Abstract
Patient: Male, 48-year-old
Final Diagnosis: Trauma induced coagulopathy
Symptoms: Chest pain • dyspnea
Clinical Procedure: —
Specialty: Surgery
Objective:
Unusual clinical course
Background:
Coagulopathy caused by trauma itself is defined as trauma-induced coagulopathy (TIC). The pathophysiology of TIC is considered to consist of coagulation activation, hyperfibrinolysis, and consumption coagulopathy, similar to disseminated intravascular coagulation (DIC). This report describes a 48-year-old man with a history of epilepsy presenting with TIC associated with multiple traumatic fractures and hemothorax.
Case Report:
A 48-year-old man with a history of epilepsy fell while working on a second-floor roof and had right rib fractures (6th to 12th rib), right hemothorax, right clavicle fracture, right elbow fracture, and pelvic fractures. The right hemothorax became exacerbated and he went into shock. We performed the emergency surgery 5 hours after the trauma. Although circulation dynamics became stable and the discharge of chest drainage became thinned at postoperative day (POD) 1 while administering blood transfusions and tranexamic acid, hemoglobin remained below 8 g/dl, platelet count was below 60 000/µl, and prothrombin time – international normalized ratio (1.22) remained prolonged. Furthermore, the right hemothorax became exacerbated and re-operation was performed on the evening of POD2. Oozing hemorrhages from multiple rib fractures were observed. Although hemostatic management was performed with electrocautery and ultrasound energy devices, the hemorrhage could not be completely managed, so hemostasis was secured using hemostatic materials.
Conclusions:
The pathophysiologic mechanism of TIC has been emphasized as being different from that of DIC, and management of severe traumatic patients with TIC should be based on an understanding of the pathophysiology of TIC.
Key words: Disseminated Intravascular Coagulation, Hemothorax
Introduction
Trauma is a leading cause of death for young age people and the mortality rate of blunt chest trauma was reported as nearly 20% [1,2]. Hemothorax has been reported to occur in approximately 30% chest trauma cases, caused by injury to the intercostal artery, laceration of the lung, great vessel injury, or diaphragmatic rupture [3]. A previous report showed that coagulopathy occurs early in the postinjury period, and increases the risk of mortality. Most deaths resulting from long prothrombin time (PT) occur early in the hospital stay, and the abnormal PT is an independent predictor of mortality [4]. The coagulopathy caused by the trauma itself has been defined as trauma-induced coagulopathy (TIC) [5], and TIC-related major trauma has been reported in some cases [6–8]. TIC is caused by a combination of hypovolemic shock and substantial tissue injury resulting in endothelial damage, glycosylation, hyperfibrinolysis, fibrinogen depletion, altered thrombin generation, and platelet dysfunction. Exogenous factors such as hypothermia, acidosis, hypokalemia, and dilution by administration of crystalloid or colloidal fluids further exacerbate TIC [9]. Furthermore, the pathophysiology of TIC is considered to consist of coagulation activation, hyperfibrinolysis, and consumption coagulopathy. These pathophysiological mechanisms cause disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype.
This report describes a 48-year-old man with a history of epilepsy presenting with trauma-induced coagulopathy which required re-operation 2 days after first surgery, associated with multiple traumatic fractures and hemothorax.
Case Report
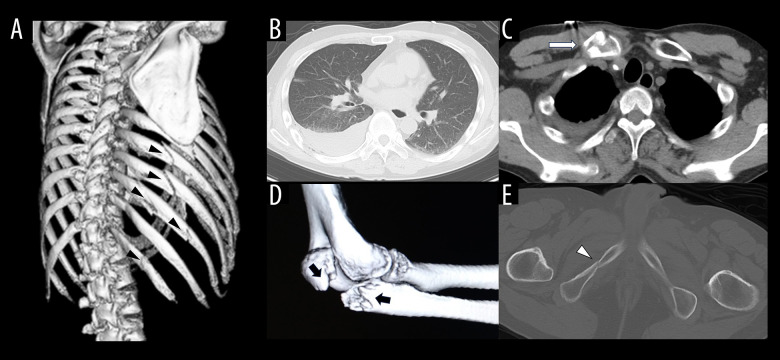
A 48-year-old man with epilepsy fell while working on a second-floor roof, and was transported to a nearby general hospital. Computed tomography (CT) showed right rib fractures (6th to 12th rib), right hemothorax, right clavicle fracture, right elbow fracture, and pelvic fractures (sacrum, acetabulum, and right ischium) (Figure 1). It was decided that intensive treatment could not be performed at the general hospital, so the patient was transported to our hospital 4 hours after the trauma. Because chest CT performed at our hospital showed exacerbation of the right hemothorax (Figure 2A) and the patient was in shock, we performed the emergency surgery 5 hours after the trauma. The operation was performed by video-assisted thoracic surgery (VATS), and a 1500 g hematoma was found in the thoracic cavity. After removal of the hematoma and observation of the bleeding points, oozing hemorrhage from multiple rib fractures site were observed. Hemostatic management was performed with electrocautery and ultrasound energy devices, while red cell concentrate (RCC) and fresh frozen plasma (FFP) transfusions were administered intraoperatively. After surgery, instable circulation dynamics was continued, and continuous noradrenaline was added, while platelet concentrate (PC) transfusion and tranexamic acid (TXA) were also administered in addition to RCC and FFP transfusion. Although the circulation dynamics became stable and the discharge of chest drainage decreased on postoperative day (POD) 1, hemoglobin (Hb) and platelet (Plt) count slowly declined and prothrombin time-international normalized ratio (PT-INR) was extended (PTINR 1.54). We also administered RCC, FFP, PC transfusion, and TXA. Despite administration of RCC, FFP, and PC, Hb remained below 8 g/dl (normal range 13–18 g/dl), Plt below 60 000/µl (normal range 150 000–450 000 µl), and PT-INR (normal range 0.85–1.15) remained prolonged. Left pneumonia occurred on POD 2 and antibacterial drug was administered. The right hemothorax was not exacerbated (Figure 2B). However, on the evening of POD 2, oxygenation suddenly became worse, and a chest X-ray showed worsening permeability of the right lung field (Figure 2C). We decided the right hemothorax was becoming exacerbated and performed a re-operation. The re-operation was also performed by VATS, and a 2240 g hematoma was found in the thoracic cavity. After removal of the hematoma and observation of the bleeding points, oozing hemorrhages from multiple rib fractures sites were observed. Although hemostatic management was performed with electrocautery and ultrasound energy devices, the hemorrhage could not be completely controlled, so hemostasis was secured by using oxidized regenerated cellulose and TachoSil® (CSL Behring, Pennsylvania, USA). Although the circulation dynamics remained stable after the re-operation, Hb (below 8 g/dl) and Plt (below 80 000/µl) count continued to be low and PT-INR prolonged (1.5) continued. RCC, FFP, PC transfusions, and TXA were also administered after the re-operation. The hemothorax was not exacerbated and left-lung pneumonia gradually improved after re-operation (Figure 2D). Because the hemothorax remained stable and the left-lung pneumonia improved, the patient was transferred to the Department of Orthopeadic Surgery for treatment of the right elbow fracture and pelvic fractures 3 weeks after the trauma. The course of treatment is shown in Figure 3.
Figure 1.
Imaging findings at the time of trauma. Computed tomography shows right rib fractures (A), right hemothorax (B), right clavicle fracture (C), right elbow fracture (D), and pelvic fractures (E).
Figure 2.
Imaging findings before and after re-operation. Chest computed tomography shows exacerbation of the right hemothorax (A). The right hemothorax did not become exacerbated, while left-lung pneumonia occurred in the morning of postoperative day (POD) 2 (B). The right lung field of chest X-ray shows worsening permeability on the evening of POD 2 (C). The right hemothorax was not exacerbated and left pneumonia gradually improved 7 days after re-operation (D).
Figure 3.
The course of the treatment. Red cell concentrate (RCC) and fresh frozen plasma (FFP) transfusions were administered from intraoperatively. After surgery, unstable circulation dynamics was continued, and continuous dose of noradrenaline was added, while platelet concentrate (PC) transfusion and tranexamic acid (TXA) were also administered in addition to RCC and FFP transfusion. Hemoglobin (Hb) and platelet (Plt) count slowly declined and prothrombin time-international normalized ratio (PT-INR) was extended (PT-INR 1.54), and then RCC, FFP, and PC transfusion and TXA were also administered. Left-lung pneumonia occurred on POD 2 and antibacterial drugs were administered [cefazolin sodium (CEZ) 2 g/day, meropenem hydrate (MEPM) 1.5 g/day, ceftriaxone sodium hydrate (CTRX) 2 g/day]. However, oxygenation suddenly became worse and a chest X-ray showed worsening permeability of the right lung field on the evening of POD 2. We decided there was exacerbation of the right hemothorax, and performed the re-operation. Although the circulation dynamics continued to be stable after the re-operation, Hb and Plt count remained low and PT-INR extension continued, and then RCC, FFP, PC transfusions, and TXA were also administered after the re-operation.
Discussion
Trauma remains a leading cause of death and disability in adults despite advances in systematic approaches to prevention, resuscitation, surgical management, and critical care [10]. Hemorrhage accounts for 30–40% of all trauma-related deaths and generally occurs within hours after injury [11]. Coagulopathy is a preventable cause of trauma mortality and is responsible for almost half of all deaths due to hemorrhage in trauma patients [9,10]. TIC is defined as coagulation activation, hyperfibrinolysis, and consumptive coagulopathy, and cause DIC with the fibrinolytic phenotype [5]. Hyperfibrinolysis is induced by coagulation activation and is recognized by elevation fibrin degradation product (FDP) levels [13]. In this case, high FDP levels (75.1 μg/ml, normal range: 5.0 μg/ml or less than) were recognized at admission to our hospital at 4 hours after the trauma, and it was considered that hyperfibrinolysis was induced by trauma.
Hyperfibrinolysis in severe trauma patients was reported to be driven by the inability of plasminogen activator inhibitor to inhibit excessive tissue-derived plasminogen activator (tPA)-mediated plasmin generation [14]. Although TXA was reported to be effective in preventing tPA-mediated fibrinolysis, TXA was also reported to stimulate fibrin-independent activation of plasminogen by urokinase plasminogen activator (uPA) [15]. Furthermore, it was reported that uPA was increased and protracted after trauma, and the administration of TXA >3 hours after trauma increased death due to bleeding. In this case, although the DIC criteria was not reached by POD 2 (FDP 7.8 μg/dl, fibrinogen 337 mg/dl, Plt 74 000/μl, PT-INR 1.22; DIC score 4), administration of TXA was continued after the first operation. Continuous administration of TXA induced prolonged hemorrhaging after the first operation and required a re-operation.
Transfusion is required for patients with severe trauma and major bleeding. The Pragmatic, Randomized Optimal Platelet and Plasma Ratios trial showed that more patients achieved hemostasis in the 1: 1: 1 transfusion ratio of FFP, PC, and RCC group compared with the 1: 1: 2 group, and fewer patients died of exsanguination [11]. Furthermore, the Prospective Observational Multicenter Major Trauma Transfusion study demonstrated that early transfusions of large amounts of plasma and platelets were associated with improved survival during the first 6 hours after admission [11,16]. In this case, we were not aware of the importance of these transfusion ratios and did not perform early administration of plasma or platelet.
Diffuse intravascular fibrin formation and deficiency of coagulation factors have been suggested to be specific findings in DIC, but intravascular fibrin formation and thrombotic occlusion of vessels have not been demonstrated, and consumptive coagulopathy leading to platelet and coagulation factor deficiency is not a common finding in TIC patients [17,18]. On the other hand, some reports showed pulmonary embolisms can occur early after major trauma [8], and that TIC largely resolves within 24 hours, after which hypercoagulability becomes increasingly more prevalent [19]. Therefore, the pathophysiologic mechanism of TIC differs from that of DIC. In this case, the re-operation was required, although the DIC criteria was not reached on POD 2. TIC has been reported to be a dynamic entity that evolves over time, and it has been suggested that no single hypothesis explains the different manifestations of coagulopathy [20].
Conclusions
We managed a case of traumatic hemothorax due to trauma-induced coagulopathy, which required re-operation 2 days after the first surgery. Treatment should be based on an understanding of the pathophysiology of TIC, including use of drug and transfusion, for management of severe traumatic patients with TIC.
Acknowledgments
We thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations:
- TIC
trauma-induced coagulopathy;
- DIC
disseminated intravascular coagulation;
- CT
computed tomography;
- VATS
video-assisted thoracic surgery;
- RCC
red cell concentrate;
- FFP
fresh frozen plasma;
- PC
platelet concentrate;
- TXA
tranexamic acid;
- POD
postoperative day;
- Hb
hemoglobin;
- Plt
platelet;
- PT-INR
prothrombin time-international normalized ratio;
- FDP
fibrin degradation product;
- tPA
tissue-derived plasminogen activator;
- uPA
urokinase plasminogen activator
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Rhee P, Joseph B, Pandit V, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 2.Veysi VT, Nikolaou VS, Paliobeis C, et al. Prevalence of chest trauma, associated injuries and mortality: A level I trauma centre experience. Int Orthop. 2009;33(5):1425–33. doi: 10.1007/s00264-009-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber S, Biberthaler P, Delhey P, et al. Predictors of poor outcomes after significant chest trauma in multiply injured patients: A retrospective analysis from the German Trauma Registry (Trauma Register DGU®) Scand J Trauma Resusc Emerg Med. 2014;22:52. doi: 10.1186/s13049-014-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa M. Pathophysiology of trauma-induced coagulopathy: Disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017;5:14. doi: 10.1186/s40560-016-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AV, Johnson ML, Tincher AM, et al. Brodifacoum contamination of synthetic cannabinoid causing unexplained coagulopathy in multiple trauma: A case report. Trauma Case Rep. 2024;51:101007. doi: 10.1016/j.tcr.2024.101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YY, Baik HJ, Lee H, et al. Heparin-free veno-venous extracorporeal membrane oxygenation in a multiple trauma patient: A case report. Medicine (Baltimore) 2020;99(5):e19070. doi: 10.1097/MD.0000000000019070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serchan P, Shorten G, Maher M, Power SP. Pulmonary embolism occurring early after major trauma. BMJ Case Rep. 2019;12(9):e228783. doi: 10.1136/bcr-2018-228783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schöchl H, Schmitt FCF, Maegele M. Pathophysiology of trauma-induced coagulopathy. Hamostaseologie. 2024;44(01):31–39. doi: 10.1055/a-2215-8936. [DOI] [PubMed] [Google Scholar]
- 10.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1: 1: 1 vs a 1: 1: 2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchesne JC, McSwain NE, Jr, Cotton BA, et al. Damage control resuscitation: The new face of damage control. J Trauma. 2010;69(4):976–90. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa M, Sawamura A, Gando S, et al. Disseminated intravascular coagulation at an early phase of trauma is associated with consumption coagulopathy and excessive fibrinolysis both by plasmin and neutrophil elastase. Surgery. 2011;149(2):221–30. doi: 10.1016/j.surg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Jessica CC, Nena M, Lisa AB, et al. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 15.Hijazi N, Fanne RA, Abramovitch R, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125(16):2558–67. doi: 10.1182/blood-2014-08-588442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Junco DJ, Holcomb JB, Fox EE, et al. Resuscitate early with plasma and platelets or balance blood products gradually: Findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl. 1):S24–30. doi: 10.1097/TA.0b013e31828fa3b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson GP, Letson HL, Sharma R, et al. Mechanisms of early trauma-induced coagulopathy: the clot thickens or not? J Trauma Acute Care Surg. 2015;79(2):301–9. doi: 10.1097/TA.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 18.Rizoli S, Nascimento B, Jr, Key N, et al. Disseminated intravascular coagulopathy in the first 24 hours after trauma: The association between ISTH score and anatomopathologic evidence. J Trauma. 2011;71(5 Suppl. 1):S441–47. doi: 10.1097/TA.0b013e318232e688. [DOI] [PubMed] [Google Scholar]
- 19.Sumislawski JJ, Kornblith LZ, Conroy AS, et al. Dynamic coagulability after injury: Is delaying venous thromboembolism chemoprophylaxis worth the wait? J Trauma Acute Care Surg. 2018;85(5):907–14. doi: 10.1097/TA.0000000000002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushimoto S, Kudo D, Kawazoe Y. Acute traumatic coagulopathy and trauma-induced coagulopathy: An overview. J Intensive Care. 2017;5(1):6. doi: 10.1186/s40560-016-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]