Abstract
This study investigates the frequency of a clinically reported variant in PMS2, NM_000535.7:c.2523G>A p.(W841∗), from next-generation sequencing studies in 2 racially diverse cohorts. We identified clinical reports of the PMS2 c.2523G>A p.(W841∗) variant in the National Precision Oncology Program’s somatic testing database (n = 25,168). We determined frequency of the variant in germline exome sequencing from the Penn Medicine BioBank (n = 44,256) and in gnomAD. The PMS2 c.2523G>A p.(W841∗) was identified as a homozygous variant on tumor testing in an adult patient of self-identified Black race/ethnicity with no evidence of constitutional mismatch repair deficiency. The variant was clinically reported on 35 total tumor and liquid biopsy tests (0.1%), and all individuals with the variant were of self-identified Black race/ethnicity (0.6% of n = 5787). In individuals of African genetic ancestry (AFR), the variant's germline frequency was reported to be 0.2% and 1.3% in the Penn Medicine BioBank (PMBB) and gnomAD, respectively. The variant cannot be found in any individuals of European genetic ancestry (EUR) from either of the databases. The variant is found in a region of PMS2 with 100% homology to the PMS2CL pseudogene. PMS2 c.2523G>A p.(W841∗), when identified, is typically an African-ancestry-specific PMS2CL pseudogene variant, which should be recognized to prevent misdiagnosis of Lynch syndrome in Blacks.
Keywords: PMS2, germline genetic testing, Lynch syndrome, PMS2CL, pseudogene interference
Introduction
Lynch syndrome (LS, OMIM 614337) is a common hereditary cancer syndrome associated primarily with increased risks for colon and endometrial cancer. Germline variants in mismatch repair genes MLH1, MSH2, MSH6, PMS2, and EPCAM cause LS.1 Diagnosis of LS occurs through germline genetic testing, although the analysis of tumor tissue through mismatch repair immunohistochemistry can aid in identifying suspected LS in cancer patients.1 Similarly, somatic genetic testing can also play an important role in detecting hereditary cancer predisposition syndromes by revealing germline variants, in addition to acquired somatic variants.2 Variant allele frequency (VAF) can be a telling indicator of germline variants identified in the somatic setting.3 When using short-read methods for germline or somatic testing, it is possible that pseudogenes can interfere with sequencing analysis, specifically read mapping; this is especially difficult for genes such as PMS2 (HGNC:9122) that have several known pseudogenes such as PMS2CL (HGNC:30061).4,5 Herein, we describe a PMS2 variant that was reported as pathogenic on somatic tumor testing only in individuals of self-reported Black race/ethnicity (Black). This variant was determined to be a PMS2CL pseudogene variant and confirmed to be carried at a higher frequency by individuals of self-reported Black race/ethnicity or genetically confirmed African ancestry. Recognition of this pseudogene interference is important to prevent the erroneous diagnosis of LS.
Materials and Methods
Durham VA Healthcare System Institutional Review Board approved the study. Informed consent was obtained for all patient volunteers who donated blood samples to the Penn Medicine BioBank (PMBB) for exome sequencing (ES) at median 65× coverage.6,7 National Precision Oncology Programs (NPOP) somatic testing database is an operational database containing identified information from patients tested through NPOP.8,9 We identified clinical reports of the NM_000535.7:c.2523G>A p.(W841∗) variant in PMS2 in the NPOP somatic testing database (n = 25,168). We determined frequency of the variant in germline ES from the PMBB (n = 44,256) and compared this with the reported population frequency in gnomAD (v2.1.1).10
Results
In an effort to identify patients with putative PMS2-related LS by tumor-only testing, the NPOP database (n = 25,156 patients) was queried and 156 reports with likely pathogenic/pathogenic (LP/P) variants in PMS2 were identified. Eighty-six different PMS2 LP/P variants were identified. Five variants were seen in 5 or less tumors, but 1 variant, a PMS2 variant denoted as “p.(W841∗),” was seen in 35 cases (PMS2 c.2523G>A). Among these tumors, a breast tumor with microsatellite stable (MSS) status and a tumor mutational burden of 0 mutations/megabase was identified where the variant was reported at an allele fraction of 100%. Given the peculiarity of the frequency of this variant and the absence of an expected MS instability signature in a tumor with VAF 100%, we investigated the variant in more detail.
Upon query to the tumor testing company, the Human Genome Variant Society nomenclature of the identified variant was c.2523G>A p.(W841∗). Query of ClinVar revealed 2 separate cDNA changes, PMS2 c.2522G>A (ClinVar ID: 234759) and PMS2 c.2523G>A (ClinVar ID: 127786), which correspond to the same predicted protein truncation reported on the somatic testing, PMS2 p.(W841∗). ClinVar had several submissions by commercial germline genetic laboratories under PMS2 c.2522G>A that were classified as pathogenic. For PMS2 c.2523G>A, ClinVar had 1 entry from a commercial germline genetic testing labs that deemed this variant pathogenic; whereas, several smaller labs labeled this a variant of uncertain significance. Germline genetic testing was carried out on blood lymphocytes from the patient with the tumor variant at VAF 100% using a large commercial laboratory. Despite the high VAF, the patient was not identified to carry any LP/P variant in PMS2 in the germline. The laboratory was contacted, and a data review was requested. Upon review, the commercial laboratory confirmed that germline PMS2 c.2523G>A p.(W841∗) was not present in PMS2, but a variant was confirmed at the homologous position of the PMS2CL pseudogene by long-range polymerase chain reaction (PCR). The PMS2CL variant was homozygous. The lab confirmed that the analysis was not confounded by allelic dropout nor coamplification.
In the NPOP database, the PMS2 c.2523G>A variant was reported in 22 patients out of 19,196 (0.11%) solid tumor samples that were successfully sequenced and reported via Foundation One CDx (Table 1). All 22 patients were of Black self-identified race/ethnicity. Microsatellite status was available for 21 of the 22 patients, with 19 of the 21 (90%) having MSS tumors, and 2 of the 21 (10%) were equivocal. Comparatively, 94 other tumors with truncating variants in PMS2 (Supplemental Table 1) and MS status available were analyzed, and 34 of the 94 (36%) were MS unstable (MSI-High) (Supplemental Figure 1A). Reviewing liquid biopsy reports, the c.2523G>A variant was reported in 2 patients out of 2990 (0.07%) liquid biopsy samples that were successfully sequenced and reported via Foundation One Liquid CDx (Table 1). Both patients who self-identified as Black and MS status were unavailable. Finally, the PMS2 c.2523G>A variant was reported in 11 patients out of 2982 (0.37%) solid tumor samples sequenced via the Personal Genome Diagnostics CP6 tumor-only test, with all 11 patients having self-identified Black race/ethnicity (Table 1). The PMS2 c.2523G>A variant was not identified in the 1361 NPOP patients who had tumor sequencing through 1396 Personalis tests (data not shown). The variant call file data were reviewed, and the reported PMS2 p.(W841∗) variants were the c.2523G>A allele in all cases. The PMS2 c.2522G>A variant was not identified in any of the NPOP patients. Overall, the PMS2 c.2523G>A p.(W841∗) was reported in 35 (0.60%) of 5787 Black individuals and none of 17,703 White individuals in the NPOP database (Table 1). Of the 35 tumor types with PMS2 p.(W841∗) reported, 28% were LS-related cancers; although this was not much lower than the rate of LS-related cancers in other truncating variants in PMS2 (36% of 121 cases) (Supplemental Figure 1B).
Table 1.
Frequency of PMS2 c.2523G>A; p.W841X in a tumor testing cohort, germline exome sequencing study, and population databases
| Cohort | All Patients |
SIRE-Black/AFRa |
SIRE-White/EURa |
Othera |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n POS | n Total | % | n POS | n Total | % | n POS | n Total | % | n POS | n Total | % | # of Homo-zygotes | |
| NPOP FoundationOne-CDx | 22 | 19196 | 0.11% | 22 | 4170 | 0.53% | 0 | 13642 | - | 0 | 1384 | - | 1 (SIRE-Black) |
| NPOP FoundationOne- LiquidCDx | 2 | 2990 | 0.07% | 2 | 886 | 0.23% | 0 | 1920 | - | 0 | 184 | - | None |
| NPOP PGDX CP6 | 11 | 2982 | 0.37% | 11 | 731 | 1.50% | 0 | 2141 | - | 0 | 110 | - | None |
| NPOP Database (total) | 35 | 25168 | 0.14% | 35 | 5787 | 0.60% | 0 | 17703 | - | 1678 | |||
| Penn Medicine BioBank | 28 | 44256 | 0.06% | 25 | 10815 | 0.23% | 0 | 29239 | - | 3 | 4112 | 0.07% | None |
| GnomAD | 97 | 96159 | 0.10% | 91 | 7106 | 1.28% | 0 | 41415 | - | 5 | 47998 | 0.01% | 3 (AFR) |
POS, number of cases with an identified PMS2 p.W841X variant; SIRE, self-identified race/ethnicity.
Patients were categorized by self-identified race/ethnicity (SIRE) as SIRE-Black or SIRE-White or “Other” using the Veterans’ Affairs electronic health record for NPOP data. In the Penn Medicine BioBank and gnomAD, samples were categorized as “AFR,” “EUR,” or “Other” by genetic informed ancestry analysis.
We next investigated research ES data from 44,256 patient samples in the PMBB. ES was performed at median 65× coverage. The PMS2 c.2523G>A p.(W841∗) variant was identified overall in 28 individuals (0.06%) (Table 1). Of the 28 individuals, 25 were of African genetic-inferred ancestry (89.3%), and 3 were of other non-White genetic ancestral background (10.7%). The frequency of the PMS2 c.2523G>A in the African genetic ancestry population in PMBB was 0.23%. This is lower than the reported variant frequency in gnomAD, which reports the overall population frequency at 0.10% and the African genetic ancestry population frequency at 1.3% (Table 1).10
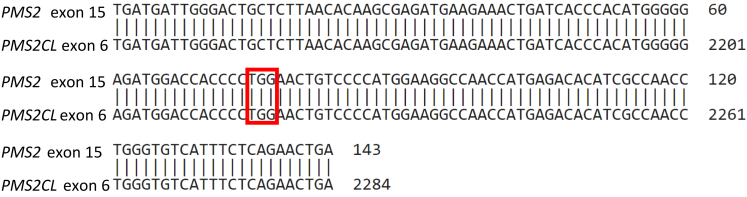
Finally, 3 commercial laboratories were contacted to review the evidence for their classifications. The 2 commercial labs that had submitted P calls under PMS2 c.2522G>A p.(W841∗) noted that PMS2 c.2522G>A p.(W841∗) had been identified by next-generation sequencing (NGS) in only a few patients, and long-range PCR confirmed that it was located in the PMS2 gene. Conversely, PMS2 c.2523G>A p.(W841∗), as was observed in this study, had been identified by NGS in over 200 patients at each lab and had never been confirmed in PMS2 by long-range PCR, with each lab concluding that PMS2 c.2523G>A p.(W841∗) was instead located in the pseudogene PMS2CL. The third commercial laboratory who had submitted a pathogenic call under PMS2 c. 2523G>A p.(W841∗) on ClinVar noted that this variant had never been confirmed in PMS2 by long-range PCR. This laboratory marked PMS2 c.2523G>A p.(W841∗) as an artifact in their pipeline, with identification of this variant by NGS always being followed up with long-range PCR. The laboratory’s justification for their pathogenic ClinVar submission was that a truncating variant in that position would be pathogenic, if confirmed to be in PMS2 by long-range PCR. Review of DNA alignment of PMS2 exon 15 and PMS2CL exon 6 showed 100% homology, with a substitution of G>A in codon 841 of PMS2 creating a stop codon (nucleotides TAG) in both the pseudogene and functional PMS2 (Figure 1), explaining the pseudogene interference at this position.
Figure 1.
PMS2 exon 15 aligned with PMS2CL exon 6. Illustrates the similarity in PMS2 exon 15 and PMS2CL exon 6 sequences. Highlighted in red is the codon corresponding to the variants of interest PMS2 c.2523G>A p.(W841∗).
Discussion
Pseudogenes are copies of functional genes that exist within the genome that do not serve as DNA templates for functional protein products. These pseudogenes can be highly similar to the functional gene across large stretches of base pairs.4 Although NGS has provided a high-throughput, efficient, and cost effective way of performing DNA sequencing, this method depends on short reads, which can make hybrid capture, read mapping, and subsequent variant calling susceptible to pseudogene interference.4 Short reads may not include areas of the gene that are distinct from the pseudogene and therefore could inadvertently capture the pseudogene sequence.4
PMS2 and the PMS2CL pseudogene contain highly similar sequences in exons 9 and 11 to 15.4,5 If using short-read sequencing, the high degree of similarity between PMS2 and PMS2CL in several adjacent exons makes this region highly susceptible to pseudogene interference. Long-read methods such as long-range PCR should be used to capture outside of the homologous regions to distinguish variants identified in PMS2CL from variants in PMS2.4
The PMS2 c. 2522G>A p.(W841∗) variant is located in exon 15, the last exon of the PMS2 gene. Exon 15 is associated with the nuclease domain, and deletions in exon 15 damage this nuclease domain that is critical for PMS2 function.11 Other downstream variants are P and LP (ClinVar). Variants in exon 15 of PMS2 may be difficult to distinguish from the pseudogene because exon 6 of PMS2CL is identical.12 The pseudogene variant c.2523G>A p.(W841∗) has been reported in several articles that all fail to differentiate this variant in PMS2CL versus PMS2.13, 14, 15, 16, 17 Because exon 6 of PMS2CL is identical to exon 15 of PMS2, a substitution of G>A in codon 841 creates a TAG stop codon in both the pseudogene and functional PMS2. Short-read sequencing could inadvertently capture the truncating PMS2CL variant while attempting to sequence PMS2.
The PMS2 variant p.(W841∗) must be distinguished by cDNA change. PMS2 c.2522G>A is a pathogenic truncating variant truly located in the PMS2 gene that has been seen infrequently by commercial laboratories and is consistent with LS. On the other hand, PMS2 c.2523G>A is a single-nucleotide variant (SNV) in the pseudogene PMS2CL commonly found in the African American population. It is important for clinicians, germline and somatic genetic testing laboratories, and bioinformaticians to recognize PMS2 c.2523G>A as a pseudogene SNV to prevent misdiagnosis of LS in Black patients carrying the SNV. This pseudogene variant has been confused for a pathogenic PMS2 variant in the literature and is also present in population databases such as gnomAD as a true PMS2 variant.10 Of note, cbioportal and AACR GENIE have not included the pseudogene variant—possibly because their bioinformatics team excluded it.18,19 This pseudogene variant c.2523G>A should be regarded as an artifact in prospective cases and should be excluded from population databases and germline and somatic genetic testing reports to limit confusion with the pathogenic PMS2 variant c.2522G>A.
Data Availability
The data are available by contacting the corresponding author. The data are available to qualified investigators with access to VA’s VINCI computing environment and an approved IRB.
ORCID
Kara N. Maxwell: http://orcid.org/0000-0001-8192-4202
Penn Medicine BioBank Members
Anurag Verma, Ph.D., Shefali S. Verma, Ph.D., Yuki Bradford, M.S., Ashlei Brock, Stephanie DerOhannessian, Scott Dudek, M.S., Joseph Dunn, Theodore Drivas, M.D., Ph.D., Ned Haubein, Khadijah Hu-Sain, Renae Judy, Ashley Kloter, Yi-An Ko, Meghan Livingstone, Linda Morrel, Colleen Morse, M.S., Afiya Poindexter, Marjorie Risman, M.S., Teo Tran, Fred Vadivieso, JoEllen Weaver, Daniel J. Rader, M.D., Marylyn D. Ritchie, Ph.D., Michael D. Feldman M.D., Ph.D.
Regeneron Genetics Center Members
Christina Beechert, Caitlin Forsythe, M.S., Erin D. Fuller, Zhenhua Gu, M.S., Michael Lattari, Alexander Lopez, M.S., John D. Overton, Ph.D., Maria Sotiropoulos Padilla, M.S., Manasi Pradhan, M.S., Kia Manoochehri, B.S., Thomas D. Schleicher, M.S., Louis Widom, Sarah E. Wolf, M.S., Ricardo H. Ulloa, B.S., Amelia Averitt, Ph.D., Nilanjana Banerjee, Ph.D., Michael Cantor, M.D., Dadong Li, Ph.D., Sameer Malhotra, M.D., Deepika Sharma, MHI, Jeffrey Staples, Ph.D. Xiaodong Bai, Ph.D., Suganthi Balasubramanian, Ph.D., Suying Bao, Ph.D., Boris Boutkov, Ph.D., Siying Chen, Ph.D., Gisu Eom, B.S., Lukas Habegger, Ph.D., Alicia Hawes, B.S., Shareef Khalid, Olga Krasheninina, M.S., Rouel Lanche, B.S., Adam J. Mansfield, B.A., Evan K. Maxwell, Ph.D., George Mitra, B.A., Mona Nafde, M.S., Sean O’Keeffe, Ph.D., Max Orelus, B.B.A., Razvan Panea, Ph.D., Tommy Polanco, B.A., Ayesha Rasool, M.S., Jeffrey G. Reid, Ph.D., William Salerno, Ph.D., Jeffrey C. Staples, Ph.D., Kathie Sun, Ph.D. Goncalo Abecasis, D.Phil., Joshua Backman, Ph.D., Amy Damask, Ph.D., Lee Dobbyn, Ph.D., Manuel Allen Revez Ferreira, Ph.D., Arkopravo Ghosh, M.S., Christopher Gillies, Ph.D., Lauren Gurski, B.S., Eric Jorgenson, Ph.D., Hyun Min Kang, Ph.D., Michael Kessler, Ph.D., Jack Kosmicki, Ph.D., Alexander Li, Ph.D., Nan Lin, Ph.D., Daren Liu, M.S., Adam Locke, Ph.D., Jonathan Marchini, Ph.D., Anthony Marcketta, M.S., Joelle Mbatchou, Ph.D., Arden Moscati, Ph.D., Charles Paulding, Ph.D., Carlo Sidore, Ph.D., Eli Stahl, Ph.D., Kyoko Watanabe, Ph.D., Bin Ye, Ph.D., Blair Zhang, Ph.D., Andrey Ziyatdinov, Ph.D. Ariane Ayer, B.S., Aysegul Guvenek, Ph.D., George Hindy, Ph.D., Giovanni Coppola, M.D., Jan Freudenberg, M.D., Jonas Bovijn M.D., Katherine Siminovitch, M.D., Kavita Praveen, Ph.D., Luca A. Lotta, M.D., Manav Kapoor, Ph.D., Mary Haas, Ph.D., Moeen Riaz, Ph.D., Niek Verweij, Ph.D., Olukayode Sosina, Ph.D., Parsa Akbari, Ph.D., Priyanka Nakka, Ph.D., Sahar Gelfman, Ph.D., Sujit Gokhale, B.E., Tanima De, Ph.D., Veera Rajagopal, Ph.D., Alan Shuldiner, M.D., Bin Ye, Ph.D., Gannie Tzoneva, Ph.D., Juan Rodriguez-Flores, Ph.D. Esteban Chen, M.S., Marcus B. Jones, Ph.D., Michelle G. LeBlanc, Ph.D., Jason Mighty, Ph.D., Lyndon J. Mitnaul, Ph.D., Nirupama Nishtala, Ph.D., Nadia Rana, Ph.D., Jaimee Hernandez, Goncalo Abecasis, PhD, Aris Baras, M.D., Michael Cantor, M.D., Giovanni Coppola, M.D., Andrew Deubler, Aris Economides, Ph.D., Luca A. Lotta, M.D., Ph.D., John D. Overton, Ph.D., Jeffrey G. Reid, Ph.D., Katherine Siminovitch, M.D., Alan Shuldiner, M.D.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
Funding
This work was supported by the National Cancer Institute (K08CA215312, K.N.M.), the Burroughs WellcomeFoundation (#1017184, K.N.M.), Basser Center for BRCA (K.N.M.), Jason and Julie Borrelli Lynch Syndrome Research Fund (B.W.K.), the King Family Fund for Lynch Syndrome Education, Outreach, and Impact (B.W.K.), and the National Oncology Program, Department of Veterans Affairs (M.J.K. and K.N.M.).
Author Information
Conceptualization: K.N.M., L.B.A.; Data Curation: K.N.M., M.J.K.; Formal Analysis: K.N.M., M.J.K., Writing-original draft: J.C., K.N.M., B.K.; Writing-review and editing: J.C., K.N.M., B.K., M.J.K.
Ethics Declaration
Durham VA Healthcare System Institutional Review Board approved the study. Obtained informed consent for all patient volunteers who donate biological samples to the Penn Medicine BioBank.13,14 https://pmbb.med.upenn.edu. National Precision Oncology Programs somatic testing database is an operational database containing identified information from patients tested through NPOP.15,16
Footnotes
The Article Publishing Charge (APC) for this article was paid by Michael J. Kelley.
The member names of the Penn Medicine BioBank Banner Members and the Regeneron Genetics Center Banner Members will appear at the end of the article.
Additional Information
The online version of this article (https://doi.org/10.1016/j.gimo.2024.101858) contains supplemental material, which is available to authorized users.
Contributor Information
Kara N. Maxwell, Email: kara.maxwell@pennmedicine.upenn.edu.
Penn Medicine BioBank, Regeneron Genetics Center:
Anurag Verma, Shefali S. Verma, Yuki Bradford, Ashlei Brock, Stephanie DerOhannessian, Scott Dudek, Joseph Dunn, Theodore Drivas, Ned Haubein, Khadijah Hu-Sain, Renae Judy, Ashley Kloter, Yi-An Ko, Meghan Livingstone, Linda Morrel, Colleen Morse, Afiya Poindexter, Marjorie Risman, Teo Tran, Fred Vadivieso, JoEllen Weaver, Daniel J. Rader, Marylyn D. Ritchie, Michael D. Feldman, Christina Beechert, Caitlin Forsythe, Erin D. Fuller, Zhenhua Gu, Michael Lattari, Alexander Lopez, John D. Overton, Maria Sotiropoulos Padilla, Manasi Pradhan, Kia Manoochehri, Thomas D. Schleicher, Louis Widom, Sarah E. Wolf, Ricardo H. Ulloa, Amelia Averitt, Nilanjana Banerjee, Michael Cantor, Dadong Li, Sameer Malhotra, Deepika Sharma, Jeffrey Staples, Xiaodong Bai, Suganthi Balasubramanian, Suying Bao, Boris Boutkov, Siying Chen, Gisu Eom, Lukas Habegger, Alicia Hawes, Shareef Khalid, Olga Krasheninina, Rouel Lanche, Adam J. Mansfield, Evan K. Maxwell, George Mitra, Mona Nafde, Sean O’Keeffe, Max Orelus, Razvan Panea, Tommy Polanco, Ayesha Rasool, Jeffrey G. Reid, William Salerno, Jeffrey C. Staples, Kathie Sun, Goncalo Abecasis, Joshua Backman, Amy Damask, Lee Dobbyn, Manuel Allen Revez Ferreira, Arkopravo Ghosh, Christopher Gillies, Lauren Gurski, Eric Jorgenson, Hyun Min Kang, Michael Kessler, Jack Kosmicki, Alexander Li, Nan Lin, Daren Liu, Adam Locke, Jonathan Marchini, Anthony Marcketta, Joelle Mbatchou, Arden Moscati, Charles Paulding, Carlo Sidore, Eli Stahl, Kyoko Watanabe, Bin Ye, Blair Zhang, Andrey Ziyatdinov, Ariane Ayer, Aysegul Guvenek, George Hindy, Giovanni Coppola, Jan Freudenberg, Jonas Bovijn, Katherine Siminovitch, Kavita Praveen, Luca A. Lotta, Manav Kapoor, Mary Haas, Moeen Riaz, Niek Verweij, Olukayode Sosina, Parsa Akbari, Priyanka Nakka, Sahar Gelfman, Sujit Gokhale, Tanima De, Veera Rajagopal, Alan Shuldiner, Bin Ye, Gannie Tzoneva, Juan Rodriguez-Flores, Esteban Chen, Marcus B. Jones, Michelle G. LeBlanc, Jason Mighty, Lyndon J. Mitnaul, Nirupama Nishtala, Nadia Rana, Jaimee Hernandez, Goncalo Abecasis, Aris Baras, Michael Cantor, Giovanni Coppola, Andrew Deubler, Aris Economides, Luca A. Lotta, John D. Overton, Jeffrey G. Reid, Katherine Siminovitch, and Alan Shuldiner
Additional Information
References
- 1.Biller L.H., Syngal S., Yurgelun M.B. Recent advances in lynch syndrome. Fam Cancer. 2019;18(2):211–219. doi: 10.1007/s10689-018-00117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman A., Sotelo J. Tumor-based genetic testing and familial cancer risk. Cold Spring Harb Perspect Med. 2020;10(8):a036590. doi: 10.1101/cshperspect.a036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng D.T., Prasad M., Chekaluk Y., et al. Comprehensive detection of germline variants by MSK-IMPACT, a clinical diagnostic platform for solid tumor molecular oncology and concurrent cancer predisposition testing. BMC Med Genomics. 2017;10(1):33. doi: 10.1186/s12920-017-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claes K.B.M., Rosseel T., De Leeneer K. Dealing with pseudogenes in molecular diagnostics in the next generation sequencing era. Methods Mol Biol. 2021;2324:363–381. doi: 10.1007/978-1-0716-1503-4_22. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn C.P., Hart K.J., Samowitz W.S., Swensen J.J. Avoidance of pseudogene interference in the detection of 3′ deletions in PMS2. Hum Mutat. 2011;32(9):1063–1071. doi: 10.1002/humu.21540. [DOI] [PubMed] [Google Scholar]
- 6.Park J., Levin M.G., Haggerty C.M., et al. A genome-first approach to aggregating rare genetic variants in LMNA for association with electronic health record phenotypes. Genet Med. 2020;22(1):102–111. doi: 10.1038/s41436-019-0625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma A., Damrauer S.M., Naseer N., et al. The Penn Medicine BioBank: towards a genomics-enabled learning healthcare system to accelerate Precision Medicine in a diverse population. J Pers Med. 2022;12(12):1974. doi: 10.3390/jpm12121974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley M.J. VA national precision oncology program. Fed Pract. 2020;37(suppl 4):S22–S27. doi: 10.12788/fp.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalal S.I., Guo A., Ahmed S., Kelley M.J. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. Published online July 19, 2022 doi: 10.1053/j.seminoncol.2022.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Karczewski K.J., Francioli L.C., MacArthur D.G. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blount J., Prakash A. The changing landscape of lynch syndrome due to PMS2 mutations. Clin Genet. 2018;94(1):61–69. doi: 10.1111/cge.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman D.S., Smith C., Liu C., et al. Efficient detection of copy number mutations in PMS2 exons with a close homolog. J Mol Diagn. 2018;20(4):512–521. doi: 10.1016/j.jmoldx.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castéra L., Krieger S., Rousselin A., et al. Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. Eur J Hum Genet. 2014;22(11):1305–1313. doi: 10.1038/ejhg.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C., Xie M., Wendl M.C., et al. Patterns and functional implications of rare germline variants across 12 cancer types. Nat Commun. 2015;6(1) doi: 10.1038/ncomms10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fostira F., Saloustros E., Apostolou P., et al. Germline deleterious mutations in genes other than BRCA2 are infrequent in male breast cancer. Breast Cancer Res Treat. 2018;169(1):105–113. doi: 10.1007/s10549-018-4661-x. [DOI] [PubMed] [Google Scholar]
- 16.Lu T., Zhou S., Wu H., Forgetta V., Greenwood C.M.T., Richards J.B. Individuals with common diseases but with a low polygenic risk score could be prioritized for rare variant screening. Genet Med. 2021;23(3):508–515. doi: 10.1038/s41436-020-01007-7. [DOI] [PubMed] [Google Scholar]
- 17.Guindalini R.S.C., Viana D.V., Kitajima J.P.F.W., et al. Detection of germline variants in Brazilian breast cancer patients using multigene panel testing. Sci Rep. 2022;12(1):4190. doi: 10.1038/s41598-022-07383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerami E., Gao J., Dogrusoz U., et al. The Cbio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AACR Project Genie: powering precision medicine through data sharing: a real-world clinico-genomic registry for clinical and translational discovery. Clin Omics. 2019;6(2) doi: 10.1089/clinomi.06.02.13. 28-28. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available by contacting the corresponding author. The data are available to qualified investigators with access to VA’s VINCI computing environment and an approved IRB.