Abstract
Background
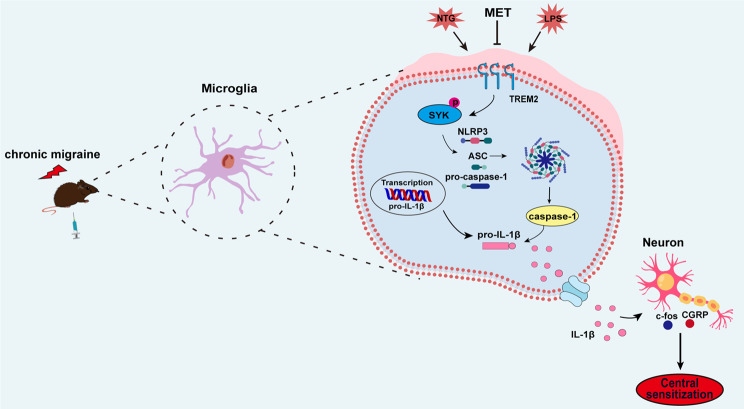
Chronic migraine (CM) is a serious neurological disorder. Central sensitization is one of the important pathophysiological mechanisms underlying CM, and microglia-induced neuroinflammation conduces to central sensitization. Triggering receptor expressed on myeloid cells 2 (TREM2) is presented solely in microglia residing within the central nervous system and plays a key role in neuroinflammation. Metformin has been shown to regulate inflammatory responses and exert analgesic effects, but its relationship with CM remains unclear. In the study, we investigated whether metformin modulates TREM2 to improve central sensitization of CM and clarified the potential molecular mechanisms.
Methods
A CM mouse model was induced by administration of nitroglycerin (NTG). Behavioral evaluations were conducted using von Frey filaments and hot plate experiments. Western blot and immunofluorescence techniques were employed to investigate the molecular mechanisms. Metformin and the SYK inhibitor R406 were administered to mice to assess their regulatory effects on neuroinflammation and central sensitization. To explore the role of TREM2-SYK in regulating neuroinflammation with metformin, a lentivirus encoding TREM2 was injected into the trigeminal nucleus caudalis (TNC). In vitro experiments were conducted to evaluate the regulation of TREM2-SYK by metformin, involving interventions with LPS, metformin, R406, siTREM2, and TREM2 plasmids.
Results
Metformin and R406 pretreatment can effectively improve hyperalgesia in CM mice. Both metformin and R406 significantly inhibit c-fos and CGRP expression in CM mice, effectively suppressing the activation of microglia and NLRP3 inflammasome induced by NTG. With the administration of NTG, TREM2 expression gradually increased in TNC microglia. Additionally, we observed that metformin significantly inhibits TREM2 and SYK expression in CM mice. Lv-TREM2 attenuated metformin-mediated anti-inflammatory responses. In vitro experiments, knockdown of TREM2 inhibited LPS-induced SYK pathway activation and alleviated inflammatory responses. After the sole overexpression of TREM2, the SYK signaling pathway is activated, resulting in the activation of the NLRP3 inflammasome and an increased expression of pro-inflammatory cytokines; nevertheless, this consequence can be reversed by R406. The overexpression of TREM2 attenuates the inhibition of SYK activity mediated by metformin, and this effect can be reversed by R406.
Conclusions
Our findings suggest that metformin attenuates central sensitization in CM by regulating the activation of microglia and NLRP3 inflammasome through the TREM2-SYK pathway.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-024-03313-2.
Keywords: Metformin, Microglia, TREM2, Chronic migraine, SYK, Central sensitization
Background
Chronic migraine (CM) is characterized as a multifaceted neurological condition that elicits pain and functional impairment [1, 2], leading to significant disability in approximately 25% of individuals affected [3]. The management of CM poses enormous challenges for neurologists due to the frequency of headache attacks, suboptimal treatment response, and limited pharmacological options available [4]. Repeated episodes of CM within a short period can lead to physical, mental, and social impairments for patients [5], imposing substantial spiritual and economic burdens on individuals as well as society. Hence, it is urgent to unveil the exact pathogenesis of CM and seek more efficient targets for interventions.
The key pathogenesis responsible for CM is the occurrence of central sensitization within the trigeminal nucleus caudalis (TNC) [6]. Central sensitization is a pathophysiological process during which the central nervous system (CNS) undergoes structural, functional, and chemical changes that render it more sensitive to stimuli [7]. Research findings have revealed that microglia activation in the TNC region releases a large number of pro-inflammatory factors and neurotrophic factors, which enhance neuronal excitability and promote central sensitization of CM through microglia-neuron interaction [8, 9]. The microglial inhibitor minocycline can significantly improve migraines in CM mice. Therefore, microglia may be considered as a potential future target for CM.
Metformin, as the first-line and long-term agent for managing type 2 diabetes, possesses various properties such as autophagy induction, anti-inflammatory effects, anticancer activity, anti-aging benefits, and antioxidant capabilities. Evidence suggests that metformin effectively improves pain chronicity [10]. Recent research has demonstrated that metformin can facilitate microglia polarization from M1 to M2 phenotype, thereby improving cognitive function in aged animals [11]. Moreover, metformin reduces the number of activated microglia following a stroke and exerts an anti-inflammatory impact [12]. However, it remains unclear whether metformin affects CM and to what extent this effect relies on neuroinflammatory mechanisms involving microglia.
In recent years, the expression of TREM2 has been discovered in microglia situated within the CNS [13]. The TREM2-mediated microglial signaling exerts a neuroprotective function by suppressing neuroinflammation [14], while it may also aggravate neuroinflammatory injury [15]. It is evident that the function of TREM2 remains controversial in various disease models, and its role in CM has yet to be explored. Metformin is known for its ability to modulate the expression of TREM2 in microglia [16]. Spleen tyrosine kinase (SYK) regulates microglia activation and inflammation [17, 18]. TREM2 binds to its signaling partner, the adaptor DNAX activating protein 12 (DAP-12), to regulate SYK signaling [19]. SYK activation can regulate the activation of the downstream NLRP3 inflammasome [20]. In addition, NLRP3 activation has been identified as a contributing factor in the advancement of central sensitization among individuals suffering from CM [21].
Based on these discoveries, we formulated a hypothesis that metformin attenuates central sensitization by regulating TNC microglia and NLRP3 inflammasome activation through the TREM2-SYK signaling cascade. We validated these findings at both in vivo and in vitro levels using lentiviruses, small interfering RNAs, and overexpression plasmids. Metformin possesses the potential to offer a novel strategy for addressing CM, with TREM2 serving as a promising therapeutic target.
Methods
Animals
The 160 male mice (C57BL/6J, 20–25 g, 8 weeks old) were procured from the Experimental Animal Center of Lanzhou University (Lanzhou, China). All procedures conformed to the National Institutes of Health guidelines and were approved by the local animal ethics committee. All mice were maintained in specific pathogen-free (SPF) environment on a 12 h/12 h light/dark schedule with unimpeded availability of nourishment and hydration.
Mouse model of chronic migraine
A mouse CM model was established based on previous reliable literature [22, 23]. NTG (Beijing Regent, China) was formulated using a solution with a stock concentration of 5.0 mg/mL from a mixture of 30% propylene glycol, 30% ethanol, and water. Prior to injecting, NTG was freshly prepared the day of use at 1 mg/ml utilizing 0.9% saline and administered intraperitoneally instantly at 10 mg/kg once alternate days for 5 consecutive injections (i.e., days 1, 3, 5, 7, and 9). In the vehicle group, mice were administered equal volumes of solvent at corresponding time points. When investigating the time-dependent alterations in TREM2 subsequent to NTG administration, TNC tissue samples were collected at 6 h post-NTG injection on days 1, 3, 5, 7, and 9. For c-fos analysis, samples were obtained at 2 h after NTG injection on day 9. Samples for other indicators were collected at the same time point (6 h) following NTG injection on day 9.
Drug administration
To illuminate the potential importance of metformin in CM, mice received intraperitoneal injections of metformin (100 mg/kg) (Sigma-Aldrich, USA). To elucidate the possible involvement of SYK in CM, mice received intraperitoneal administration of R406 (5 mg/kg) (MCE, USA), a SYK antagonist. Metformin and R406 were given 30 min before each NTG or solvent injection, with a frequency of alternate days for a total of five administrations. Metformin was diluted in 0.9% saline while R406 underwent dissolution within 10% DMSO, 40% PEG300, 5% Tween-80 and 45% saline. The control group received a comparable volume of 0.9% saline. The dosages of metformin and R406 were determined based on previous literature [18, 24].
A bilateral injection of 0.5 µl lentivirus encoding TREM2 (BrainVTA, Wuhan) was administered into the TNC (AP: -8.0 mm; ML: 1.5 mm; DV: 4.5 mm) [25] utilizing a 33-gauge needle, the substance was performed at 0.1 µl/min. Subsequently, the needle was left undisturbed for a duration of 10 min prior to its gradual extraction.
Culture and treatment of primary microglia
Preparation of primary microglia has been previously described [26, 27]. All experimental procedures were ethically approved and strictly adhering to the Guidelines for Laboratory Animals. Briefly, adult male C57BL/6J mice were anesthetized, subjected to cervical dislocation to isolate the TNC. After removing the meninges, the TNC underwent fragmentation and was submerged in phosphate buffered saline (PBS, pH 7.4). The tissue was subsequently dissociated using 0.25% trypsin at 37 °C for approximately 10–15 min, and then incubated with EDTA and 100 µg/ml DNase I (Roche). Subsequently, the tissue underwent a gentle grinding procedure and was then filtered through a custom-made nylon mesh specifically designed with a diameter of 50 μm. The cells were suspended in DMEM supplemented with 10% FBS and then seeded onto flasks coated with poly-L-lysine. Microglia were detached using gentle oscillation cycles over 10–14 days before being transferred to appropriate culture plates.
Cell transfection and treatment
To perform TREM2 knockdown experiments, two siRNA sequences were formulated: their specific oligonucleotide sequences were provided below: siRNA-1 (GACTTCTGTTTCTGCTACT) and siRNA-2 (CTGTCAACTTCTGCACTTT). A negative control siRNA was included for experimental control. The siRNA exhibiting the utmost efficacy was chosen for subsequent experiments after evaluating its knockdown efficiency using Western blot. We synthesized pcDNA3.1-TREM2 overexpression plasmid (oe-TREM2) and pcDNA3.1-NC vector (oe-NC), followed by Western blot analysis for TREM2 overexpression. The RNA interference and TREM2 plasmid were constructed by Sangon Biotechnology (Shanghai, China). For transfection, Lipofectamine® 3000 (Invitrogen) was employed as the transfection reagent. To determine the optimal concentration of LPS, we established four concentration gradients (0, 1, 5, and 10 µg/ml). siTREM2 or TREM2 transfection was performed 24 h prior to LPS treatment. Subsequently, cells were incubated with metformin (2 mM) or R406 (1 mM) for a duration of 2 h followed by a 24-hour LPS treatment period before sample collection. Each experiment was independently repeated at least six times.
Behavioral assessment
All animal behavioral examinations were conducted from 9:00 to 15:00, with mice undergoing 3 days of behavioral training prior to the experiment. The experimenter remained blinded to both group assignments and the analysis of behavioral data. Despite NTG being a vasodilator, no significant antihypertensive effect was observed throughout the experiment. Similarly, although metformin is a hypoglycemic agent, no clear indications of hypoglycemia were detected during the course of the experiment.
Severe headache is a hallmark feature of migraine, while unusual skin discomfort suggests the presence of central sensitization. In order to replicate the manifestations of migraine, we assessed mechanical hindpaw and head-specific (periorbital) pain thresholds, alongside measuring thermal withdrawal latency. Prior to the experiment, mice were acclimated for half an hour in a quiet acrylic cage with a wire mesh flooring. Pain thresholds for mechanical and thermal stimuli were assessed prior to and within a 2-h timeframe following each administration. To measure the mechanical threshold of hind paws, a specially designed glass enclosure with an upper opening was utilized. Von Frey filaments (0.008–2 g) were employed to vertically stimulate the central region of their hind paw on the corresponding side. A positive response was characterized by paw withdrawal, trembling, or paw licking subsequent to stimulation. To assess periorbital mechanical sensitivity, mice were confined in a enclosure with only their head exposed to prevent movement. The von Frey monofilament was applied in an upright manner to elicit stimulation on the periorbital region, including the midline vicinity. A favorable reaction was defined as an immediate withdrawal of the head upon being exposed to stimulation or receiving forelimb facial scratching on the corresponding side.
For thermal sensitivity evaluation, mice were acclimated on a hot plate apparatus prior to testing. Upon activation of the heat source, mice exhibited responses such as paw raising, foot licking, or shaking, which were considered positive reactions. Three repetitions were performed for each experiment, and the final assessment was determined by calculating the mean outcome.
Western blot analysis
TNC tissue was obtained 2 h post-drug administration to identify c-fos expression, while for other targets it was extended to 6 h subsequent to drug injection. The mice were given a 5% pentobarbital solution to induce deep anesthesia, after which the TNC was swiftly extracted and kept frozen at a temperature of -80 °C. The tissue samples underwent lysis using RIPA lysis buffer under refrigeration at 4 °C for a duration of 1 h. Subsequently, centrifugation was performed at the same temperature and speed of 12,000×g for a period of 15 min. The BCA Protein Assay kit (Beyotime, China) was utilized to determine supernatant protein concentration. An equal quantity of protein (40 µg) was applied onto SDS-polyacrylamide gels for electrophoresis, followed by subsequent transfer onto NC membranes. The membranes were obstructed using 5% skim milk under typical ambient temperature conditions, enduring for a period of 1 h. Afterwards, they were exposed to their corresponding primary antibodies (Table 1) and left to incubate overnight at 4 °C. After completing the washing procedure, incubation was carried out with secondary antibodies that were linked to horseradish peroxidase (Table 1). Immunoreactive bands were revealed by the ECL kit (Thermo Fisher Scientific, USA), visualized and analyzed using an imaging system (Bio-Rad, USA). To ensure consistency in the levels of target proteins across various groups, β-actin was utilized as a reference for normalization purposes.
Table 1.
Antibody used in Western blotting and immunofluorescence staining
| Antibody | Manufacturer | Catalog number | Host | Dilution |
|---|---|---|---|---|
| For western blot | ||||
| TREM2 | Abcam, UK | ab305103 | Rabbit | 1:1000 |
| c-fos | Santa Cruz, USA | sc166940 | Mouse | 1:500 |
| CGRP | Abcam, UK | ab139264 | Rabbit | 1:1000 |
| SYK | CST, USA | 2712 | Rabbit | 1:1000 |
| p-SYK | CST, USA | 2710 | Rabbit | 1:1000 |
| NLRP3 | CST, USA | 15,101 | Rabbit | 1:1000 |
| ASC | CST, USA | 67,824 | Rabbit | 1:1000 |
| Cleaved-Caspase-1 | Proteintech, China | 22915-1-AP | Rabbit | 1:2000 |
| Mature IL-1β | Abcam, UK | ab234437 | Rabbit | 1:1000 |
| β-actin | Santa Cruz, USA | sc-47,778 | Mouse | 1:1000 |
| HRP conjugated anti-rabbit | Proteintech, China | SA00001-2 | Goat | 1:100000 |
| HRP conjugated anti-mouse | Proteintech, China | SA00001-1 | Goat | 1:100000 |
| For immunofluorescence | ||||
| TREM2 | Abcam, UK | ab305103 | Rabbit | 1:1000 |
| CGRP | Santa Cruz, USA | sc-57,053 | Mouse | 1:100 |
| Iba1 | Abcam, UK | ab5076 | Goat | 1:500 |
| Iba1 | Abcam, UK | ab178846 | Rabbit | 1:500 |
| GFAP | Santa Cruz, USA | sc-33,673 | Mouse | 1;200 |
| NeuN | Abcam, UK | ab104224 | Mouse | 1:1000 |
| Alexa Fluor 488 Donkey anti-mouse IgG | Jackson ImmunoResearch, USA | 715-545-150 | Donkey | 1:200 |
| Alexa Fluor 594 Donkey anti-rabbit IgG | Jackson ImmunoResearch, USA | 711-585-152 | Donkey | 1:500 |
| Alexa Fluor 488 Donkey anti-goat IgG | Jackson ImmunoResearch, USA | 705-545-147 | Donkey | 1:200 |
Immunofluorescence staining
After anesthesia, mice were transcardially perfused with pre-cooled PBS (pH 7.4), and then 4% pre-cooled paraformaldehyde (PFA). Whole brains were extracted and fixed in 4% PFA for 6 h at a temperature of 4 °C, followed by gradient dehydration using PBS solutions infused with sucrose (20% and 30%). Subsequently, the tissue underwent embedding, freezing, and subsequent coronal slicing into sections with a thickness of 10 μm using a cryostat (Leica, Japan). These sections were subsequently subjected to a time-limited restriction of 1 h under normal environmental temperature conditions utilizing a solution comprising 5% BSA. Following this, the samples underwent an overnight incubation at 4 °C with primary antibodies that corresponded to those mentioned in (Table 1). After a PBS wash, the sections underwent incubation using the appropriate fluorescent secondary antibody (Table 1) under ambient conditions for 1 h and then stained with DAPI at room temperature for a duration of 10 min. The confocal imaging microscope (Nikon AXR, Japan) was used to acquire the images. The TNC regions were determined by referencing the mouse brain atlas, and quantitative immunofluorescence analysis was carried out using ImageJ software (version: 2.3.0, USA). The CGRP-positive region was quantified to analyze the immunoreactivity exhibited by CGRP. To measure amount of Iba1+ microglia, a 193.75 × 193.75 μm^2 square image centered on the TNC surface layer was captured, all cells showing immunoreactivity were enumerated within this area. Morphological features of microglia were further facilitated using the Neuron J plug-in to measure the overall length of microglial processes and the average dimensions of their extensions originating from the cellular soma, with data expressed as mean ± SEM. Three sections were taken from each mouse, encompassing 4–6 visual perspectives within each section (n = 5). The average values of the left and right portions of every slice were computed for further statistical examination.
Statistical analysis
Prism 9.5.1 software (GraphPad Software Inc., San Diego, CA, USA) was utilized for conducting statistical analysis. Mean ± SEM are used to present the data. Pairwise comparisons were performed by conducting one-way ANOVA, followed by the implementation of post-hoc Tukey’s test. To facilitate comparisons between multiple groups, we employed a two-way ANOVA and performed post-hoc Tukey’s test. Behavioral results were analyzed using a two-way repeated-measures ANOVA with a Bonferroni post hoc test. A p-value < 0.05 was chosen to determine statistical significance.
Results
Metformin attenuates NTG-induced central sensitization
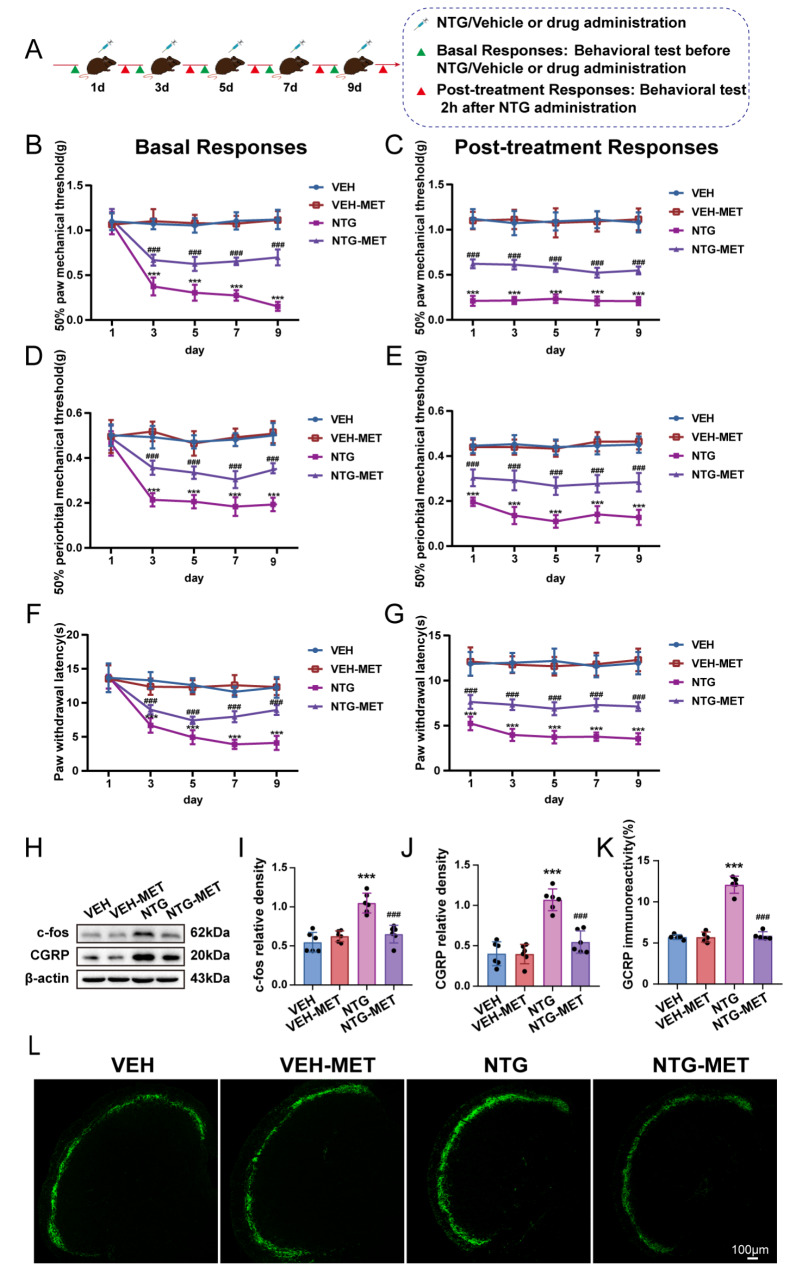
Central sensitization is a crucial pathophysiological mechanism of CM. CM patients frequently present with hyperalgesia. c-fos and CGRP are employed as markers for central sensitization. We assessed the impact of metformin on central sensitization in CM. Behavioral data showed that basal and acute thresholds for mechanical and thermal pain were considerably upregulated in NTG-MET mice contrasted with NTG mice, while pain thresholds did not differ significantly between VEH and VEH-MET mice (Fig. 1b-g). The Western blot analysis disclosed a substantial decrease in the levels of c-fos and CGRP protein in the TNC region of CM mice upon treatment with metformin (Fig. 1h-j). Immunofluorescence staining demonstrated that pretreatment with metformin significantly reduced the immunoreactivity of CGRP in comparison with the NTG group (Fig. 1k, l). These findings present convincing evidence supporting the significant involvement of metformin in regulating central sensitization in CM.
Fig. 1.
Metformin significantly attenuates NTG-induced central sensitization. (a) Schematic timeline for drug treatment and behavioral assessment. Metformin efficaciously inhibited the reduction of the mechanical thresholds of both basal and post-treatment in hind paw plantar (b, c) and periorbital regions (d, e), while also prolonging the latency for withdrawal to heat (f, g) (n = 10); two-way RM ANOVA with Bonferroni post hoc test. (h-j) Assessment of c-fos and CGRP using immunoblotting technique (n = 6). (k, l) The intensity of immunoreactivity against CGRP (n = 5). Scale bars = 100 μm; Data are depicted as mean ± SEM; two-way ANOVA with post-hoc Tukey’s test; ***p < 0.001 compared with the VEH group; ###p < 0.001 compared with the NTG group
Metformin regulates TNC microglial activation and inflammatory responses following NTG administration
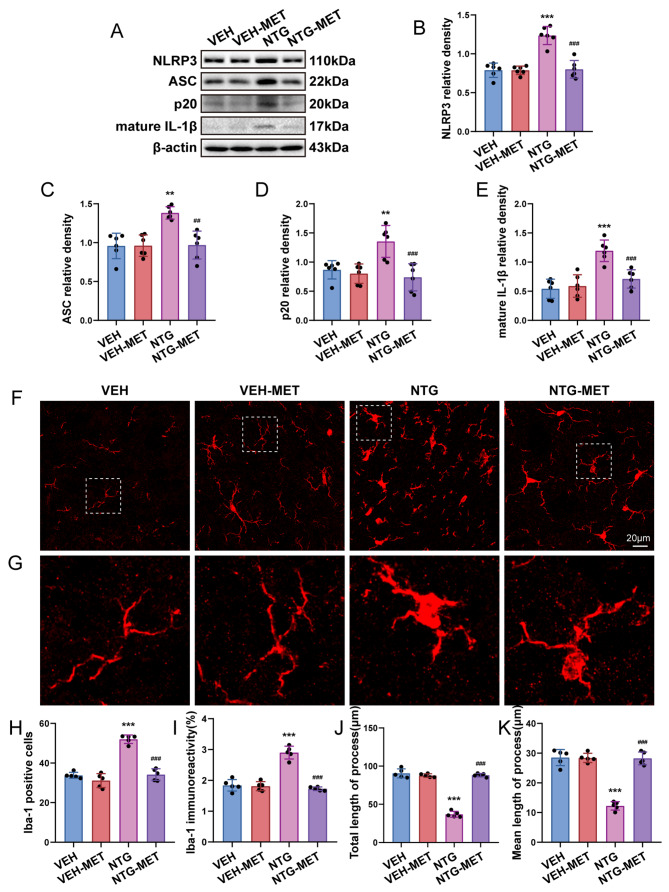
Microglia-mediated neuroinflammation is widely acknowledged as a significant contributor to central sensitization of CM. To elucidate the regulatory effect of metformin on microglia, we assessed the levels of NLRP3 inflammasome-related factors and mature IL-1β expression through Western blot. Additionally, we examined the cell number, immunoreactivity and morphological alterations of microglia using immunofluorescence techniques. The results suggested that the administration of metformin leads to a significant reduction in NLRP3 expression, as well as p20, ASC, and mature IL-1β, when contrasted to NTG group (Fig. 2a-e). Additionally, quantitative analysis of immunofluorescence staining revealed a rise in both the number and immunoreactivity of Iba1+ microglia following NTG administration. Morphologically, there was observed enlargement in the cell body of microglia, accompanied by a reduction in both total initial process length and average initial process length per cell (Fig. 2f-k). Notably, metformin effectively diminished the number of Iba1+ microglia, reduced immunoreactivity, and mitigated the morphological alterations of microglia induced by NTG. Our collective findings indicate that metformin effectively suppresses microglia activation and mitigates inflammation in the TNC in CM mice.
Fig. 2.
Metformin inhibits TNC microglial activation and mitigates inflammatory responses following NTG application. a-e Immunobloting detection of NLRP3, ASC, p20 and mature IL-1β subsequent to NTG and metformin management (n = 6). f Images depicting Iba1 immunostaining treated with NTG and metformin. g The enlarged view of Iba-1 aligns with the area framed with a dashed white line in f. h–k Immunofluorescent analysis of Iba-1+ microglia, Iba1 immunostaining, the overall length of processes as well as the average length of initial processes for each microglia (n = 5). Analysis was carried out on three mouse samples, each consisting of 4–6 FOVs. Scale bar = 20 μm. Data are depicted as mean ± SEM; two-way ANOVA with post-hoc Tukey’s test; **p < 0.01, ***p < 0.001 compared with the VEH group; ##p < 0.001, ###p < 0.001 compared with the NTG group
TREM2 expression in microglia was significantly upregulated after repeated NTG administration
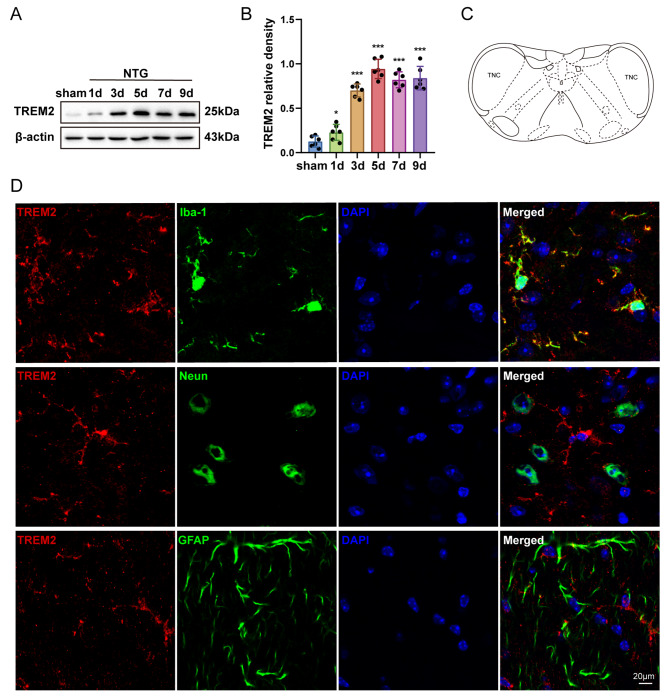
To explore the mechanistic involvement of TREM2 in CM, we examined alterations in TREM2 protein levels throughout the advancement of NTG administration. Utilizing the Western blot method, a progressive increase in TREM2 expression was observed over a period of time, when compared to the sham group. Furthermore, TREM2 expression remained consistently high throughout the duration of NTG administration (Fig. 3a-b). To elucidate the cellular distribution of TREM2, double immunofluorescence labeling was performed to co-localize TREM2 with Iba1, NeuN, and GFAP. The results revealed that TREM2 is exclusively localized in microglia rather than neurons and astrocytes within the TNC of CM mice (Fig. 3d). Consequently, it can be inferred that repeated administration of NTG leads to considerable enhancement of TREM2 expression specifically in TNC microglia.
Fig. 3.
TREM2 expression is notably increased in TNC microglia subsequent to repetitive NTG injections. a, b Immunoblot examination of TREM2 at specified intervals after NTG treatment (n = 6). c Schematic diagram of the TNC. d Co-localization of TREM2 with microglia (Iba1), neurons (NeuN), and astrocytes (GFAP) in the TNC. Scale bar = 20 μm. Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test; *p < 0.05 and ***p < 0.001 compared with the sham group
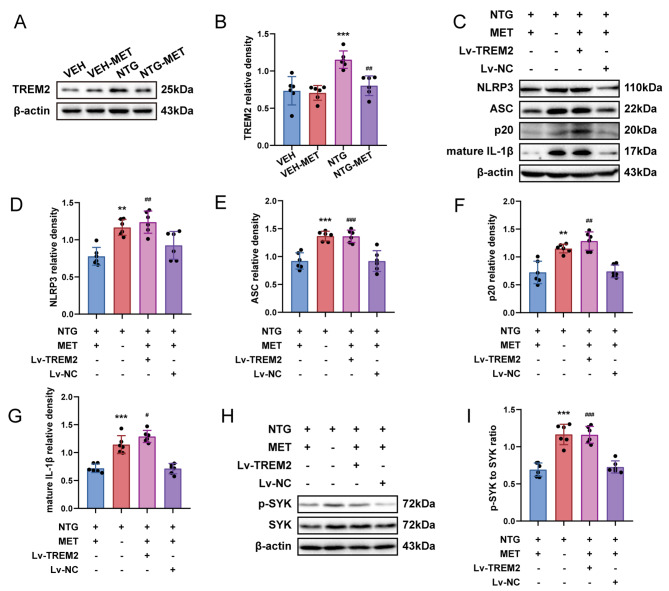
Metformin regulates the inflammatory response in the TNC of CM mice via TREM2
We investigated the mechanistic role of metformin involvement in CM. Western blot analysis uncovered an apparent decrease in TREM2 expression at the site of TNC following metformin administration in contrast to the NTG group, indicating the effectiveness of metformin in regulating TREM2 expression in the TNC of CM mice (Fig. 4a, b). Additionally, the overexpression of TREM2 in the TNC significantly attenuated the anti-inflammatory efficacy of metformin in comparison to NTG-MET mice (Fig. 4c-g, Fig. S1a). Metformin significantly inhibited the NTG-induced enhancement of SYK activity, which could be reversed by overexpression of TREM2 (Fig. 4h, i). These results indicate that metformin regulates the inflammatory response via TREM2, which might be accomplished through SYK.
Fig. 4.
Metformin modulates the inflammatory response in the TNC of CM mice via TREM2. a, b Immunoblot analysis of TREM2 (n = 6); two-way ANOVA with post-hoc Tukey’s test. c-g Immunoblot detection of NLRP3, ASC, p20, and mature IL-1β. h, i Immunoblot identification of the ratio of p-SYK/SYK in the designated groups (n = 6). Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test; a ***p < 0.001 compared with the VEH group; ##p < 0.01 compared with the NTG group. c-i **p < 0.01, ***p < 0.001 compared with the NTG-MET group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the NTG-MET-Lv-NC group
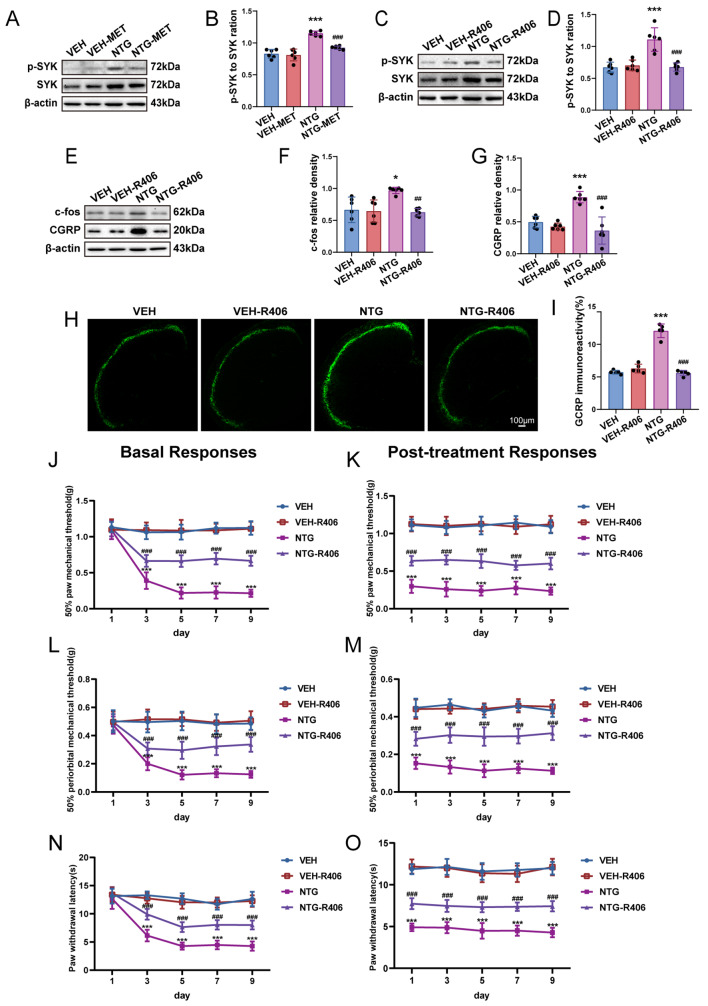
SYK mediates the regulation of central sensitization induced by NTG
We investigated the role of the SYK pathway in central sensitization of CM. Western blot analysis showed an evident elevation of the phosphorylated SYK (p-SYK) to total SYK ratio following repeated NTG administration, indicating activation of the SYK pathway, which was significantly attenuated by pretreatment with metformin (Fig. 5a, b). R406 manifestly suppressed the p-SYK to total SYK ratio compared with NTG group (Fig. 5c, d), resulting in reduced c-fos and CGRP expression (Fig. 5e-g). Furthermore, immunofluorescence analysis revealed that R406 notably reduced CGRP immunoreactivity in comparison to the NTG group (Fig. 5h, i). Behavioral results demonstrated that R406 patently augmented basal and acute pain thresholds in terms of mechanical and thermal stimuli in contrast to the NTG group (Fig. 5j-o). Drawing from the findings acquired, it can be inferred that SYK plays a crucial function in governing central sensitization in CM-affected mice.
Fig. 5.
Inhibition of SYK significantly alleviates the central sensitization induced by NTG. a-d Immunoblot identification of the ratio of p-SYK/SYK was performed in the designated groups (n = 6). e-g Immunoblotting of c-fos and CGRP protein levels were identified (n = 6). h, i The intensity of immunoreactivity against CGRP (n = 5). Two-way ANOVA with post-hoc Tukey’s test. R406 efficaciously inhibited the reduction of mechanical thresholds of both basal and post-treatment in hind paw plantar (j, k) and periorbital regions (l, m), while also prolonging the latency for withdrawal to heat (n, o); two-way RM ANOVA with Bonferroni post hoc test. Data are depicted as mean ± SEM; *p < 0.05, ***p < 0.001 compared with the VEH group; ##p < 0.01 and ###p < 0.001 compared with the NTG group
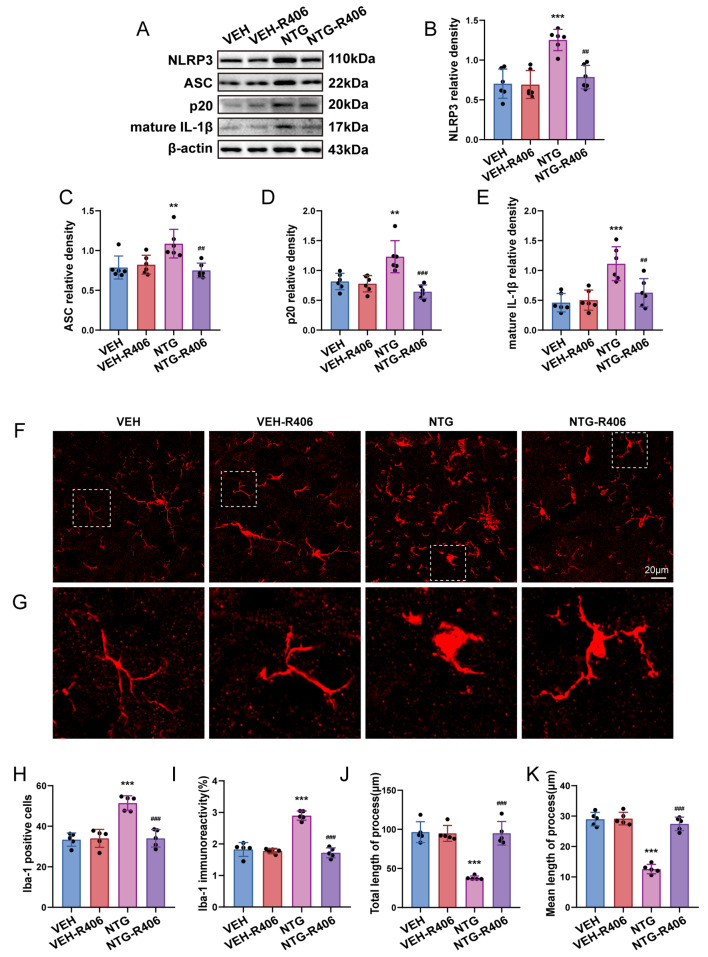
SYK activity regulates microglial activation and inflammatory responses subsequent to NTG administration
We proceeded to examine the essential role of SYK in microglial activation and inflammatory response induced by NTG injection. Mice were injected with R406, a SYK inhibitor, as mentioned earlier. Western blot analysis argued that pretreatment with R406 markedly reduced the enhancement of NLRP3 inflammasome-related factors and mature IL-1β expression levels, which were induced by NTG (Fig. 6a-e). In addition, our immunofluorescence analysis revealed that R406 significantly decreased the quantity and immune response of Iba1+ microglia, simultaneously restoring morphological alterations compared to the NTG group (Fig. 6f-k). These results suggest that SYK may assume a pivotal function in modulating microglial activation and inflammatory response within the TNC of CM mice.
Fig. 6.
SYK activity governs activation of microglia and infammatory response after NTG utilization. a-e Immunobloting detection of NLRP3, ASC, p20, and mature IL-1β subsequent to NTG and R406 management (n = 6). f Images depicting Iba1 immunostaining treated with NTG and R406. g The enlarged view of Iba-1 aligns with the area framed with a dashed white line in f. h–k Immunofluorescent analysis of Iba1+ microglia, Iba1 immunostaining, the overall length of processes as well as the average length of initial processes for each microglia (n = 5). Analysis was carried out on three mouse samples, each consisting of 4–6 FOVs. Scale bar = 20 μm; Data are depicted as mean ± SEM; two-way ANOVA with post-hoc Tukey’s test; **p < 0.01, ***p < 0.001 compared with the VEH group; ##p < 0.01, ###p < 0.001 compared with the NTG group
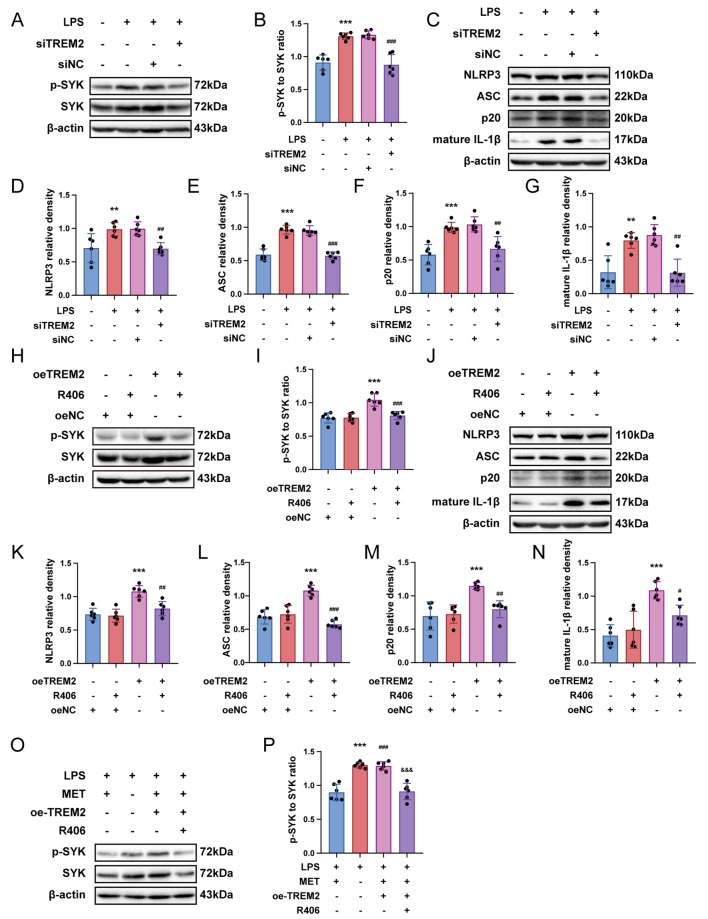
Metformin regulates the inflammatory response through the TREM2-SYK axis
Based on previous references, the inflammatory cell model was established by stimulating primary microglia with LPS. Upon LPS treatment, an incremental rise in TREM2 expression was observed with increasing concentrations of LPS, reaching its maximum value at 5 µg/ml compared to 0 µg/ml, 1 µg/ml, and 10 µg/ml. Therefore, a density of 5 µg/ml of LPS was selected for this study (Fig. S2a, b). We utilized small interfering RNA to silence TREM2 gene expression in microglia. Western immunoblot analysis demonstrated efficient knockdown of TREM2 by small interfering RNA (Fig. S2c, d), which effectively reversed the significant increase in the p-SYK to SYK ratio induced by LPS treatment (Fig. 7a, b). Furthermore, it was observed that TREM2 knockdown repressed the enhanced quantities of NLRP3 inflammasome along with mature IL-1β when contrasted to the LPS group (Fig. 7c-g). Therefore, our discoveries imply a pivotal role for TREM2 in regulating SYK function and NLRP3 inflammasome activation in primary microglia exposed to LPS. Subsequently, our objective was to determine whether TREM2 could independently modulate SYK activity in microglia, thereby regulating the inflammatory response. We conducted overexpression experiments of TREM2 in primary microglia. Western blot analysis showed significant elevation of TREM2 expression levels upon overexpression (Fig. S2e, f). Moreover, when contrasting against the control group, overexpression of TREM2 significantly enhanced the p-SYK to SYK ratio, indicating activation of SYK signaling in microglia with elevated levels of TREM2. In addition, treatment with R406 effectively suppressed the activation of the SYK pathway in microglia overexpressing TREM2 (Fig. 7h, i). Furthermore, we observed increase of NLRP3 inflammasome components and also mature form of IL-1β in the TREM2 overexpression group, which was reversed by R406 (Fig. 7j-n). We investigated the regulatory effect of metformin on TREM2-SYK. The results revealed that, in comparison with the LPS-MET group, the activity of SYK in the LPS group was significantly enhanced. Overexpression of TREM2 antagonized the regulatory effect of metformin on SYK, and this phenomenon was inhibited by R406. (Fig. 7o, p). Taken together, our findings suggest that Metformin regulates the inflammatory response through the TREM2-SYK axis.
Fig. 7.
Metformin regulates the inflammatory response through the TREM2-SYK axis. (a, b, h, I, o, p) Immunoblot assay of p-SYK to SYK ratio was carried out in the designated groups (n = 6) (c-g, j-n) Immunoblots were implemented to evaluate the quantities involved NLRP3, ASC, p20, and mature IL-1β expression. Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test. a-g **p < 0.01, ***p < 0.001 compared with the control group; ##p < 0.01, ###p < 0.001 compared with the LPS group. h-n ***p < 0.001 compared with the oeNC group; ##p < 0.01, ###p < 0.001 compared with the oeTREM2 group. o, p ***p < 0.001, ###p < 0.001 compared with the LPS-MET group; &&&p < 0.001 compared with the LPS-MET-oeTREM2 group
Discussion
Our study presents new findings that offer novel directions for the management of CM. Firstly, we demonstrated that metformin significantly ameliorated hyperalgesia in CM mice. Secondly, metformin effectively reduced the levels of c-fos and CGRP protein expression within the TNC of CM mice, thereby alleviating central sensitization. Finally, our study revealed that metformin mediated microglial activation and inflammatory response through the TREM2-SYK axis, thus elucidating the paramount importance of the TREM2-SYK pathway in CM.
Metformin is widely recognized as the preferred treatment option for individuals diagnosed with type 2 diabetes. Recent studies have demonstrated its potential role in managing Alzheimer’s disease [28], Parkinson’s disease [29], multiple sclerosis [30], stroke [31], and several other neurological dysfunctions. These studies offer substantiation for the anti-inflammatory properties exhibited by metformin [32]. The findings of other studies have demonstrated that metformin exhibits a significant capacity to mitigate inflammation, thereby improving hyperalgesia [10, 33]. The results of our study demonstrate that metformin effectively alleviated both mechanical and thermal hyperalgesia in mice with CM. In line with its demonstrated analgesic efficacy in previous studies, metformin holds considerable promise as a prospective preventive drug for CM. It is imperative to investigate the precise mechanism underlying metformin’s analgesic effects.
The pathogenesis of CM is a multifactorial and intricate process, with central sensitization being recognized as one of the crucial pathophysiological mechanisms [6, 34–36]. The most recent research suggests that the CGRP receptor antagonist, Rimegepant, is capable of effectively preventing and treating migraines [37–39]. Given its involvement in various migraine mechanisms, CGRP has been proposed as a dependable indicator for central sensitization in migraine [40]. In the current investigation, CGRP expression within the TNC was notably increased in CM mice. Immunofluorescence staining showed that CGRP was arc-shaped and distributed in the outermost stratum of TNC. This is in accordance with the previous study of our team [41] and the results of Zhou et al. [42]. c-fos functions as a reliable indicator for neuronal activation and exhibits a strong correlation with central sensitization [43]. In our study, the administration of metformin exhibited a notable decrease in c-fos and CGRP expression levels at TNC sites in mice with CM. This discovery offers robust evidence to support the potential impact of metformin on regulating central sensitization among individuals afflicted by CM. As CGRP and c-fos originate from neurons, metformin possesses the capacity to mediate microglial activation and mitigate inflammatory responses [11, 44]. We postulated that the suppressive impact of metformin on both CGRP and c-fos could potentially be attributed to a crosstalk mechanism between microglia and neurons. Previous studies have indicated that microglia modulate neuronal activity by releasing diverse cytokines [45–47]. Consequently, investigations into the underlying molecular mechanisms of metformin in CM have primarily centered around microglial activation as well as inflammatory response.
Microglia activation triggers the initiation of proinflammatory cytokines release, which contribute towards central sensitization in CM [48]. In the current investigation, we observed an increased number and enhanced fluorescence reactivity of Iba1+ microglia present in mice experiencing CM linked to TNC region, along with alterations in their morphology such as cell body hypertrophy and shortened processes. These findings indicate the activation of microglia, congruent with prior research [47, 49]. Our data report that metformin effectively inhibits the activation of TNC microglia and NLRP3 while alleviating the inflammatory response.
Studies have demonstrated that LPS stimulation causes an elevation of TREM2 expression in mouse primary cultured microglia and facilitates neuroinflammatory processes [50]. In Alzheimer’s disease, TREM2 is essential in mediating the phenotypic transformation of microglia [51]. TREM2, an important member of the TREM family, serves as a novel regulator of microglia phenotype and plays various roles in different disease models. In ischemic stroke models, TREM2 exhibits neuroprotective effects by reducing neuroinflammatory responses [14]. However, limited investigations have been conducted regarding its involvement in pain regulation. In neuropathic pain models, the activation of microglia mediated by TREM2 contributes to hyperalgesia [15, 52]. In this research, TREM2 protein expression in TNC showed an apparent elevation in mice suffering from CM, and TREM2 exhibited a distinct distribution pattern primarily within microglia rather than neurons and astrocytes. However, we only investigated the role of TREM2 in regulating neuroinflammation at the TNC site in CM mice and its corresponding mechanisms without evaluating its effect on the behavioral responses, which is a limitation of our study.
To our understanding, this research marks the initial exploration of TREM2 expression and distribution at TNC locations in CM mice. Furthermore, metformin exerted a profound suppressive effect on TREM2 expression at the TNC site in CM mice. The lentivirus encoding TREM2 markedly suppresses the anti-inflammatory effects of metformin. Additionally, knockdown of TREM2 effectively attenuated LPS-induced NLRP3 inflammasome activation in primary microglia. Our previous study [41] verified that NLRP3 is mainly distributed in TNC microglia of CM mice, thus contributing to the central sensitization associated with CM. In conclusion, both the in vitro and in vivo experiments imply that metformin may mediate the activation of microglia and NLRP3 inflammasomes through TREM2 to regulate neuroinflammatory response and participate in the regulation of central sensitization of CM.
Studies have proved that SYK participates in the modulation of neuropathic pain [53]. We observed SYK activation within the TNC vicinity among mice with CM, which was obviously inhibited by metformin. Pretreatment with R406, a highly selective SYK inhibitor, significantly ameliorated pain hypersensitivity in CM mice and decreased the protein levels of both CGRP and c-fos in the vicinity of TNC. Furthermore, it has come to light that SYK is engaged in regulating the activation of the NLRP3 inflammasome in macrophages [54]. In our study, we noticed a notable inhibition of microglia activation and NLRP3 inflammasome at TNC sites in CM mice upon treatment with R406, accompanied by a down-regulation of mature IL-1β expression. Furthermore, in vitro studies demonstrated an enhancement of LPS-stimulated SYK activity in primary microglia, which was attenuated upon knockdown of TREM2. Conversely, overexpression of TREM2 induced an enhanced SYK pathway activity that could be reversed by R406 treatment. Metformin markedly inhibited the elevated activity of SYK induced by NTG and LPS, and this could be reversed by the overexpression of TREM2. These discoveries reveal metformin modulates the activation of microglia and also NLRP3 inflammasomes through the TREM2-SYK pathway to ameliorate central sensitization in CM.
The present study has certain limitations. Firstly, we concentrated on investigating the intervention of metformin in the chronicization process of migraine, and its therapeutic effect on CM awaits further exploration. Secondly, gender differences exist in CM and to avoid estrogen’s impact on pain mediator transmission [55], male C57BL/6J mice were employed to conduct the CM model; therefore, additional research is required to investigate TREM2’s role in female CM models. Thirdly, while peripheral sensitization of the trigeminal ganglion (TG) has been linked with CM [56, 57], it remains unknown whether TREM2 is expressed in TG and further involved in regulating CM; given our systemic delivery approach, other pain-related regulatory regions’ involvement cannot be excluded. Fourthly, we established cell models using LPS for in vitro experiments; however, the responses initiated by LPS differ significantly from those triggered by endogenous agents; therefore, it is highly necessary to develop more effective in vitro models for CM.
Conclusions
In summary, our study confirms the role of metformin in CM. Metformin exerts anti-inflammatory effects by mediating microglia activation through the TREM2-SYK axis, thereby reducing central sensitization of CM. Although further clarification is needed regarding the mechanism of action of metformin in CM, our research results provide new evidence for its role and offer new avenues for clinical management of CM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional file 1: Fig. S1. Immunoblot analysis of TREM2. Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test; ***p < 0.001 compared with the control group
Supplementary Material 2: Additional file 2: Fig. S2. Immunoblot analysis of TREM2. Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test; a-f ***p < 0.001 compared with the control group
Acknowledgements
We express our gratitude to the other personnel affiliated with the Cuiying Medical Center of Lanzhou University Second Hospital.
Abbreviations
- CM
Chronic migraine
- TREM2
Triggering receptor expressed on myeloid cells 2
- NTG
Nitroglycerin
- SYK
Spleen tyrosine kinase
- pSYK
Phosphorylated spleen tyrosine kinase
- CGRP
Calcitonin gene-related peptide
- TNC
Trigeminal nucleus caudalis
- LPS
Lipopolysaccharide
- MET
Metformin
- CNS
Central nervous system
- ASC
Apoptosis-associated speck-like protein containing CARD
- IL-1β
Interleukin-1β
- SPF
Specific pathogen-free
- DAP-12
DNAX activating protein 12
- PBS
Phosphate buffered saline
- PFA
Paraformaldehyde
- AD
Alzheimer’s disease
- SNI
Spared nerve injury
- SDH
Spinal dorsal horn
- TG
Trigeminal ganglion
- FOVs
Fields of view
- ANOVA
Analysis of variance
Author contributions
ZF designed and conducted the study, performed data analysis, and drafted the manuscript. DS and ZL provided valuable suggestions and contributed to the execution of the experiment. SS offered technical support for the experiment. ZG supervised the experimental progress and revised the manuscript. All authors reviewed the manuscript.
Funding
This work was funded by the Innovation project in educational technology in Gansu Province. (Grant No. 2022B-054), Doctoral Research Foundation of Lanzhou University Second Hospital (Grant No. YJS-BD-05), Clinical Medical Research Center of Neurology Department of Gansu Province (Grant No. 2020-0411-SFC-0025), and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (Grant No. CY2021-QN-B06), and Cuiying Scientific Training Program for Undergraduates of The SecondHospital & Clinical Medical School, Lanzhou University (Grant No. CYXZ2022-03).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All experimental procedures strictly adhered to the guidelines established by the National Institutes of Health and received approval from the Animal Ethics Committee of Lanzhou University Second Hospital with an approval number of D2023-342.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Songtang Sun and Zhaoming Ge contributed equally to this work.
Contributor Information
Songtang Sun, Email: sstlucky@163.com.
Zhaoming Ge, Email: GZMcourage1136@163.com.
References
- 1.Hovaguimian A, Roth J. Management of chronic migraine. BMJ. 2022;379:e067670. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, et al. Migraine Nat Rev Dis Primers. 2022;8(1):2. [DOI] [PubMed] [Google Scholar]
- 3.May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–64. [DOI] [PubMed] [Google Scholar]
- 4.Scher AI, Buse DC, Fanning KM, Kelly AM, Franznick DA, Adams AM, et al. Comorbid pain and migraine chronicity: the chronic migraine epidemiology and outcomes study. Neurology. 2017;89(5):461–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwedt TJ. Chronic migraine. BMJ. 2014;348:g1416. [DOI] [PubMed] [Google Scholar]
- 6.Boyer N, Dallel R, Artola A, Monconduit L. General Trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain. 2014;155(7):1196–205. [DOI] [PubMed] [Google Scholar]
- 7.Volcheck MM, Graham SM, Fleming KC, Mohabbat AB, Luedtke CA. Central sensitization, chronic pain, and other symptoms: better understanding, better management. Cleve Clin J Med. 2023;90(4):245–54. [DOI] [PubMed] [Google Scholar]
- 8.Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–90. [DOI] [PubMed] [Google Scholar]
- 9.Magni G, Pedretti S, Audano M, Caruso D, Mitro N, Ceruti S. Glial cell activation and altered metabolic profile in the spinal-trigeminal axis in a rat model of multiple sclerosis associated with the development of trigeminal sensitization. Brain Behav Immun. 2020;89:268–80. [DOI] [PubMed] [Google Scholar]
- 10.Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, Murbartián J, Torres-López JE, Granados-Soto V. Metformin: a prospective alternative for the treatment of Chronic Pain. Front Pharmacol. 2020;11:558474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodali M, Attaluri S, Madhu LN, Shuai B, Upadhya R, Gonzalez JJ, et al. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell. 2021;20(2):e13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemgulyte G, Tanaka S, Hide I, Sakai N, Pampuscenko K, Borutaite V et al. Evaluation of the effectiveness of Post-stroke Metformin Treatment Using Permanent Middle Cerebral Artery Occlusion in rats. Pharmaceuticals (Basel). 2021;14(4). [DOI] [PMC free article] [PubMed]
- 13.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71(3):656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma WY, Wang SS, Wu QL, Zhou X, Chu SF, Chen NH. The versatile role of TREM2 in regulating of microglia fate in the ischemic stroke. Int Immunopharmacol. 2022;109:108733. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Konishi H, Sayo A, Takai T, Kiyama H. TREM2/DAP12 Signal elicits Proinflammatory Response in Microglia and exacerbates Neuropathic Pain. J Neurosci. 2016;36(43):11138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdi M, Pasbakhsh P, Shabani M, Nekoonam S, Sadeghi A, Fathi F, et al. Metformin Therapy attenuates pro-inflammatory microglia by inhibiting NF-κB in Cuprizone Demyelinating Mouse Model of multiple sclerosis. Neurotox Res. 2021;39(6):1732–46. [DOI] [PubMed] [Google Scholar]
- 17.Ennerfelt H, Frost EL, Shapiro DA, Holliday C, Zengeler KE, Voithofer G, et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell. 2022;185(22):4135–e5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10(8):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun H, Feng J, Tang L. Function of TREM1 and TREM2 in liver-related diseases. Cells. 2020;9(12). [DOI] [PMC free article] [PubMed]
- 20.Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Long T, Pan Q, Zhang S, Zhang Y, Zhang D, et al. Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J Neuroinflammation. 2019;16(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou TM, Chen SP. Animal models of chronic migraine. Curr Pain Headache Rep. 2018;22(6):44. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan AA, Smith ML, Mcguire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155(2):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adedeji HA, Ishola IO, Adeyemi OO. Novel action of metformin in the prevention of haloperidol-induced catalepsy in mice: potential in the treatment of Parkinson’s disease? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:245–51. [DOI] [PubMed] [Google Scholar]
- 25.Shu H, Liu S, Crawford J, Tao F. A female-specific role for trigeminal dynorphin in orofacial pain comorbidity. Pain. 2023;164(12):2801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhary P, Marracci G, Pocius E, Galipeau D, Morris B, Bourdette D. Effects of lipoic acid on primary murine microglial cells. J Neuroimmunol. 2019;334:576972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue K, Morimoto H, Ueki T. Modulation of microglial activity by salt load and SGK1. NeuroReport. 2020;31(7):571–77. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Sun Y, Cen X, Shan B, Zhao Q, Xie T, et al. Metformin activates chaperone-mediated autophagy and improves disease pathologies in an Alzheimer disease mouse model. Protein Cell. 2021;12(10):769–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mor DE, Sohrabi S, Kaletsky R, Keyes W, Tartici A, Kalia V, et al. Metformin rescues Parkinson’s disease phenotypes caused by hyperactive mitochondria. Proc Natl Acad Sci U S A. 2020;117(42):26438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negrotto L, Farez MF, Correale J. Immunologic effects of Metformin and Pioglitazone Treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 2016;73(5):520–8. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Zhang Z, Georgakis MK, Karhunen V, Liu D. The impact of genetically proxied AMPK activation, the target of Metformin, on functional outcome following ischemic stroke. J Stroke. 2023;25(2):266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loan A, Syal C, Lui M, He L, Wang J. Promising use of metformin in treating neurological disorders: biomarker-guided therapies. Neural Regen Res. 2024;19(5):1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei J, Wei Y, Huang M, Wang P, Jia S. Is metformin a possible treatment for diabetic neuropathy? J Diabetes. 2022;14(10):658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su M, Yu S. Chronic migraine: a process of dysmodulation and sensitization. Mol Pain. 2018;14:1744806918767697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74(18):3275–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shemer A, Erny D, Jung S, Prinz M. Microglia Plasticity during Health and Disease: an immunological perspective. Trends Immunol. 2015;36(10):614–24. [DOI] [PubMed] [Google Scholar]
- 37.Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51–60. [DOI] [PubMed] [Google Scholar]
- 38.Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737–45. [DOI] [PubMed] [Google Scholar]
- 39.Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for Migraine. N Engl J Med. 2019;381(2):142–49. [DOI] [PubMed] [Google Scholar]
- 40.Russo AF, Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev. 2023;103(2):1565–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun S, Fan Z, Liu X, Wang L, Ge Z. Microglia TREM1-mediated neuroinflammation contributes to central sensitization via the NF-κB pathway in a chronic migraine model. J Headache Pain. 2024;25(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long T, He W, Pan Q, Zhang S, Zhang Y, Liu C, et al. Microglia P2X4 receptor contributes to central sensitization following recurrent nitroglycerin stimulation. J Neuroinflammation. 2018;15(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaltenecker D, Al-Maskari R, Negwer M, Hoeher L, Kofler F, Zhao S, et al. Virtual reality-empowered deep-learning analysis of brain cells. Nat Methods. 2024;21(7):1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu YQ, Xiong J, He ZL, Yuan Y, Wang BN, Xu JY, et al. Metformin promotes microglial cells to facilitate myelin debris clearance and accelerate nerve repairment after spinal cord injury. Acta Pharmacol Sin. 2022;43(6):1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinhard L, Di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9(1):1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367(6477):528–37. [DOI] [PubMed] [Google Scholar]
- 47.Jing F, Zhang Y, Long T, He W, Qin G, Zhang D, et al. P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine. J Neuroinflammation. 2019;16(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma L, Li J, Zhou J, Zhang D, Xiao Z, Yu T, et al. Intravenous lidocaine alleviates postherpetic neuralgia in rats via regulation of neuroinflammation of microglia and astrocytes. iScience. 2021;24(2):102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Zhang Y, Jing F, Long T, Qin G, Zhang D, et al. P2X7R-mediated autophagic impairment contributes to central sensitization in a chronic migraine model with recurrent nitroglycerin stimulation in mice. J Neuroinflammation. 2021;18(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Gao X, Zhao K, Chen H, Xu M, Wang K. Effect of TREM2 on release of inflammatory factor from LPS-stimulated Microglia and its possible mechanism. Ann Clin Lab Sci. 2019;49(2):249–56. [PubMed] [Google Scholar]
- 51.Wang S, Sudan R, Peng V, Zhou Y, Du S, Yuede CM, et al. TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways. Cell. 2022;185(22):4153–e6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Shi Y, Huang Y, Liu W, Cai G, Huang S, et al. Resveratrol mediates mechanical allodynia through modulating inflammatory response via the TREM2-autophagy axis in SNI rat model. J Neuroinflammation. 2020;17(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu D, Zhang Y, Zhao C, Li Q, Zhang J, Han J, et al. Disruption of C/EBPβ-Clec7a axis exacerbates neuroinflammatory injury via NLRP3 inflammasome-mediated pyroptosis in experimental neuropathic pain. J Transl Med. 2022;20(1):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin YC, Huang DY, Wang JS, Lin YL, Hsieh SL, Huang KC, et al. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol. 2015;97(5):825–35. [DOI] [PubMed] [Google Scholar]
- 55.Marcus R. Study provides New Insight into Female Sex hormones and migraines. JAMA. 2023;329(17):1439–40. [DOI] [PubMed] [Google Scholar]
- 56.Cropper HC, Pradhan AA. Seq-ing the mechanisms of migraine. Neuron. 2022;110(11):1745–46. [DOI] [PubMed] [Google Scholar]
- 57.Ryu S, Liu X, Guo T, Guo Z, Zhang J, Cao YQ. Peripheral CCL2-CCR2 signalling contributes to chronic headache-related sensitization. Brain. 2023;146(10):4274-91. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Additional file 1: Fig. S1. Immunoblot analysis of TREM2. Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test; ***p < 0.001 compared with the control group
Supplementary Material 2: Additional file 2: Fig. S2. Immunoblot analysis of TREM2. Data are depicted as mean ± SEM; one-way ANOVA with post-hoc Tukey’s test; a-f ***p < 0.001 compared with the control group
Data Availability Statement
No datasets were generated or analysed during the current study.