Abstract
Purpose
The Australian Genomics Cardiovascular Disorders Flagship was a national multidisciplinary collaboration. It aimed to investigate the feasibility of genome sequencing (GS) and functional genomics to resolve variants of uncertain significance (VUS) in the clinical management of patients and families with cardiomyopathies, primary arrhythmias, and congenital heart disease (CHD).
Methods
Between April 2019 and December 2021, 600 probands meeting cardiovascular disorder criteria from 17 cardiology and genetics clinics across Australia were enrolled in the Flagship and underwent GS. The Flagship adopted a tiered approach to GS analysis. Tier 1 analysis assessed genes with established clinical validity for each cardiovascular condition. Tier 2 analysis assessed lesser-evidenced research-based genes. Tier 3 analysis assessed the functional impact of VUS that remained after tier 1 and tier 2 analysis.
Results
Overall, a pathogenic or likely pathogenic variant was identified in 41% of participants with a cardiomyopathy, 40% with an arrhythmia syndrome, and 15% with a familial CHD/CHD+Extra Cardiac Anomalies. A VUS outcome ranged from 13% for arrhythmias to 34% for CHD/CHD+Extra Cardiac Anomalies participants. Tier 2 research analysis identified a likely pathogenic/pathogenic variant for a further 15 participants and a VUS for an additional 15 participants.
Conclusion
The Flagship successfully facilitated a model of care that harnesses clinical GS and functional genomics for the resolution of VUS in the clinical setting. This valuable data set can be used to inform clinical practice and facilitate research into the future.
Keywords: Australian Genomics, Cardiovascular genetic disorders, Genome sequencing, Specialized multidisciplinary care
Introduction
Cardiovascular genetic disorders are a major cause of morbidity and account for more than half of all sudden deaths in people under age 35.1,2 The most common inherited heart disorders include cardiomyopathies, primary arrhythmia syndromes, and congenital heart disease (CHD).1,3, 4, 5 In Australia, clinicians from a range of specialties are involved in the care of patients and families with these disorders. In some health care centers, these services are integrated into dedicated multidisciplinary clinics that provide detailed assessment, determine appropriate treatment and family screening advice, and coordinate genetic testing when appropriate. The results of genetic testing are used to inform ongoing patient evaluation and management and to facilitate genetic testing for family members in whom a disease-causing variant is identified.
The reported diagnostic yield after clinical genetic testing of individuals with inherited cardiomyopathy and arrhythmia syndromes is up to 40% using cardiac gene panel approaches in selected cohorts and specialist clinical settings.6, 7, 8, 9 Because genetic testing for these conditions has become more commonplace, and eligibility criteria broader over time, lower detection rates for disease-causing variants have been reported.9 For CHD, the diagnostic yield from gene panels is not well described. In recent years, comprehensive tests, such as exome sequencing and genome sequencing (GS), have become available with the advantage of increased sensitivity in variant detection.10, 11, 12 GS provides the most comprehensive analysis because it covers the entirety of the genome, including noncoding regions (such as introns) and mitochondrial DNA. It provides improved assessment of complex structural variation and enables future reanalysis to further increase diagnostic yields.11,13, 14, 15
Previous genomics studies have often involved cohorts that are highly selective, retrospective, and ascertained through specialized research programs or tertiary care clinics. The highly selected individuals who were tested in specialist services over a decade ago are not representative of what can be expected in a modern program that uses genetic testing as a standard tool for clinical assessment. Whether GS can be used prospectively as a first-line test in clinical practice for patients with a mixture of phenotypes from within general clinical genetics and cardiology services remains an important question.
Australian Genomics is a national initiative to build evidence and develop a framework to support the utilization of genomic medicine into Australian health care through the prospective recruitment of patients into 19 clinical Flagship projects.16,17 The Cardiovascular Genetic Disorders Flagship of Australian Genomics is a collaboration of cardiologists, clinical geneticists, genetic counselors, nurses, clinical scientists, bioinformaticians, health economists, and research scientists. These experts in the genetics of human disease and functional genomics have a common goal to improve outcomes for Australian families with inherited cardiac disorders. The Flagship aimed to investigate the feasibility and benefits of multitiered GS analysis in clinical practice by (1) performing GS as part of the clinical care of 600 individuals and families with cardiomyopathies, primary arrhythmias, or CHD; (2) investigating the clinical and health service implications of integrating GS into cardiovascular health care18,19; and (3) integrating functional genomics to resolve variants of uncertain significance (VUS) into a patient’s clinical care pipeline. Here, we describe the overall design and initial findings of the Australian Genomics Cardiovascular Genetic Disorders Flagship.
Materials and Methods
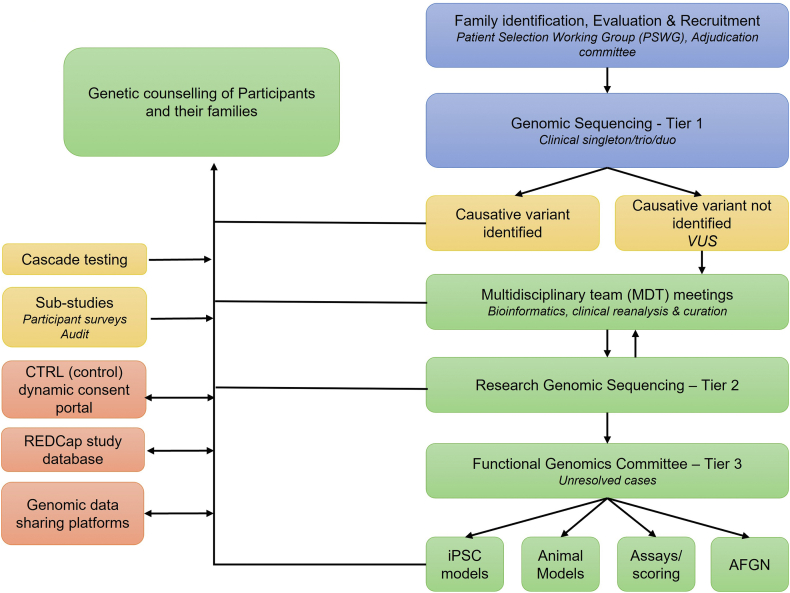
An overview of the pathway from participant recruitment to the return of GS and further research results is summarized in Figure 1.
Figure 1.
Schema of participant recruitment, approach to clinical and research sequencing, variant curation, and the resolution of VUS. Stages of feedback to participants are also illustrated.
Selection criteria
A Participant Selection Working Group, consisting of cardiologists, clinical geneticists, and genetic counselors was established to develop the Flagship’s clinical selection criteria for each specific clinical diagnosis within the 3 main diagnostic categories. Consensus criteria were established over several multidisciplinary meetings and were designed to balance a high detection rate with the consideration of areas of clinical benefit for participants and families. The criteria were regularly reviewed and amended in the initial stages of participant recruitment (Table 120, 21, 22, 23). Exclusion criteria included deceased individuals/unascertained sudden death, a previous next-generation sequencing–based test performed from 2013 onward, and individuals from families with a known likely pathogenic (LP) or pathogenic (P) variant.
Table 1.
Selection criteria for Flagship participants
| Cardiomyopathies | ||||
|---|---|---|---|---|
| MUST Meet Diagnostic Criteria for dx | AND Must be Able to Tick At Least 1 Box from At Least 2 Columns (or FHx Criteria can be Considered as Standalone if Confirmed) |
|||
| Age Criteriaa | FHx Criteria | Clinical Criteria | ||
| HCM |
Adults: a wall thickness ≥15 mm in one or more LV myocardial segments Children: LV wall thickness more than 2 standard deviations greater than the predicted mean (z-score 2, where a z-score is defined as the number of standard deviations from the population mean).20 |
|
|
|
| DCMb | LVEDD (% predicted) >112% + LVEF <45%. Note: Worst echo report can be used. Note: Please be mindful of overlap with left dominant ARVC. |
|
|
|
| ARVC | Modified taskforce criteria – at least borderline21 If borderline, must have FHx of ≥1 first-degree relative with documented ARVC or sudden cardiac death. Note: Please forward patients suspected of left dominant ARVC to the adjudication committee. |
|
|
|
| LVNC | Multiple trabeculations, deep intertrabecular recesses seen on color flow Doppler and a 2-layered structure of the myocardium with ratio of noncompacted to compacted myocardium of >2:1 in systole. This includes patients with varying pathophysiology (DFCM, HCM, RCM) and those with entirely normal cardiac function. |
|
|
|
| RCM | Evidence of primary myocardial disease comprising LV diastolic dysfunction with normal/near-normal wall thickness and systolic function. |
|
||
| Arrhythmia Syndromes | |||
|---|---|---|---|
| MUST Meet Diagnostic Criteria for dx | AND Must be Able to Tick At Least 1 Box from At Least 2 Columns (or FHx Criteria can be Considered as Standalone if Confirmed) |
||
| FHx Criteria | Clinical Criteria | ||
| BrS | HRS/EHRA/APHRS Expert Consensus Statement22 |
|
|
| LQTS | LQTS risk score ≥3.5 in the absence of a secondary cause for QT prolongation and/or a QTc interval ≥500 ms in repeated 12-lead ECG and in the absence of a secondary cause for QT prolongation.23 | No additional criteria required, provided diagnostic criteria are met. | |
| CPVT | Structurally normal heart, normal ECG, and unexplained catecholamine-induced bidirectional VT or polymorphic ventricular beats or VT in an individual <40 or relative of a CPVT case with normal heart who manifests exercise induced PVCs or bidirectional polymorphic VT.22 | Must be dx ≤ 40 y old and meet diagnostic criteria—no additional criteria required. | |
| CHDC | |
|---|---|
| Familial CHD | Nonsyndromic CHD at any age AND ≥1 first- or second-degree relative with documented CHD ± additional affected family members |
| CHD + ECA | CHD at any age AND ≥1 documented major structural noncardiac anomalyd AND normal chromosomal microarray any age |
APHRS, Asia Pacific Heart Rhythm Society; ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CHD, congenital heart disease; CM, cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; DFCM, dilated functional cardiomyopathy; dx, diagnosis; ECA, extra cardiac anomalies; ECHO, echocardiogram; ECG, electrocardiogram; EHRA, European Heart Rhythm Association; FHx, family history; HCM, hypertrophic cardiomyopathy; HRS, Heart Rhythm Society; LQTS, long QT syndrome; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVNC, left ventricular noncompaction; OHCA, out-of-hospital cardiac arrest; PVC, premature ventricular contractions; RCM, restrictive cardiomyopathy; SD, standard deviation; yo, years of age; VT, ventricular tachycardia.
If less than 18, no additional criteria needed.
Metabolic, infective, and syndromic causes must be excluded.
For the purpose of this project, CHD includes patients who require surgical or catheter-based intervention, so as not to capture individuals with trivial valvular disease, minor septal defects, small patent ductus arteriosus, etc.
Extra cardiac anomalies include major structural noncardiac anomalies, eg, diaphragmatic hernia, cleft lip/palate, intestinal atresia, midline defects, structural brain abnormalities, and/or functional neurological impairment (when diagnosed by a neurologist/geneticist/pediatrician and fitting criteria for moderate neurodevelopmental disability on the basis of formal testing, for example, Bayley Scales in children or neurocognitive assessments in older children and adults).
Adjudication committee
An adjudication committee was established to decide on the inclusion/exclusion of cases in which further expertise was required to determine the participant’s eligibility to be enrolled into the study. The committee consisted of cardiologists, cardiac electrophysiologists, a cardiac surgeon, a clinical geneticist, and genetic counselors with expertise across the 3 diagnostic categories. The clinical details of cases (Supplemental Table 1) requiring adjudication were reviewed in detail, and participants were invited into the Flagship based on the majority view of the committee.
The adjudication committee reviewed 117 cases; 46% were accepted into the Flagship. CHD was the most common diagnosis at 54% of adjudicated cases (Supplemental Table 2).
Recruitment process of Flagship cohort
Patients referred to a participating clinical genetics or cardiology clinic between April 2019 to December 2021 who met the selection criteria for one of the 3 diagnostic groups (or approved by the adjudication committee) were invited to participate in the Flagship. Eligible patients were identified at the time of preclinic workup and, if possible, were provided with study information and consent forms (hard copy, PDF, or REDCap e-consent) before their consultation. Eligible patients were also given the option of consenting via a dynamic digital portal called Control (CTRL).24 They were then formally invited to participate during their in-person, telehealth, or telephone cardiology or genetics consultation (Figure 1).
Data collection
Study data were collected and managed using the electronic data capture software, REDCap, and stored at the Murdoch Children’s Research Institute.25,26 The data fields (Supplemental Table 3) were established through multidisciplinary consultation and included demographics, family history, clinical, GS, and variant information. The Flagship REDCap software was also used to invite participants to complete surveys collecting psychosocial feedback and economic data on the testing process at 5 time points (at time of recruitment [Baseline], and 1-, 6-, 12-, and 24-months post result disclosure) for a substudy—the Effectiveness of a Standardized Cardiac Genetic Testing Approach in Australia.
GS and analysis pipeline
The Flagship adopted a multitiered approach to GS analysis. The genes included for curation in tier 1 and tier 2 for each condition were determined by a Gene Selection Working Group, consisting of cardiologists, clinical geneticists, genetic counselors, and research scientists.
Genome sequencing—Tier 1
GS was performed by 3 Australian laboratories accredited by the National Association of Testing Authorities: Victorian Clinical Genetics Service (88% of reports issued), Genome.One (6% of reports issued), and SA Pathology (6% of reports issued). Supplemental Table 4 lists the methods for sample collection, DNA extraction, storage, sequencing, and bioinformatic processing. The genes selected for tier 1 analysis included genes with established clinical validity for each cardiovascular condition as of November 2019 (Supplemental Table 5). If a well-established disease-causing variant was identified, or if no variant was identified, a report was generated and returned to the clinic team for return to the participant. When a VUS was identified, the case was presented at a multidisciplinary team (MDT) meeting of clinical and laboratory experts, including the participant’s clinical team and the scientists who performed the sequencing. At the MDT, the clinical history was presented in a deidentified format, and the tier 1 results reviewed. The final classification of each variant was based on American College of Medical Genetics (ACMG) guidelines.27 The suitability for reporting each variant was determined, and any additional information or investigations with the potential to alter the classification were identified. The outcome was returned to the participant for further clinical management (ie, cascade testing of family members). Variant data were entered into the Flagship’s REDCap database by a scientist and the Flagship’s coordinators.
Secondary research analysis—Tier 2
After the completion of the tier 1 process, participant’s sequence data underwent a wider tier 2 analysis, which included an additional list of lesser-evidenced research-based genes (Supplemental Table 5). Tier 2 analysis was performed by research teams at the Centenary Institute (all primary arrhythmias and all cardiomyopathies except dilated cardiomyopathy [DCM]) and the Victor Chang Cardiac Research Institute (all DCM and CHD). Cases in which no additional secondary research findings were identified were summarized in a research report and returned to the clinical team and the information given to the participant. Cases in which the tier 2 analysis identified additional variants that were LP/P or were a VUS, the case was presented at a tier 2 MDT meeting for further discussion and action.
Tier 2 MDT meetings were similarly conducted as the tier 1 MDT meetings, but with a focus on research-based cardiac genes. If the tier 2 MDT agreed that a variant identified through tier 2 analysis was LP/P, it was returned to the original reporting laboratory for recuration. It then went back to a tier 1 MDT to determine the final classification and clinical relevance of the variant for the final report before it was returned to the clinical teams and participants.
Given the genetic complexity of CHD, 89 parental samples were also collected because trio analysis increases the likelihood of identifying CHD-causing variants.
Functional genomics analysis—Tier 3
Unresolved cases, in which a VUS remained after tier 1 and tier 2 analysis, were considered for functional analysis by the Flagship’s Functional Genomics Committee. The identification of eligible variants was facilitated through REDCap with assistance from the tailored results reports (and accumulating evidence against ACMG criteria27). A call for expressions of interest to functionally assess the impact of the VUS on gene/protein function was distributed through the Australian Functional Genomics Network and the Flagship. Eight Flagship-funded functional genomics platforms were used to analyze VUS. The MDT evaluated whether a VUS with significantly altered function compared with the reference will affect the classification of the variant according to ACMG guidelines. Cases in which the reclassification of variants was not possible, evidence that the variant is functionally altered was useful because it prioritized the case for follow-up. Outcomes were discussed at tier 3 MDT meetings for clinical curation and final reporting. When possible, blood samples were also collected from participants for peripheral blood mononuclear cell (PBMC) isolation. PBMCs were extracted from whole-blood patient samples and stored using a standard extraction protocol.
Data sharing
Sequencing data, including reclassification, have been made available to other diagnostic laboratories via genomics data sharing platforms such as the Australian Genomics Shariant platform28 and variant atlas (Figure 1).
Deidentified genomic and associated data from this study are available for ethically approved research. Data access requests are accepted via an online application form that will require approval from the Australian Genomics Data Access Committee. For access to the data, please email ag-datarequest@mcri.edu.au. Data access requests are reviewed by the committee once a month. Access to the data will require a Data Transfer Agreement (DTA). Once a DTA is signed, the data will be transferred to the requestor from AWS S3 storage.
Results
Study participants
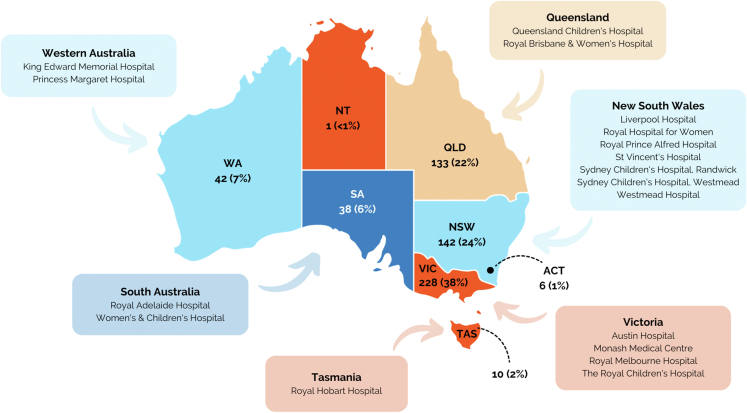
A total of 600 participants were recruited from 17 clinical sites across all 8 states and territories of Australia (Figure 2). At the time of consent, participants ranged in age from 0 to 82 years with a mean age of 37 years (median 40 years). Eighty-two percent were older than 18 years. Of the pediatric cases, 5.6% (n = 3 CHD, n = 2 cardiomyopathy, and n = 1 arrythmia) were newborn (≤28 days) at the time of recruitment. Fifty-four percent of participants were male. From self-reported ethnicity, European accounted for 67%, followed by Asian (19%). Six percent indicated Australian Aboriginal ethnicity. Most participants had a clinical diagnosis of a cardiomyopathy (67%), 20% had an arrhythmia syndrome, and 13% had a diagnosis of familial CHD or CHD+ECA. Tables 2 and 3 provide a summary of clinical and intervention characteristics of the study cohort at the time of recruitment.
Figure 2.
Number of participants recruited from each Australian State and Territory.
Table 2.
Clinical characteristics of cohort for each diagnosis at time of recruitmenta (n = 600)
| Cardiomyopathies | HCM | DCM | ARVC | LVNC | RCM | Total |
|---|---|---|---|---|---|---|
| Total | 172 | 159 | 39 | 26 | 5 | 401 |
| Pediatric (%) | 14 (8.1%) | 8 (5%) | 1 (2.6%) | 6 (23.1%) | 4 (80%) | 33 (8.2%) |
| Adult (%) | 158 (91.8%) | 151 (94.9%) | 38 (97.4%) | 20 (76.9%) | 1 (20%) | 368 (91.7%) |
| Mean age at diagnosis in years (median, range) | 41 (42, 0-81) | 45 (47, 0-77) | 44 (46, 17-82) | 30 (32, 0-72) | 15 (13, 6-33) | 42 (44, 0-82) |
| Family history of disease in first-degree/second-degree relative(s), n (%) | 98 (57) | 113 (71) | 13 (33) | 13 (50) | 1 (20) | - |
| Family history of sudden death (<50 y) in first-degree/second-degree relative(s), n (%) | 31 (18.0) | 53 (33) | 7 (18) | 3 (11.5) | 0 | - |
| History of syncope, n (%) | 21 (12.2) | 6 (3.8) | 7 (17.9) | 2 (7.7) | 0 | 36 (9.0) |
| Out-of-hospital cardiac arrest, n (%) | 14 (8) | 10 (6.3) | - | - | - | - |
| Mean IVSd in mm (median, range) - adult | 20 (20, 0-40.9) | - | - | - | - | - |
| Mean IVSd in mm (median, range) - pediatric | 12.5 (13, 0-23) | - | - | - | - | - |
| Mean LVPWd in mm (median, range) - adult | 9 (10, 0-30) | - | - | - | - | - |
| Mean LVPWd in mm (median, range) - pediatric | 9 (8, 0-17) | - | - | - | - | - |
| % predicted LVEDD, mean (median, range) | - | 135.2 (132.7, 12- 245.8) | - | - | - | - |
| Mean worst LVEF % (median, range) | - | 30 (32, 8-57) | - | 49 (46, 30-68) | - | - |
| Conduction diseaseb | - | 70 | - | - | - | - |
| Primary Arrythmias | BrS | LQTS | CPVT | Total |
|---|---|---|---|---|
| Total | 16 | 94 | 9 | 119 |
| Pediatric, n (%) | 1 (6.2) | 14 (14.9) | 2 (22) | 17 (14.28) |
| Adult, n (%) | 15 (93.8) | 79 (84.0) | 7 (78) | 101 (84.9) |
| Mean age at diagnosis in years (median, range) | 42 (44, 9-70) | 34 (36, 0-66) | 26 (18, 11-60) | - |
| Family history of disease first- or second-degree relative(s), n (%) | 6 (37.5) | 32 (34) | 1 (11) | - |
| Family history of sudden death (<50 y old) first- or second-degree relative(s), n (%) | 7 (43.8) | 23 (24.5) | 5 (55) | - |
| History of syncope, n (%) | 3 (18.8) | 38 (40.4) | 8 (88.9) | 49 (41.2) |
| BrS previous OHCA, n (%) | 2 (12.5) | - | - | - |
| BrS spontaneous type 1 pattern, n (%) | 10 (62.5) | - | - | - |
| BrS induced type 1 pattern, n (%) | 8 (50) | - | - | - |
| LQTS risk score ≥3.5 in absence of secondary cause for QT prolongation, n (%) | - | 68 (72.3) | - | - |
| LQTS QTc interval ≥ 500 ms in repeated 12-lead ECG and in the absence of a secondary cause for QT prolongation, n (%) | - | 52 (55.3) | - | - |
| Resting QTc at diagnosis off QT prolonging drugs and not immediately post arrest, mean (median, range) | - | 509.5 (500, 400-642) | - | - |
| Catecholamine-induced bidirectional VT or polymorphic ventricular premature beats or VT, n (%) | - | - | 9 (100) | - |
| Relative of a CPVT case with normal heart who manifests exercise-induced PVCs or bidirectional polymorphic VT, n (%) | - | - | 1 (11.1) | - |
| CHD | Familial CHD | CHD + ECA | Total |
|---|---|---|---|
| Total | 54 | 26 | 80 |
| Pediatric, n (%) | 36 (66.6) | 20 (76.9) | 56 (70) |
| Adult, n (%) | 18 (33.3) | 6 (23) | 24 (30) |
| Mean age at diagnosis in years (median, range) | 3 (0, 0-57) | 0.15 (0, 0-2) | - |
| Family history of CHD in ≥ first-degree relative(s), n (%) | 52 (96.3) | - | - |
| Additional CHD affected family members, n (%) | 24 (44.4) | - | - |
| Mean number of defects diagnosed for each Pt (median, range) | 2 (2, 1-5) | 2 (2, 1-5) | - |
| Most common CHD diagnoses, n (%) | Atrial septal defect 19 (35.2) Ventricular septal defect 14 (25.9) Tetralogy of Fallot 8 (14.8) |
Patent ductus arteriosus 7 (26.9) Atrial septal defect 5 (19.2) Ventricular septal defect 4 (15.4) |
- |
| Mean number of ECA (median, range) | - | 2 (2, 1-5) | - |
| Most common ECA diagnoses, n (%) | - | Skeletal 10 (38.5) Liver/renal 7 (26.9) Dysmorphisms 7 (26.9) |
- |
ARVC, arrhythmogenic right ventricular cardiomyopathy; AV block, atrioventricular block; BrS, Brugada syndrome; CHD, congenital heart disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ECA, extra cardiac anomalies; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; IVSd, intraventricular septal thickness at diastole; LQTS, long QT syndrome; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVNC, left ventricular noncompaction; LVPWd, left ventricular posterior wall thickness at end diastole; mm, millimeters; OHCA, out-of-hospital cardiac arrest; pediatric, <18 years old (2 HCM pediatric participants were newborn, 1 LQTS pediatric participant was newborn, and 2 CHD and 1 CHD+ECA participants were newborn); RCM, restrictive cardiomyopathy; VT, ventricular tachycardia; “-”, data not applicable.
Totals differ because of missing data or data not applicable to the diagnosis.
Second- or third-degree AV block.
Table 3.
Interventions reported for each diagnosis at time of recruitmenta
| Cardiomyopathies | HCM | DCM | ARVC | LVNC | RCM |
|---|---|---|---|---|---|
| PPM, n (%) | 20 (11.6) | 19 (11.9) | 3 (7.7) | 1 (3.8) | 0 |
| Mean age in years of PPM (median, range) | 43.2 (40.5, 26-77) | 41.7 (46, <1-63) | 50.3 (50.0, 49.0-52.0) | 38 | - |
| ICD, n (%) | 72 (41.9) | 53 (33.3) | 20 (51.3) | 3 (11.5) | 0 |
| Mean age in years of ICD (median, range) | 41 (42, 13-78) | 46 (47, 12-67) | 42 (44, 17-66) | 30 (27, 26-38) | - |
| CRT, n (%) | 0 | 10 (6.3) | 0 | 0 | 0 |
| Mean age in years of CRT (median, range) | - | 54.1 (55.5, 39-69) | - | - | - |
| LVAD, n (%) | 0 | 6 (3.8) | 0 | 0 | 0 |
| Mean age in years of LVAD (median, range) | - | 29.7 (25.5, 14-53) | - | - | - |
| Cardiac transplant, n (%) | 1 (0.6) | 12 (7.5) | 0 | 0 | 1 |
| Mean age in years of transplant (median, range) | 66 | 39.1 (41, 15-63) | - | - | 32 |
| Arrhythmias | BrS | LQTS | CPVT |
|---|---|---|---|
| PPM, n (%) | 1 (6.2) | 5 (5.3) | 1 (11.1) |
| Mean age in years of PPM (median, range) | 46 | 34.6 (36, <1-65) | 30 |
| ICD, n (%) | 4 (25) | 34 (36.1) | 6 (66.7) |
| Mean age in years of ICD (median, range) | 38.1 (38.0, 20-60) | 39.4 (38, 18-65) | 37.2 (38, 18-60) |
| Congenital Heart Disease | Familial CHD | CHD + ECA |
|---|---|---|
| PPM, n (%) | 4 (7.4) | 1 (3.8) |
| Mean age in years of PPM (median, range) | 1 (1, 0-1) | 13 |
| ICD, n (%) | 0 | 0 |
| Mean age in years of ICD (median, range) | - | - |
| Cardiac transplant, n (%) | 0 | 0 |
| Mean age in years of transplant (median, range) | - | - |
| Open heart surgery, n (%) | 40 (74) | 24 (92.3) |
| Cardiac catheterization, n (%) | 10 (18.5) | 5 (19.2) |
| Mean number of procedures (median, range) | 1.8 (1, 1-4) | 1.8 (1, 1-5) |
| Most common procedures, n (%) | Closure of atrial septal defect 6 (11.1) Repair of tetralogy of Fallot 6 (11.1) Repair of coarctation of aorta 5 (9.2) |
Modified Blalock-Taussig shunt 6 (23.1) Closure of atrial septal defect 5 (19.2) Banding of pulmonary artery 3 (11.5) Cardiac catheterization NOS 3 (11.5) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CRT, cardiac resynchronization therapy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; LVNC, left ventricular noncompaction; NOS, not otherwise specified; PPM, permanent pacemaker; RCM, restrictive cardiomyopathy; “-”, data not applicable.
Totals differ because of missing data or data not applicable to the diagnosis.
GS diagnostic rates across clinical diagnoses—Tier 1
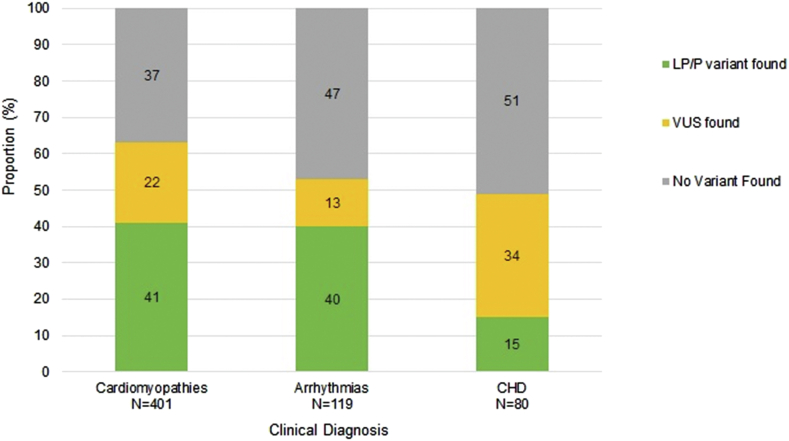
Table 4 summarizes the genetic diagnostic rate among different clinical diagnoses. After GS and tier 1 variant and clinical curation, a genetic cause for the participant’s diagnosis was found in 38% of participants. Of these, 41% had cardiomyopathy, 40% had arrhythmia, and 15% of participants had familial CHD/CHD+ECA (Figure 3). Supplemental Table 6 lists all LP/P variants identified in tier 1 analysis for each diagnosis.
Table 4.
Summary of participants’ clinical diagnosis and their tier 1 clinically curated outcomes (n = 600)
| Clinical Diagnosis | Reported | No Variants Found, n (%) | VUS Found, n (%) | LP/P Variant Found, n (%) |
|---|---|---|---|---|
| Cardiomyopathies | ||||
| HCM | 172 | 46 (27) | 41 (24) | 85 (49) |
| DCM | 159 | 68 (43) | 38 (24) | 53 (33)a |
| ARVC | 39 | 19 (49) | 2 (5) | 18 (46) |
| LVNC | 26 | 15 (58) | 5 (19) | 6 (23) |
| RCM | 5 | 1 (20) | 1 (20) | 3 (60) |
| Total | 401 | 149 (37) | 87 (22) | 165 (41) |
| Arrhythmias | ||||
| BrS | 16 | 10 (63) | 3 (19) | 3 (19) |
| LQTS | 94 | 44 (47) | 10 (11) | 40 (43) |
| CPVT | 9 | 2 (22) | 2 (22) | 5 (56) |
| Total | 119 | 56 (47) | 15 (13) | 48 (40) |
| CHD | ||||
| Familial CHD | 54 | 24 (44) | 20 (37) | 10 (19) |
| CHD + ECA | 26 | 17 (65) | 7 (27) | 2 (8) |
| Total | 80 | 41 (51) | 27 (34) | 12 (15) |
| Overall participant Total | 600 | 246 (41) | 129 (22) | 225 (38) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CHD, congenital heart disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ECA, extra cardiac anomalies; HCM, hypertrophic cardiomyopathy; LP, likely pathogenic; LQTS, long QT syndrome; LVNC, left ventricular noncompaction; P, pathogenic; RCM, restrictive cardiomyopathy; VUS, variants of uncertain significance.
Numbers shown include TTNtv VUS that were reclassified as LP/P following reanalysis after Flagship completion.
Figure 3.
Summary of tier 1 clinically curated diagnostic rate (%) for the 3 clinical diagnosis categories (n = 600).
A VUS was found in 22% of cardiomyopathy participants, 13% of those had an arrhythmia syndrome, and 34% were CHD/CHD+ECA participants (Figure 3).
At completion of the Flagship, it was apparent that there had been significant evolution in approaches to curation of truncating variants in the TTN gene (TTNtv). To standardize TTNtv classification and align with international practice, TTNtv previously clinically curated as VUS were reviewed. This resulted in reclassification of 18TTNtv from VUS to LP and 1 TTNtv VUS to P (Table 4).
Secondary research analysis—Tier 2
For participants with no LP/P variant and/or a VUS identified in tier 1 analysis, tier 2 analysis with an expanded gene panel identified LP/P variants for an additional 15 participants and also a VUS for an additional 15 participants.
Table 5 summarizes the genes in which all LP/P variants were found for each clinical diagnosis following the tiered 1 and 2 analysis approach.
Table 5.
Total number of LP/P variants (N) identified from tier 1 and tier 2 analysis each gene and clinical diagnosis
| Diagnosis | Gene | Tier 1 LP/P Variants, n | Tier 2 LP/P Variants, n |
|---|---|---|---|
| HCM | MYBPC3 | 59 | 2 |
| MYH7 | 17 | ||
| ALPK3 | 1 | ||
| TNNI3 | 3 | ||
| CACNA1C | 2 | ||
| PTPN11 | 2 | ||
| JPH2 | 1 | ||
| KCNQ1 | 0 | ||
| LAMP2 | 1 | ||
| PRKAG2 | 1 | ||
| TNNT2 | 1 | ||
| TPM1 | 1 | ||
| DCM | TTNa | 29 | 1 |
| LMNA | 4 | ||
| BAG3 | 3 | ||
| DMD | 2 | ||
| DSP | 2 | ||
| FLNC | 2 | ||
| MYBPC3 | 2 | ||
| MYH7 | 2 | ||
| RBM20 | 2 | ||
| TNNT2 | 2 | ||
| PKP2 | 1 | 1 | |
| DES | 1 | ||
| SCN5A | 1 | ||
| ARVC | PKP2 | 9 | |
| DSP | 5 | ||
| DSG2 | 4 | ||
| BMPR2 | 1 | ||
| FLCN | 1 | ||
| LVNC | MYH7 | 2 | |
| MYBPC3 | 1 | ||
| TAZ | 1 | ||
| TBX20 | 1 | ||
| RYR2 | 1 | ||
| TTN | 2 | ||
| DSP | 1 | ||
| MT-TI | 1 | ||
| PRDM16 | 1 | ||
| RBM20 | 1 | ||
| TBX20 | 1 | ||
| RCM | MYBPC3 | 1 | |
| MYH7 | 1 | ||
| TNNI3 | 1 | ||
| BrS | SCN5A | 3 | |
| LQTS | KCNQ1 | 28 | |
| KCNH2 | 10 | ||
| SCN5A | 1 | ||
| TTN | 1 | ||
| CPVT | RYR2 | 4 | |
| CASQ2 | 1 | ||
| TECRL | 2 | ||
| MYBPC3 | 1 | ||
| Familial CHD | JAG1 | 2 | |
| CDK13 | 1 | ||
| ELN | 1 | ||
| FLT4 | 1 | ||
| GATA4 | 1 | ||
| NKX2 | 1 | ||
| NODAL | 1 | ||
| NOTCH1 | 1 | ||
| PTPN11 | 1 | ||
| DNAH11 | 1 | ||
| PKD2 | 1 | ||
| CHD + ECA | CHD7 | 2 | |
| NFE2L2 | 1 | ||
| SETD5 | 1 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CHD, congenital heart disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ECA, extra cardiac anomalies; HCM, hypertrophic cardiomyopathy; LP, likely pathogenic; LQTS, long QT syndrome; LVNC, left ventricular noncompaction; P, pathogenic; RCM, restrictive cardiomyopathy; VUS, variants of uncertain significance.
Numbers shown includes the TTNtv VUS that were reclassified as LP following reanalysis after Flagship completion.
Functional genomics analysis—Tier 3
After tier 1 and tier 2 analyses, VUS were selected for tier 3 functional analysis to identify variants that affect protein function and potentially lead to the reclassification of the VUS based on the obtained information. Furthermore, PBMCs were extracted from a total of 205 participants. Tier 3 analysis is ongoing with functional platforms and results to be reported on completion.
Time required for the GS testing process
The turnaround time in weeks for the GS process was calculated for each diagnosis (Supplemental Table 7). This turnaround time included from when the GS test was ordered until a results report was issued. Overall, it took an average of 21 weeks (median 19 and range 3-150) until the clinical GS (tier 1) results report was issued. Cardiomyopathies and arrhythmia syndromes had a similar turnaround time, with a mean of 19 weeks (median 18), and CHD had the longest turnaround time (mean 30, median 20, and range 8-150).
Participants survey results
Survey response outcomes are to be reported in a future publication. Supplemental Figure 1 flow diagram shows the analysis plan for the substudy—Effectiveness of a Standardized Cardiac Genetic Testing Approach in Australia.
Discussion
The Australian Genomics Cardiovascular Genetic Disorders Flagship successfully facilitated clinical GS in 600 people with a suspected inherited cardiovascular disorder across Australia. The establishment of clinical and research networks and collaborations and the incorporation of additional research testing enabled a real-time evaluation of genomic testing as part of the participants’ care in a clinical setting.
The Flagship achieved the integration of the most up-to-date sequencing and functional technologies into a standard care pathway within a routine clinical testing time frame. The extension of the testing pathway into the secondary tier 2 analysis and functional tier 3 analyses will be reported when completed. These analyses, usually considered as purely research activities, resulted in the tier 2 identification of additional LP/P variants that were then verified and reported by the clinical genetics service to clarify that the additional LP/P variants were clinical-grade findings. Furthermore, it was a highly participatory program that involved more than 150 clinical and laboratory personnel across specialties and disciplines from a range of health services, research facilities, and geographic locations to achieve national representation. The result was broad input into elements such as consensus criteria for testing eligibility and gene lists, and inclusion of clinical input at all stages of genetic test interpretation, to maximize the utility of the test results in clinical care. Beyond these elements, the interaction between the typically distinct clinical and research communities also had obvious benefits to the activities of both groups, and the Flagship has provided valuable experience and expertise on a national scale to improve the utilization of genomics in the care of individuals and families with inherited cardiac disorders.
Overall, 38% of participants were found to have an LP/P variant that explained their cardiovascular disorder (rates ranged from 41% for cardiomyopathies to 15% for CHD). This diagnostic rate is in keeping with the rates previously reported for cardiomyopathies and arrythmias by specialist clinics and laboratories.6, 7, 8 Genomic testing in the Flagship’s CHD cohort had a diagnostic rate of 15%, which falls at the lower end of the reported rates.12,29, 30, 31 However, these studies used a range of different ascertainment criteria that included either all CHD types or specific subgroups of isolated CHD and/or CHD+ECA, and different combinations of sporadic and/or familial cases. As a result, it is difficult to draw useful comparisons between studies. In some patients with CHD, later onset of comorbidities and/or comorbidities that may not be apparent in early infancy further complicate genetic testing and variant interpretation in this patient group. Since the commencement of Flagship, a recent best practice document on cardiac genetic testing has been published in 2022,32 which provides further direction regarding which genes to test in patients with cardiomyopathy, arrhythmia, and CHD.
Tier 2 analysis increased the overall yield of LP/P variants over that of tier 1, but it is difficult to make useful comparisons with reported studies because of the diversity in approaches. One explanation could lie in the tier 1 gene lists that were selected. The lists were designed to be comprehensive and used where additional tier 2 findings occurred; these were often in known cardiovascular disorder genes associated with a related phenotype. For instance, it was noted that tier 2 testing of the left ventricular noncompaction cohort identified diagnostic variants that were included in the DCM tier 1 gene list. Although the diagnostic yield of clinical genetic testing was only modestly improved by the addition of research-focused tier-2 testing, there was an important clinical impact to those families for whom a diagnosis was made. The role of research in improving diagnostic yield over time will be something the Cardiovascular Genetic Disorders Flagship intends to monitor and report on. Additionally, we anticipate that continued careful stratification of gene lists according to clinical phenotype will better define the diagnostic yield.
For CHD, beyond a limited selection of well-defined phenotypes, the pathway to genetic testing varies considerably and is mostly restricted to a research setting.33,34 Patients with CHD, similar to other patients with cardiovascular disorders, benefit from multidisciplinary care involving both clinical genetics and cardiology specialists to improve the accessibility and diagnostic rate of genomic tests in this cohort. Incorporating research testing (tier 2 and tier 3) analysis and segregation studies for VUS has the potential to further increase the diagnostic rate.34 Recommendations garnered from the ClinGen consortium35 will further establish evidence for the optimal clinical testing in the CHD cohort and optimization of gene lists and variant curation. As expected, the utilization of trios greatly assisted with variant analyses and classification in this patient group.
Despite the complexity of GS as a first-line test into standard clinical care, it is reassuring that the turnaround time for the Flagship GS process was similar to the non-Flagship testing process (unpublished clinic data). A minority of Flagship participants had significantly longer turnaround times, but this was typically related to organizational or workflow issues rather than the technical demands of GS.
A more detailed assessment of the average hours per diagnosis contributed by different members of the clinical and laboratory teams across the GS pathway would provide further information on the feasibility of introducing GS into standard clinical care. This type of data was not collected in the Flagship; however, Christensen et al36 reported that the impact of GS on cardiologists’ clinical work was minimal. A detailed analysis of the costs associated with Flagship GS and outcomes will be possible as the cohort is followed up. Cost analysis of clinical GS in the MedSeq project found that the greatest proportion of the costs was incurred in the short term, related to sequencing, variant interpretation, and disclosure, and was expected to decrease with advancing sequencing technology and improvements in data sharing between laboratories. Importantly, GS did not increase downstream health care costs.36
The Flagship’s data and expertise, including pick-up rates of diagnostic genetic testing and actionable findings, contributed to 2 successful Australian Medical Services Advisory Committee applications by demonstrating the feasibility, safety, effectiveness, and cost-effectiveness of genetic testing in the Australian health care setting. This led to 2 new Medicare Benefit Scheme item numbers being approved to provide Australian Commonwealth Government funding for cardiac genetic testing in patients with inherited arrhythmias or cardiomyopathies.
Continuing analysis of the Flagship’s data, including the participant survey data and further tier 2 and tier 3 outcomes, will elucidate the effectiveness of this multitiered cardiac GS analysis approach from both participant and health service perspectives and detail the clinical utility of the functional genomic evaluation.
Conclusions
The Australian Genomics Cardiovascular Genetic Disorders Flagship successfully facilitated GS in the clinical setting and provided evidence of the feasibility and benefits of offering GS to patients with cardiac phenotypes indicating a genetic cause. It enabled a model of care that harnessed clinical and functional genomics for the resolution of VUS. It also contributed to the approval of further publicly funded genetic tests. This Flagship will inform future clinical practice on a national scale, as well as encourage the broader utilization of cardiac genetic testing in the diverse Australian population and national collaboration to improve the quality of care and outcomes for families.
Data Availability
Study data sets have not been deposited in a public repository because of consent restrictions. Deidentified data from this study are available for ethically approved research. The online access application process is administered by the Australian Genomics Data Access Committee.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the families who participated in this Flagship and the clinical teams involved in their care.
Funding
The Australian Genomics Cardiovascular Genetic Disorders Flagship was funded by the Medical Research Future Fund (EPCD000028). Australian Genomics is funded by The National Health and Medical Research Council (NHMRC; GNTIER 1113531 and GNTIER 2000001). S.L.D. was supported by NHMRC Fellowship/Investigator ID 1135886, ID2007896 and Z.S. by GHFM APP2008820.
Author Information
Conceptualization: T.B., D.W., E.H., G.M.B., J.V., J.J.A., L.B., R.G.W., P.J., D.F., I.M., J.I., S.L.D., C.S., J.M.; Data Curation: D.D., E.G., E.M.R., K.C., Z.S., R.D.B., S.-J.P., S.L., P.D.F., Y.C., D.F., J.I., J.V., M.S.; Funding Acquisition: T.B., C.S., J.M.; Investigation: A.D., A.E.H., E.H., D.W., E.G., G.M.B., K.v.S.-Z., M.W., N.P., N.K.P., T.T., P.J., I.M., J.I., C.S., J.M.; Project Administration: R.A., J.S.B., T.M., T.B., M.H., M.C.J.Q.; Software: S.C., E.O.M., A.M., M.H.; Writing-review and editing: R.A., M.C.J.Q., J.M., C.S., D.F., P.J., S.L.D., J.J.A., J.S.B.
Ethics Declaration
Ethics approval was provided by the Melbourne Human Research Ethics Committee (HREC2016.224), and all participants provided written informed consent.
The Australian Genomics Cardiovascular Disorders Flagship
Lesley Ades, Annabel Enriquez, Alison McLean, Renee Smyth, Dimithu Alankarage, Diane Fatkin, James McNamara, Magdalena Soka, Morgan almog, Vanessa Fear, Caroline Medi, Zornitza Stark, Mohammad Al-Shinnag, Miriam Fine, Alejandro Metke, Raymond Sy, John J. Atherton, Keri Finlay, Di Milnes, Dotti Tang, Rachel Austin, Denisse Garza, Michael Milward, Jessica Taylor, Richard D. Bagnall, Eleni Giannoulatou, Ansley Morrish, Shelby Taylor, Chris Barnett, Laura Gongolidis, Jim Morwood, Michel Tchan, Gillian M. Blue, Belinda Gray, Helen Mountain, Tina Thompson, Simon Bodek, Cassie Greer, David Mowat, Jordan Thorpe, Kirsten Boggs, Eric Haan, Chai-Ann Ng, Alison Trainer, Michael Bogwitz, Mathilda Haas, Natalie Nowak, Gunjan Trivedi, Tiffany Boughtwood, Bernadette Hanna, Noelia Nunez Martinez, Giulia Valente, Alessandra Bray, Richard Harvey, Monique Ohanian, Karin van Spaendonck-Zwarts, Marie-Jo Brion, Janette Hayward, Sinead O’Sullivan, Jamie Vandenberg, Jaye Brown, Carmen Herrera, Angela Overkov, Kunal Verma, Rob Bryson Richardson, Adam Hill, Nicholas Pachter, Miranda Vidgen, Leslie Burnett, Georgie Hollingsworth, Chirag Patel, Jitendra Vohra, Charlotte Burns, Georgina Hollway, Mark Perrin, Kathryn Waddel-Smith, Michelle Cao, Ari E. Horton, Matthew Perry, Mathew Wallis, Will Carr, Denise Howting, Andreas Pflaumer, Robert G. Weintraub, Sarah Casauria, Jodie Ingles, Peta Phillips, Meredith Wilson, Heather Chalinor, Joanne Isbister, Thuan Phuong, David Winlaw, Yuchen Chang, Matilda Jackson, Rachel Pope-Couston, Lisa Worgan, Gavin Chapman, Paul James, Nicola K. Poplawski, Linda Wornham, Theosodia Charitou, Sarah Jane-Pantaleo, Preeti Punni, Kathy Wu, Belinda Chong, Renee Johnson, Michael C.J. Quinn, Laura Yeates, Felicity Collins, Andrew Kelly, Dominica Zentner, Gemma Correnti, Sarah King-Smith, Sulekha Rajagopalan, Kathy Cox, Edwin Kirk, Hariharan Raju, Fiona Cunningham, Sarah Kummerfeld, Emma M. Rath, Debjani Das, Timo Lassman, Matthew Regan, Jason Davis, Jonathon Lipton, Jonathan Rogers, Andrew Davis, Sebastian Lunke, Mark Ryan, Paul De Fazio, Ivan Macciocca, Sarah Sandaradura, Michelle de Silva, Paul MacIntyre, Nicole Schonrock, Nicola Den Elzen, Evanthia O. Madelli, Paul Scuffham, Sophie Devery, Amali Mallawaarachchi, Chris Semsarian, Julia Dobbins, Julia Mansour, Isabella Sherburn, Sally L. Dunwoodie, Ellenore Martin, Mary-Clare Sherlock, Nathan Dwyer, Jacob Mathew, Emma Singer, Stefanie Elbracht-Leong, Tessa Mattiske, Carla Smerdon, David Elliott, Julie McGaughran, Janine Smith
Footnotes
The Article Publishing Charge (APC) for this article was paid by the Centenary Institute.
Please note a full list of Flagship members’ names are included at the end of this document.
Additional Information
The online version of this article (https://doi.org/10.1016/j.gimo.2024.101842) contains supplemental material, which is available to authorized users.
Contributor Information
Chris Semsarian, Email: c.semsarian@centenary.org.au.
Julie McGaughran, Email: julie.mcgaughran@health.qld.gov.au.
Australian Genomics Cardiovascular Disorders Flagship:
Lesley Ades, Annabel Enriquez, Alison McLean, Renee Smyth, Dimithu Alankarage, Diane Fatkin, James McNamara, Magdalena Soka, Morgan almog, Vanessa Fear, Caroline Medi, Zornitza Stark, Mohammad Al-Shinnag, Miriam Fine, Alejandro Metke, Raymond Sy, John J. Atherton, Keri Finlay, Di Milnes, Dotti Tang, Rachel Austin, Denisse Garza, Michael Milward, Jessica Taylor, Richard D. Bagnall, Eleni Giannoulatou, Ansley Morrish, Shelby Taylor, Chris Barnett, Laura Gongolidis, Jim Morwood, Michel Tchan, Gillian M. Blue, Belinda Gray, Helen Mountain, Tina Thompson, Simon Bodek, Cassie Greer, David Mowat, Jordan Thorpe, Kirsten Boggs, Eric Haan, Chai-Ann Ng, Alison Trainer, Michael Bogwitz, Mathilda Haas, Natalie Nowak, Gunjan Trivedi, Tiffany Boughtwood, Bernadette Hanna, Noelia Nunez Martinez, Giulia Valente, Alessandra Bray, Richard Harvey, Monique Ohanian, Karin van Spaendonck-Zwarts, Marie-Jo Brion, Janette Hayward, Sinead O’Sullivan, Jamie Vandenberg, Jaye Brown, Carmen Herrera, Angela Overkov, Kunal Verma, Rob Bryson Richardson, Adam Hill, Nicholas Pachter, Miranda Vidgen, Leslie Burnett, Georgie Hollingsworth, Chirag Patel, Jitendra Vohra, Charlotte Burns, Georgina Hollway, Mark Perrin, Kathryn Waddel-Smith, Michelle Cao, Ari E. Horton, Matthew Perry, Mathew Wallis, Will Carr, Denise Howting, Andreas Pflaumer, Robert G. Weintraub, Sarah Casauria, Jodie Ingles, Peta Phillips, Meredith Wilson, Heather Chalinor, Joanne Isbister, Thuan Phuong, David Winlaw, Yuchen Chang, Matilda Jackson, Rachel Pope-Couston, Lisa Worgan, Gavin Chapman, Paul James, Nicola K. Poplawski, Linda Wornham, Theosodia Charitou, Sarah Jane-Pantaleo, Preeti Punni, Kathy Wu, Belinda Chong, Renee Johnson, Michael C.J. Quinn, Laura Yeates, Felicity Collins, Andrew Kelly, Michael Quinn, Dominica Zentner, Gemma Correnti, Sarah King-Smith, Sulekha Rajagopalan, Kathy Cox, Edwin Kirk, Hariharan Raju, Fiona Cunningham, Sarah Kummerfeld, Emma M. Rath, Debjani Das, Timo Lassman, Matthew Regan, Jason Davis, Jonathon Lipton, Jonathan Rogers, Andrew Davis, Sebastian Lunke, Mark Ryan, Paul De Fazio, Ivan Macciocca, Sarah Sandaradura, Michelle de Silva, Paul MacIntyre, Nicole Schonrock, Nicola Den Elzen, Evanthia O. Madelli, Paul Scuffham, Sophie Devery, Amali Mallawaarachchi, Chris Semsarian, Julia Dobbins, Julia Mansour, Isabella Sherburn, Sally L. Dunwoodie, Ellenore Martin, Mary-Clare Sherlock, Nathan Dwyer, Jacob Mathew, Emma Singer, Stefanie Elbracht-Leong, Tessa Mattiske, Carla Smerdon, David Elliott, Julie McGaughran, and Janine Smith
Additional Information
Flow diagram of analysis plan for the sub-study - Effectiveness of a Standardized Cardiac Genetic Testing Approach in Australia.
Excel document titled ‘Supplementary tables 02022024’ contains supplementary tables 1-7.
References
- 1.Girolami F., Frisso G., Benelli M., et al. Contemporary genetic testing in inherited cardiac disease: tools, ethical issues, and clinical applications. J Cardiovasc Med (Hagerstown) 2018;19(1):1–11. doi: 10.2459/JCM.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isbister J., Semsarian C. Cardiovascular genomics and sudden cardiac death in the young. Aust J Gen Pract. 2019;48(3):90–95. doi: 10.31128/AJGP-09-18-4715. [DOI] [PubMed] [Google Scholar]
- 3.Pierpont M.E., Brueckner M., Chung W.K., et al. Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation. 2018;138(21):e653–e711. doi: 10.1161/CIR.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giudicessi J.R., Ackerman M.J., Fatkin D., Kovacic J.C. Precision medicine approaches to cardiac arrhythmias: JACC focus seminar 4/5. J Am Coll Cardiol. 2021;77(20):2573–2591. doi: 10.1016/j.jacc.2021.03.325. [DOI] [PubMed] [Google Scholar]
- 5.Fatkin D., Calkins H., Elliott P., James C.A., Peters S., Kovacic J.C. Contemporary and future approaches to precision medicine in inherited cardiomyopathies: JACC focus seminar 3/5. J Am Coll Cardiol. 2021;77(20):2551–2572. doi: 10.1016/j.jacc.2020.12.072. [DOI] [PubMed] [Google Scholar]
- 6.Alfares A.A., Kelly M.A., McDermott G., et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet Med. 2015;17(11):880–888. doi: 10.1038/gim.2014.205. [DOI] [PubMed] [Google Scholar]
- 7.Gal D.B., Morales A., Rojahn S., et al. Comprehensive genetic testing for pediatric hypertrophic cardiomyopathy reveals clinical management opportunities and syndromic conditions. Pediatr Cardiol. 2022;43(3):616–623. doi: 10.1007/s00246-021-02764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofman N., Tan H.L., Alders M., et al. Yield of molecular and clinical testing for arrhythmia syndromes: report of 15 years’ experience. Circulation. 2013;128(14):1513–1521. doi: 10.1161/CIRCULATIONAHA.112.000091. [DOI] [PubMed] [Google Scholar]
- 9.Dellefave-Castillo L.M., Cirino A.L., Callis T.E., et al. Assessment of the diagnostic yield of combined cardiomyopathy and arrhythmia genetic testing. JAMA Cardiol. 2022;7(9):966–974. doi: 10.1001/jamacardio.2022.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giudicessi J.R., Kullo I.J., Ackerman M.J. Precision cardiovascular medicine: state of genetic testing. Mayo Clin Proc. 2017;92(4):642–662. doi: 10.1016/j.mayocp.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagnall R.D., Ingles J., Dinger M.E., et al. Whole genome sequencing improves outcomes of genetic testing in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2018;72(4):419–429. doi: 10.1016/j.jacc.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 12.Alankarage D., Ip E., Szot J.O., et al. Identification of clinically actionable variants from genome sequencing of families with congenital heart disease. Genet Med. 2019;21(5):1111–1120. doi: 10.1038/s41436-018-0296-x. [DOI] [PubMed] [Google Scholar]
- 13.Cirino A.L., Harris S., Lakdawala N.K., et al. Role of genetic testing in inherited cardiovascular disease: a review. JAMA Cardiol. 2017;2(10):1153–1160. doi: 10.1001/jamacardio.2017.2352. [DOI] [PubMed] [Google Scholar]
- 14.Minoche A.E., Horvat C., Johnson R., et al. Genome sequencing as a first-line genetic test in familial dilated cardiomyopathy. Genet Med. 2019;21(3):650–662. doi: 10.1038/s41436-018-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton A.E., Lunke S., Sadedin S., Fennell A.P., Stark Z. Elusive variants in autosomal recessive disease: how can we improve timely diagnosis? Eur J Hum Genet. 2023;31(4):371–374. doi: 10.1038/s41431-023-01293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark Z., Boughtwood T., Phillips P., et al. Australian genomics: a federated model for integrating genomics into healthcare. Am J Hum Genet. 2019;105(1):7–14. doi: 10.1016/j.ajhg.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark Z., Boughtwood T., Haas M., et al. Australian Genomics: outcomes of a 5-year national program to accelerate the integration of genomics in healthcare. Am J Hum Genet. 2023;110(3):419–426. doi: 10.1016/j.ajhg.2023.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin R., Quinn M.C.J., Afoakwah C., et al. Investigation of current models of care for genetic heart disease in Australia: a national clinical audit. Int J Cardiol. 2021;330:128–134. doi: 10.1016/j.ijcard.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Blue G.M., Smith J., Sholler G.F., Semsarian C., Winlaw D.S., Australian Genomics Cardiovascular Genetic Disorders Flagship Current Practice of Genetic Testing and Counselling in Congenital Heart Disease: An Australian Perspective. Heart Lung Circ. 2020;29(11):1733–1736. doi: 10.1016/j.hlc.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Authors/Task Force members. Elliott P.M., Anastasakis A., et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(39):2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 21.Marcus F.I., McKenna W.J., Sherrill D., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31(7):806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz P.J., Crotti L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124(20):2181–2184. doi: 10.1161/CIRCULATIONAHA.111.062182. [DOI] [PubMed] [Google Scholar]
- 24.Haas M.A., Teare H., Prictor M., et al. ‘CTRL’: an online, Dynamic Consent and participant engagement platform working towards solving the complexities of consent in genomic research. Eur J Hum Genet. 2021;29(4):687–698. doi: 10.1038/s41431-020-00782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tudini E., Andrews J., Lawrence D.M., et al. Shariant platform: enabling evidence sharing across Australian clinical genetic-testing laboratories to support variant interpretation. Am J Hum Genet. 2022;109(11):1960–1973. doi: 10.1016/j.ajhg.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin E.L., Nees S.N., Morton S.U., et al. Evidence-based assessment of congenital heart disease genes to enable returning results in a genomic study. Circ Genom Precis Med. 2023;16(2) doi: 10.1161/CIRCGEN.122.003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shikany A.R., Landis B.J., Parrott A., et al. A comprehensive clinical genetics approach to critical congenital heart disease in infancy. J Pediatr. 2020;227:231–238.e14. doi: 10.1016/j.jpeds.2020.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mone F., Eberhardt R.Y., Morris R.K., et al. COngenital heart disease and the diagnostic yield with exome sequencing (CODE) study: prospective cohort study and systematic review. Ultrasound Obstet Gynecol. 2021;57(1):43–51. doi: 10.1002/uog.22072. [DOI] [PubMed] [Google Scholar]
- 32.Wilde A.A.M., Semsarian C., Márquez M.F., et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Heart Rhythm. 2022;19(7):e1–e60. doi: 10.1016/j.hrthm.2022.03.1225. [DOI] [PubMed] [Google Scholar]
- 33.Chaix M.A., Andelfinger G., Khairy P. Genetic testing in congenital heart disease: a clinical approach. World J Cardiol. 2016;8(2):180–191. doi: 10.4330/wjc.v8.i2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrish A.M., Smith J., Enriquez A., et al. A new era of genetic testing in congenital heart disease: a review. Trends Cardiovasc Med. 2022;32(5):311–319. doi: 10.1016/j.tcm.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Rehm H.L., Berg J.S., Brooks L.D., et al. ClinGen--the clinical genome resource. N Engl J Med. 2015;372(23):2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen K.D., Vassy J.L., Phillips K.A., et al. Short-term costs of integrating whole-genome sequencing into primary care and cardiology settings: a pilot randomized trial. Genet Med. 2018;20(12):1544–1553. doi: 10.1038/gim.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of analysis plan for the sub-study - Effectiveness of a Standardized Cardiac Genetic Testing Approach in Australia.
Excel document titled ‘Supplementary tables 02022024’ contains supplementary tables 1-7.
Data Availability Statement
Study data sets have not been deposited in a public repository because of consent restrictions. Deidentified data from this study are available for ethically approved research. The online access application process is administered by the Australian Genomics Data Access Committee.