which is available to authorized users.
Additional Information
References
- 1.Centers for Disease Control and Prevention (CDC) Tier 1 Genomic Applications Toolkit for Public Health Departments. Centers for Disease Control and Prevention (CDC); 2014. [Google Scholar]
- 2.Biesecker L.G., Green R.C. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014;370(25):2418–2425. doi: 10.1056/NEJMra1312543. [DOI] [PubMed] [Google Scholar]
- 3.Godino L., Jackson L., Turchetti D., Hennessy C., Skirton H. Decision making and experiences of young adults undergoing presymptomatic genetic testing for familial cancer: a longitudinal grounded theory study. Eur J Hum Genet. 2018;26(1):44–53. doi: 10.1038/s41431-017-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zardavas D., Baselga J., Piccart M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol. 2013;10(4):191–210. doi: 10.1038/nrclinonc.2013.29. [DOI] [PubMed] [Google Scholar]
- 5.Garcia C., Wendt J., Lyon L., et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2014;132(2):428–433. doi: 10.1016/j.ygyno.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Ding H., Sutton A.L., Hurtado-de-Mendoza A., Sheppard V.B. The role of psychosocial factors in Black women’s self-efficacy in receiving genetic counseling and testing. J Genet Couns. 2021;30(6):1719–1726. doi: 10.1002/jgc4.1439. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaidis C., Duquette D., Mendelsohn-Victor K.E., et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet Med. 2019;21(6):1363–1370. doi: 10.1038/s41436-018-0349-1. [DOI] [PubMed] [Google Scholar]
- 8.Mai P.L., Vadaparampil S.T., Breen N., McNeel T.S., Wideroff L., Graubard B.I. Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 national health interview surveys. Am J Prev Med. 2014;46(5):440–448. doi: 10.1016/j.amepre.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolb B., Wallace A.M., Hill D., Royce M. Disparities in cancer care among racial and ethnic minorities. Oncol (Williston Park) 2006;20(10):1256–1261. [PubMed] [Google Scholar]
- 10.Link B.G., Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995:80–94. doi: 10.2307/2626958. Spec No. [DOI] [PubMed] [Google Scholar]
- 11.Kwate N.O.A., Goodman M.S. Cross-sectional and longitudinal effects of racism on mental health among residents of black neighborhoods in New York City. Am J Public Health. 2015;105(4):711–718. doi: 10.2105/AJPH.2014.302243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman M., Lyons S., Dean L.T., Arroyo C., Hipp J.A. How segregation makes us fat: food behaviors and food environment as mediators of the relationship between residential segregation and individual body mass index. Front Public Health. 2018;6:92. doi: 10.3389/fpubh.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell E.F., Kramer M.R., Cooper H.L.F., Gabram-Mendola S., Senior-Crosby D., Jacob Arriola K.R. Metropolitan area racial residential segregation, neighborhood racial composition, and breast cancer mortality. Cancer Causes Control. 2012;23(9):1519–1527. doi: 10.1007/s10552-012-0029-4. [DOI] [PubMed] [Google Scholar]
- 14.Dai D. Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health Place. 2010;16(5):1038–1052. doi: 10.1016/j.healthplace.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Haas J.S., Earle C.C., Orav J.E., et al. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008;113(8):2166–2172. doi: 10.1002/cncr.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajpal N., Muñoz J., Peshkin B.N., Graves K.D. Insights into BRCA1/2 genetic counseling from ethnically diverse Latina breast cancer survivors. J Genet Couns. 2017;26(6):1221–1237. doi: 10.1007/s10897-017-0096-5. [DOI] [PubMed] [Google Scholar]
- 17.United States Preventive Services Task Force, Owens D.K., Davidson K.W., et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322(7):652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 18.Madlensky L., Trepanier A.M., Cragun D., Lerner B., Shannon K.M., Zierhut H. A rapid systematic review of outcomes studies in genetic counseling. J Genet Couns. 2017;26(3):361–378. doi: 10.1007/s10897-017-0067-x. [DOI] [PubMed] [Google Scholar]
- 19.Chetty R., Hendren N. The impacts of neighborhoods on intergenerational mobility I: Childhood exposure effects. Q J Econ. 2018;133(3):1107–1162. doi: 10.1093/qje/qjy007. [DOI] [Google Scholar]
- 20.Kaphingst K.A., Blanchard M., Milam L., Pokharel M., Elrick A., Goodman M.S. Relationships between health literacy and genomics-related knowledge, self-efficacy, perceived importance, and communication in a medically underserved population. J Health Commun. 2016;1(suppl 1):58–68. doi: 10.1080/10810730.2016.1144661. 21suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan J.H., Lyons S.A., Goodman M.S., Blanchard M.S., Kaphingst K.A. Relationship between health literacy and unintentional and intentional medication nonadherence in medically underserved patients with type 2 diabetes. Diabetes Educ. 2016;42(2):199–208. doi: 10.1177/0145721715624969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert K.L., Elder K., Lyons S., Kaphingst K., Blanchard M., Goodman M. Racial composition over the life course: examining separate and unequal environments and the risk for heart disease for African American men. Ethn Dis. 2015;25(3):295–304. doi: 10.18865/ed.25.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman M.S., Griffey R.T., Carpenter C.R., Blanchard M., Kaphingst K.A. Do subjective measures improve the ability to identify limited health literacy in a clinical setting? J Am Board Fam Med. 2015;28(5):584–594. doi: 10.3122/jabfm.2015.05.150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson D.L., Kaphingst K.A., Croston M.A., Blanchard M.S., Goodman M.S. Estimates of mental health problems in a vulnerable population within a primary care setting. J Health Care Poor Underserved. 2016;27(1):308–326. doi: 10.1353/hpu.2016.0012. [DOI] [PubMed] [Google Scholar]
- 25.Seo J., Goodman M.S., Politi M., Blanchard M., Kaphingst K.A. Effect of health literacy on decision-making preferences among medically underserved patients. Med Decis Making. 2016;36(4):550–556. doi: 10.1177/0272989X16632197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride C.M., Alford S.H., Reid R.J., Larson E.B., Baxevanis A.D., Brody L.C. Characteristics of users of online personalized genomic risk assessments: implications for physician-patient interactions. Genet Med. 2009;11(8):582–587. doi: 10.1097/GIM.0b013e3181b22c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashida S., Goodman M., Pandya C., et al. Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics. 2011;14(4-5):307–316. doi: 10.1159/000316234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Neill S.C., White D.B., Sanderson S.C., et al. The feasibility of online genetic testing for lung cancer susceptibility: uptake of a web-based protocol and decision outcomes. Genet Med. 2008;10(2):121–130. doi: 10.1097/GIM.0b013e31815f8e06. [DOI] [PubMed] [Google Scholar]
- 29.Furr L.A., Kelly S.E. The genetic knowledge index: developing a standard measure of genetic knowledge. Genet Test. 1999;3(2):193–199. doi: 10.1089/gte.1999.3.193. [DOI] [PubMed] [Google Scholar]
- 30.Kaphingst K.A., Lachance C.R., Gepp A., D’Anna L.H., Rios-Ellis B. Educating underserved latino communities about Family Health history using lay health advisors. Public Health Genomics. 2011;14(4-5):211–221. doi: 10.1159/000272456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrott R., Silk K., Raup Krieger J., Harris T., Condit C. Behavioral health outcomes associated with religious faith and media exposure about human genetics. Health Commun. 2004;16(1):29–45. doi: 10.1207/S15327027HC1601_3. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Awareness of family health history as a risk factor for disease—United States, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(44):1044–1047. [PubMed] [Google Scholar]
- 33.Kaphingst K.A., Goodman M., Pandya C., Garg P., Stafford J., Lachance C. Factors affecting frequency of communication about family health history with family members and doctors in a medically underserved population. Patient Educ Couns. 2012;88(2):291–297. doi: 10.1016/j.pec.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Cancer Institute Health information national trends survey. 2007. https://hints.cancer.gov/docs/hints2007finalreport.pdf Published online.
- 35.Krysan M., Farley R. The residential preferences of blacks: do they explain persistent segregation? Soc Forces. 2002;80(3):937–980. doi: 10.1353/sof.2002.0011. [DOI] [Google Scholar]
- 36.Haga S.B., Barry W.T., Mills R., et al. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomarkers. 2013;17(4):327–335. doi: 10.1089/gtmb.2012.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molster C., Charles T., Samanek A., O’Leary P. Australian study on public knowledge of human genetics and health. Public Health Genomics. 2009;12(2):84–91. doi: 10.1159/000164684. [DOI] [PubMed] [Google Scholar]
- 38.Bass P.F., Wilson J.F., Griffith C.H. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18(12):1036–1038. doi: 10.1111/j.1525-1497.2003.10651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team . R Foundation for Statistical Computing; 2023. R: a Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 40.Goel N., Westrick A.C., Bailey Z.D., et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275(4):776–783. doi: 10.1097/SLA.0000000000005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulson M., Cornell E., Madiedo A., et al. The impact of racial residential segregation on colorectal cancer outcomes and treatment. Ann Surg. 2021;273(6):1023–1030. doi: 10.1097/SLA.0000000000004653. [DOI] [PubMed] [Google Scholar]
- 42.Hovick S.R., Yamasaki J.S., Burton-Chase A.M., Peterson S.K. Patterns of Family Health history communication among older African American adults. J Health Commun. 2015;20(1):80–87. doi: 10.1080/10810730.2014.908984. [DOI] [PubMed] [Google Scholar]
- 43.Jones L.M., Moss K.O., Wright K.D., Rosemberg M.A., Killion C. ‘Maybe This Generation Here Could Help the Next Generation’: older African American women’s perceptions on information sharing to improve health in younger generations. Res Gerontol Nurs. 2018;11(1):39–47. doi: 10.3928/19404921-20171129-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menendian S., Gambhir S., Gailes A. Twenty-First Century Racial Residential Segregation in the United States. 2021. https://belonging.berkeley.edu/roots-structural-racism
- 45.Hemming K., Haines T.P., Chilton P.J., Girling A.J., Lilford R.J. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. doi: 10.1136/bmj.h391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are from the Survey of Center for Outpatient Health Patients, and can be obtained by making a request to M.S.G. and K.A.K. through the following link: https://wp.nyu.edu/collegeofglobalpublichealth-goodman_mle_lab/survey-of-center-for-outpatient-health-patients/. Access to the data requires IRB approval because the data contains information (eg, medical records) that could compromise the privacy of study participants.