Abstract
Background
Family caregivers of gastric cancer (GC) patients after gastrectomy have a strong demand for nutrition knowledge. This study investigates the knowledge, attitudes, and practices (KAP) of primary caregivers of GC patients regarding postoperative dietary management.
Methods
We conducted a cross-sectional study, collecting data through questionnaire distribution. Demographic information of the respondents and KAP scores were assessed and analyzed.
Results
Of 508 included participants, majority were female (59.84%) urban residents (78.94%), aged 40–60 years (53.15%). Caretakers were primarily spouses of GC patient (50.39%) or parents (10.43%), only child (12.99%) or non-only child (24.21%). Notable percentage of poor knowledge and practice was found among participants (45.05% and 40.55%, respectively), while attitude was predominantly positive (99.41%). Correlation analysis revealed a weak positive correlation between knowledge and attitude scores (r = 0.150, P < 0.001) and negative link to practice scores (r=-0.228, P < 0.001); attitude scores were positively correlated with practice (r = 0.117, P = 0.008). Multivariate logistic regression analysis found that higher attitude scores were independently associated with higher practice scores (OR = 1.360; 95%CI, 1.223–1.513), P < 0.001), while higher knowledge scores (OR = 0.684; 95%CI, 0.575–0.815), P < 0.001), older age (OR = 0.951; 95%CI, 0.918–0.985), P = 0.005), duration of caregiving > 3 months (3–6 months (OR = 0.415; 95%CI, 0.193–0.894, P = 0.025); 6 months-1 year (OR = 0.269; 95%CI, 0.120–0.606), P = 0.002); >1 year (OR = 0.290; 95%CI, 0.120–0.705), P = 0.006), and follow-up location after patient’s surgery (OR = 0.072 (0.033–0.160), P < 0.001) were independently associated with lower practice scores.
Conclusions
Family caregivers of GC patients that participated in this study demonstrated moderate knowledge and practice, but positive attitude towards dietary management after gastrectomy.
Keywords: Gastric cancer, Caregivers, Knowledge, Attitudes, Practices
Background
Gastric cancer (GC) is highly heterogeneous disease that remains a major unmet clinical problem worldwide [1, 2]. During the last decade GC was reported as the third or fourth leading cause of cancer deaths, and 1.1 million new cases were diagnosed in 2020 (~ 6% of all cancer cases globally) [3, 4]. The lifetime risk of GC is about 1 in 54 men and 1 in 126 women, progressing with age [5]. Different regions report specific patterns of the GC presentation, with the highest incidence reported in Eastern Asia, followed by eastern and central Europe [6]. Thereby, Asia presents approximately 75% of all new cases or deaths from GC [4]. To combat this challenge, GC diagnosis and treatment possibilities are rapidly evolving along with the progress of modern medicine, allowing for better recognition and recovery. Besides surgical resection, fundamental treatment methods include systemic chemoradiation or chemotherapy, as well as novel targeted and immune therapies [7]. Comprehensive surgical resection with lymphadenectomy is regarded as the choice strategy aimed to cure GC [1, 3]. However, although the 5-year survival rate of early GC can reach 90%, late stage patients undergoing surgical resection in many cases have a 5-year survival rate less than 30% [1, 8].
Among the reasons for negative outcome, the functional and anatomical changes of the digestive tract after gastrectomy play the major role, leading to the appearance of “post-gastrectomy syndromes“ [9, 10]. Malnutrition, consistently associated with unfavorable prognosis, occurs in more than half of patients after surgery for gastric cancer, particularly after discharge from hospital [11, 12]. Micronutrients deficiency and reduction of albumin levels are found in about 2/3 of GC patients after gastrectomy, which is highly correlated with infectious complications, longer hospital stay, and, as the result, higher treatment costs and mortality [13–15]. Recent studies demonstrated that early nutritional support might significantly reduce postoperative complications discussed above, but to date there are no specific evidence-based guidelines in this field [13, 16].
The effectiveness of nutrition education was discussed before in changing practice habits associated with gastric cancer [17], as well as other types of cancer [18, 19]. Knowledge, Attitude and Practice (KAP) study is a valuable tool to evaluate the impact of education on understanding and habits. Previous KAP studies reported low knowledge regarding the post-surgery nutritional support not only in cancer patients [20], but in surgeons [21, 22] and nurses [23], calling for a discussion on the possible ways to improve this knowledge. Moreover, recent study reported the lack of acceptance of novel technologies for delivering nutrition care by health professionals [24]. In this scenario, family caregivers of GC patients have a strong demand for nutrition knowledge, driven by the necessity to battle malnourishment and arrange the adequate diet after discharge [25]. To the best of our knowledge, no previous study assessed the KAP towards nutrition support after gastrectomy among primary caregivers of GC patients.
Based on the above, this study aimed to investigate the KAP of primary caregivers of GC patients regarding postoperative dietary management, based on hypothesis that there are differences between the knowledge that caregivers have, their attitudes toward diet management, and the practices they implement, with demographics of caregivers and their relationship with patients influencing KAP scores. Obtained results might help to identify potential gaps in caregiver knowledge and practice to enable the development of targeted educational interventions and support services to optimize postoperative nutritional care for patients with GC.
Methods
Study design and participants
This cross-sectional study was conducted from August 21, 2023, to November 15, 2023, at the author’s Hospital, focusing on primary caregivers of GC patients. The research received ethical approval from the Ethics Committee of the author’s Hospital and informed consent was obtained from all participants.
Inclusion Criteria: 1. Caregivers of patients with GC confirmed by our hospital’s pathological examination. 2. Age between 18 and 75 years. 3. Adequate language communication abilities with a reasonable level of reading and writing skills. 4. Willingness to participate in the study and sign informed consent form.
Exclusion Criteria: 1. History of psychiatric disorders. 2. Refusal to participate or withdrawal during the study. 3. Incomplete questionnaire during data collection. 4. Death or withdrawal of GC patient.
We distributed questionnaire QR codes to invite family members of gastric cancer patients to participate in a survey through WeChat follow-up groups for gastric cancer patients and WeChat groups for family members. Simultaneously, we placed questionnaire QR codes in the outpatient waiting areas to encourage family members accompanying gastric cancer patients for follow-up visits to fill out the survey.
Questionnaire introduction
The questionnaire design was guided by relevant guidelines [26, 27] and recent published KAP studies that included participants providing nutrition care to patients on treatment for upper gastrointestinal cancers [21, 24]. Following the initial design, feedback from a panel of 5 experts (2 specialists in gastrointestinal surgery, 2 specialists in gastroenterology, and 1 expert in public health) was incorporated to refine the questionnaire. After the initial draft, a pilot study involving 30 participants was conducted. The reliability of the pre-experimental feedback questionnaire, assessed by Cronbach’s α coefficient, was 0.906 (with Cronbach’s α values of 0.651 for knowledge, 0.885 for attitude, and 0.907 for practice dimensions).
The final questionnaire comprises four sections: basic information, knowledge dimension, attitude dimension, and practice dimension. The knowledge dimension consisted of 12 questions, with a score range of 0–12 points, where correct answers were awarded 1 point and incorrect or unclear responses received 0 points. The attitude dimension included 8 questions, utilizing a five-point Likert scale ranging from very positive (5 points) to very negative (1 point), with a total score range of 8–40 points. The practice dimension consisted of 10 questions, employing a five-point Likert scale ranging from always (5 points) to never (1 point), with a total score range of 10–50 points. After scores were calculated, following qualitative (truncated) interpretation was used: Good (> 75% of maximal); Upper middle (75%−50% of maximal); Middle and lower (50%−25% of maximal); Poor (25% or less of maximal). Percentage of participants with “good” scores, signifying “good knowledge”, “positive attitude” and “proactive practice”, was calculated and compared between different categories, suggesting areas where caregivers may need more focused educational interventions to optimize patient care.
Statistical methods
Descriptive analysis was conducted for demographic data and KAP scores: continuous variables were presented using Mean ± SD. Categorical variables and responses to each question were described using frequency counts and percentages.
Differences in knowledge (K), attitude (A), and practice (P) scores among subjects with different demographic characteristics was assessed using independent sample analysis: group comparisons were performed using Mann-Whitney U test or Kruskal-Wallis H test.
Spearman correlation analysis was employed to explore the correlation between knowledge, attitude, and practice scores.
Single and multiple logistic regression analyses were conducted to investigate factors influencing practice.
All statistical results with a p-value less than 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 26.0.
Results
Basic characteristics of survey participants
A total of 532 questionnaires were collected. Of them 23 invalid questionnaires were excluded: (1) Disagreement with the study (3 cases); (2) Response time less than 43 s (15 cases) or greater than 2580 s (2 cases); (3) Age outliers (3 cases). A total of 508 remaining valid questionnaires were analyzed in this study, with the Cronbach’s α for the formal experiment feedback scale of 0.764 (Knowledge: 0.596, Attitude: 0.629, Practice: 0.764). The Kaiser-Meyer-Olkin (KMO) value was 0.844.
Characteristics of included participants are demonstrated in Table 1. The majority of responders were female (59.84%) urban residents (78.94%), aged 40–60 years (53.15%). Based on the relationship with GC patients, caretakers were primarily spouses (50.39%); other relationships included parents (10.43%), only child (12.99%) or non-only child (24.21%) of GC patient. Only 22.83% were sole caregivers, others reported to receive assistance from family members. Strick adherence to 3-month follow-up frequency was demonstrated by 81.69% of participants.
Table 1.
Basic information of participants and their knowledge, attitude and practice scores
| N (%) | Good* knowledge (6–9) N (%) | Knowledge, mean ± SD | P | Positive* attitude (24–40) N (%) | Attitude, mean ± SD | P | Proactive* practice (30–50) N (%) | Practice, mean ± SD | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| N = 508 | ||||||||||
| Total Score | 6.40 ± 1.96 | 32.87 ± 2.81 | 33.24 ± 6.38 | |||||||
| Gender | 0.258 | 0.343 | 0.134 | |||||||
| Male | 204 (40.16) | 92 (45.10) | 6.28 ± 1.82 | 203 (99.51) | 33.01 ± 2.77 | 121 (59.31) | 33.75 ± 6.69 | |||
| Female | 304 (59.84) | 151 (49.67) | 6.48 ± 2.05 | 302 (99.34) | 32.77 ± 2.84 | 176 (57.89) | 32.89 ± 6.16 | |||
| Age | 0.001 | 0.860 | < 0.001 | |||||||
| 18–40 years | 165 (32.48) | 88 (53.33) | 6.71 ± 2.08 | 163 (98.79) | 32.95 ± 2.94 | 124 (75.15) | 36.07 ± 6.88 | |||
| 40–60 years | 270 (53.15) | 129 (47.78) | 6.41 ± 1.78 | 269 (99.63) | 32.86 ± 2.51 | 137 (50.74) | 31.89 ± 5.61 | |||
| > 60 years | 73 (14.37) | 26 (35.62) | 5.64 ± 2.14 | 73 (100.00) | 32.74 ± 3.53 | 36 (49.32) | 31.82 ± 5.86 | |||
| Marital Status | < 0.001 | 0.237 | < 0.001 | |||||||
| Married | 427 (84.06) | 220 (51.52) | 6.61 ± 1.92 | 425 (99.53) | 32.93 ± 2.78 | 222 (51.99) | 32.15 ± 6.01 | |||
| Unmarried/ divorced/ widowed | 81 (15.94) | 23 (28.40) | 5.31 ± 1.83 | 80 (98.77) | 32.53 ± 2.98 | 75 (92.59) | 38.98 ± 5.14 | |||
| Education | < 0.001 | 0.886 | < 0.001 | |||||||
| Junior high school and below | 149 (29.33) | 55 (36.91) | 5.83 ± 1.94 | 149 (100.00) | 32.79 ± 3.19 | 83 (27.95) | 32.95 ± 6.49 | |||
| High school/ technical school | 200 (39.37) | 110 (50.65) | 6.68 ± 1.85 | 199 (99.50) | 32.87 ± 2.57 | 93 (46.50) | 31.34 ± 5.52 | |||
| Associate/ bachelor’s degree and above | 157 (30.31) | 78 (50.65) | 6.58 ± 2.02 | 157 (100.00) | 32.95 ± 2.75 | 121 (77.07) | 35.90 ± 6.42 | |||
| Residence | < 0.001 | 0.393 | 0.031 | |||||||
| Urban | 401 (78.94) | 209 (52.12) | 6.65 ± 1.84 | 398 (99.25) | 32.93 ± 2.71 | 243 (60.60) | 33.55 ± 6.33 | |||
| Rural | 107 (21.06) | 34 (31.78) | 5.48 ± 2.11 | 107 (100.00) | 32.66 ± 3.17 | 54 (50.47) | 32.06 ± 6.49 | |||
| Occupation | < 0.001 | 0.763 | < 0.001 | |||||||
| Enterprise employee | 101 (19.88) | 64 (63.37) | 7.21 ± 1.57 | 101 (100.00) | 33.09 ± 2.47 | 52 (51.49) | 31.92 ± 5.18 | |||
| Farmer | 114 (22.44) | 41 (35.97) | 5.61 ± 2.10 | 114 (100.00) | 32.82 ± 3.11 | 54 (47.37) | 31.69 ± 6.43 | |||
| Public official | 66 (12.99) | 31 (46.97) | 6.88 ± 2.05 | 66 (100.00) | 33.17 ± 2.45 | 54 (81.82) | 36.23 ± 6.01 | |||
| Worker | 85 (16.73) | 30 (35.29) | 5.79 ± 1.73 | 84 (98.82) | 32.86 ± 2.76 | 55 (64.71) | 34.71 ± 6.55 | |||
| Self-employed/ businessperson | 60 (11.81) | 36 (60.00) | 6.78 ± 1.84 | 60 (100.00) | 32.73 ± 2.84 | 39 (65.00) | 34.08 ± 7.31 | |||
| Other | 82 (16.14) | 41 (50.00) | 6.46 ± 1.88 | 80 (97.56) | 32.55 ± 3.11 | 43 (52.44) | 32.45 ± 5.97 | |||
| Monthly per capita income | < 0.001 | 0.607 | 0.650 | |||||||
| 4000 | 64 (12.60) | 19 (29.69) | 5.16 ± 2.35 | 63 (98.44) | 32.73 ± 3.94 | 34 (53.13) | 32.75 ± 7.47 | |||
| 4000–6000 | 310 (61.02) | 147 (47.42) | 6.41 ± 1.80 | 308 (99.35) | 32.81 ± 2.65 | 177 (57.10) | 33.17 ± 6.24 | |||
| 6000 | 134 (26.38) | 77 (57.46) | 6.96 ± 1.85 | 134 (100.00) | 33.07 ± 2.53 | 86 (64.18) | 33.61 ± 6.17 | |||
| Relationship with gastric cancer patient | < 0.001 | 0.026 | < 0.001 | |||||||
| Spouse | 256 (50.39) | 126 (49.22) | 6.57 ± 1.59 | 255 (99.61) | 32.98 ± 2.35 | 113 (44.14) | 30.98 ± 5.08 | |||
| Parent | 53 (10.43) | 12 (22.64) | 4.77 ± 2.35 | 53 (100.00) | 31.72 ± 3.71 | 43 (81.13) | 36.15 ± 6.92 | |||
| Only child | 66 (12.99) | 35 (53.03) | 6.65 ± 2.05 | 66 (100.00) | 33.29 ± 2.65 | 55 (63.41) | 36.77 ± 6.35 | |||
| Non-only child | 123 (24.21) | 65 (52.85) | 6.63 ± 1.96 | 121 (98.37) | 32.87 ± 2.98 | 78 (63.41) | 34.54 ± 6.79 | |||
| Other | 10 (1.97) | 5 (50.00) | 6.00 ± 3.59 | 10 (100.00) | 33.30 ± 5.42 | 8 (80.00) | 36.00 ± 7.38 | |||
| Duration of caregiving | < 0.001 | 0.263 | < 0.001 | |||||||
| < 3 months | 92 (18.11) | 30 (32.61) | 5.50 ± 2.29 | 91 (98.91) | 33.26 ± 3.40 | 72 (78.26) | 37.22 ± 6.72 | |||
| 3–6 months | 161 (31.69) | 66 (40.99) | 6.14 ± 1.65 | 159 (98.76) | 32.65 ± 2.63 | 93 (57.76) | 33.57 ± 6.50 | |||
| 6 months-1 year | 168 (33.07) | 94 (55.95) | 6.77 ± 1.84 | 168 (100.00) | 32.73 ± 2.51 | 79 (47.02) | 31.21 ± 6.51 | |||
| > 1 year | 87 (17.13) | 53 (60.92) | 7.10 ± 1.92 | 87 (100.00) | 33.14 ± 2.99 | 53 (60.92) | 32.31 ± 6.51 | |||
| Assistance in caregiving | 0.026 | 0.012 | 0.334 | |||||||
| Yes | 392 (77.17) | 195 (49.74) | 6.51 ± 1.98 | 390 (99.49) | 33.04 ± 2.83 | 228 (58.16) | 33.39 ± 6.37 | |||
| No | 116 (22.83) | 48 (41.38) | 6.04 ± 1.84 | 115 (99.14) | 32.29 ± 2.71 | 69 (59.48) | 32.73 ± 6.44 | |||
| Living with the patient | < 0.001 | 0.075 | 0.020 | |||||||
| Yes | 403 (79.33) | 175 (43.42) | 6.17 ± 1.84 | 401 (99.50) | 32.76 ± 2.70 | 240 (59.55) | 33.57 ± 6.45 | |||
| No | 105 (20.67) | 68 (64.76) | 7.28 ± 2.15 | 33.30 ± 3.20 | 104 (99.05) | 31.94 ± 5.99 | 57 (54.29) | |||
| Follow-up location after patient’s surgery | < 0.001 | 0.004 | < 0.001 | |||||||
| Country and below | 121 (23.82) | 21 (17.36) | 4.99 ± 2.01 | 120 (99.17) | 32.22 ± 3.31 | 105 (86.78) | 38.50 ± 6.09 | |||
| City and above | 387 (76.18) | 222 (57.36) | 6.84 ± 1.72 | 385 (99.48) | 33.07 ± 2.61 | 192 (49.61) | 31.59 ± 5.53 | |||
| Strict adherence to 3-month follow-up frequency | < 0.001 | < 0.001 | 0.461 | |||||||
| Yes | 415 (81.69) | 219 (52.77) | 6.63 ± 1.86 | 413 (99.52) | 33.08 ± 2.75 | 240 (57.83) | 33.14 ± 6.19 | |||
| No | 93 (18.31) | 24 (25.81) | 5.37 ± 2.06 | 92 (98.92) | 31.95 ± 2.92 | 57 (61.29) | 33.68 ± 7.22 | |||
*Percentage of participants in upper quartile, with the scores > 75% of maximal
Knowledge, attitude, and practice patterns
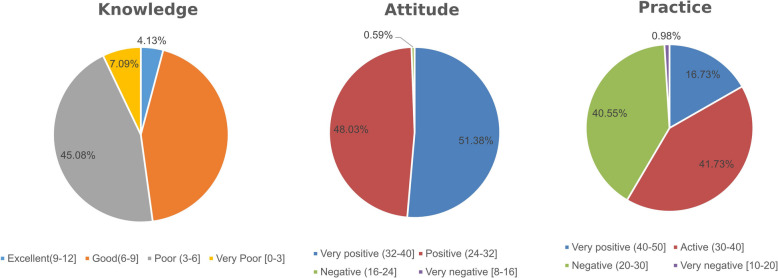
The mean knowledge score was 6.40 ± 1.96 (53.33% from maximum 12 points), with 43.70% of participants characterized by “good knowledge” according to quartile distribution, and 45.08 by “poor knowledge”. Attitude scores were 32.87 ± 2.81 (82.18% from maximum 40 points), with the majority of participants demonstrating positive (48.03%) or very positive (51.38%) attitudes. The mean practice score was 33.24 ± 6.38 (66.48% from maximum 50 points), with 41.73% characterized by proactive practice, while 40.55% had scores corresponding to inactive practice. Distribution of scores according to quartiles was demonstrated in Fig. 1.
Fig. 1.
Knowledge, Attitude, and Practice (KAP) Score Distribution Percentage, According to the 4 parts of the total range: >75%; 75%−50%; 50%−25%; 25% or less
Knowledge scores significantly differed according to age (p = 0.001), marriage status (p < 0.001), education (p < 0.001), residence (p < 0.001), occupation (p < 0.001), income (p < 0.001) and relationship to the patient (p < 0.001). Among participants who performed caregiving duty for more than 1 year, number of responders with good knowledge was almost twice higher compared to < 3 months (60.92% VS 32.61%); percentage of “good knowledge” was also higher in those not living with the patient (64.76% VS 43.42%), and participants who strictly adhered to 3-month follow-up frequency (52.77% VS 25.81%) (Table 1). The least known points included the potential of liquid diet after surgery (17.32% correct answers) and nutritional value of liquid dishes (25.79% correct answers) (Table 2).
Table 2.
Distribution of answers in the knowledge dimension
| Accuracy N (%) | |
|---|---|
| K1.After tumor resection, surgical doctors will perform digestive tract reconstruction for gastric cancer patients. | 405 (79.72) |
| K2.Gastric cancer patients are prone to anorexia due to immune response imbalance and metabolic disorders. | 405 (79.72) |
| K3. Anti-tumor treatments such as surgery and chemotherapy can cause digestive absorption disorders, leading to insufficient dietary intake and resulting in malnutrition. | 345 (67.91) |
| K5. Within 1–2 days after gastric cancer surgery, patients are in the postoperative trauma period and should wait until anal gas is discharged before eating. | 459 (90.35) |
| K6. The dietary care process for gastric cancer patients after surgery usually involves transitioning gradually from clear liquid diet to liquid diet, semi-liquid diet, soft diet, and finally to a regular diet. | 425 (83.66) |
| K7.Generally, it is recommended that gastric cancer patients change from liquid diet to semi-liquid foods, such as lotus root powder and steamed egg custard, starting from the fifth day after surgery. | 363 (71.46) |
| K9. It is advisable for gastric cancer patients to avoid eating fruits as they are considered “cooling” in nature. | 188 (37.01) |
| K10. Compared to meat (such as chicken or fish), soup (such as chicken soup or fish soup) is more nutritious. | 131 (25.79) |
| K11. After gastric cancer surgery, it is important to supplement iron elements appropriately to prevent iron-deficiency anemia. | 415 (81.69) |
| K12. It is preferable for gastric cancer patients to stick to a liquid diet to nourish the stomach after surgery. | 88 (17.32) |
| Multiple choices | N (%) |
| K4. The following dietary or eating habits are risk factors for gastric cancer: (multiple choices) | |
| High-salt diet | 231 (45.47) |
| Pickled food | 399 (78.54) |
| Fried food | 355 (69.88) |
| Roasted food | 195 (38.39) |
| Red meat and processed meat | 81 (15.94) |
| Leftover food | 132 (25.98) |
| Heavy alcohol consumption | 208 (40.94) |
| Fast eating | 26 (5.12) |
| Skipping breakfast | 51 (10.04) |
| Adequate intake of vegetables and fruits | 9 (1.77) |
| Binge eating | 234 (46.06) |
| K8. Principles of postoperative diet for gastric cancer patients include: (multiple choices) | |
| Small, frequent meals at regular intervals | 431 (84.84) |
| No alcohol consumption | 208 (40.94) |
| Light, soft diet | 224 (44.09) |
| Balanced nutrition | 223 (43.90) |
| Intake of high-protein foods | 134 (26.38) |
| Intake of large amounts of high-sugar foods | 19 (3.74) |
| Intake of fibrous foods to promote gastrointestinal motility | 31 (6.10) |
| Chewing food thoroughly | 327 (64.37) |
Attitude scores significantly differed in participants with different relationship to the patient (p = 0.026). Percentage of participants with “positive attitude” was also higher among those who strictly adhered to 3-month follow-up frequency (99.52% VS 98.92%) (Table 1). Points with less positive attitudes included the capability of dietary management to reduce the tumor recurrence rate and extend the survival period (31.89% neutral, 4.92% disagree), as well as to reduce the risk of adverse drug reactions (13.39% neutral, 0.98% disagree) (Table 3).
Table 3.
Distribution of answers in the attitude dimension
| N (%) | |||||
|---|---|---|---|---|---|
| Strongly agree | Agree | Neutral | Disagree | Strongly disagree | |
| A1. Gastric cancer patients should follow a regulated diet after surgery to prevent malnutrition. (P) | 283 (55.71) | 173 (34.06) | 50 (9.84) | 2 (0.39) | / |
| A2. Healthy dietary management after gastric cancer surgery enhances the body’s defense against pathogenic microorganisms, reducing the risk of infection. (P) | 151 (29.72) | 315 (62.01) | 40 (7.87) | 1 (0.2) | 1 (0.2) |
| A3. Healthy dietary management after gastric cancer surgery reduces the risk of adverse drug reactions. (P) | 161 (31.69) | 274 (53.94) | 68 (13.39) | 5 (0.98) | / |
| A4. Healthy dietary management after gastric cancer surgery lowers the risk of postoperative complications. (P) | 134 (26.38) | 308 (60.63) | 61 (12.01) | 5 (0.98) | / |
| A5. Healthy dietary management after gastric cancer surgery increases the chemotherapy tolerance. (P) | 120 (23.62) | 278 (54.72) | 103 (20.28) | 4 (0.79) | 3 (0.59) |
| A7. Healthy dietary management after gastric cancer surgery reduces the tumor recurrence rate and extends the survival period. (P) | 79 (15.55) | 241 (47.44) | 162 (31.89) | 25 (4.92) | 1 (0.2) |
| A6. Gastric cancer patients should prioritize hospital outpatient nutrition consultations, scheduling at least one every three months. (P) | 124 (24.41) | 315 (62.01) | 67 (13.19) | 1 (0.2) | 1 (0.2) |
| A8. Support from primary caregivers promotes gastric cancer patients’ adherence to healthy dietary management after surgery. (P) | 106 (20.87) | 342 (67.32) | 59 (11.61) | 1 (0.2) | / |
Practice scores significantly differed according to age (p < 0.001), marriage status (p < 0.001), education (P < 0.001), residence (p = 0.031), occupation (p < 0.001) and relationship to the patient (P < 0.001). Scores were significantly decreased, and percentage of participants with “proactive practice” lower in those who performed caregiving duty for more than 6 month compared to < 3 months (47.02% VS 78.26%), and those not living with the patient (54.29% VS 59.55%) (Table 1). Of all participants 45.67% seldom or never assessed the patient’s dietary calorie and other nutrient intake, while 27.16% seldom or never seek nutritional knowledge by themselves, accessing various websites or platforms (Table 4).
Table 4.
Practice dimension of the participants
| N (%) | |||||
|---|---|---|---|---|---|
| Always | Often | Sometimes | Seldom | Never | |
| K1. Assist patients in keeping a food diary to thoroughly document their food intake over several days (3 or 7 days). (P) | 66 (12.99) | 103 (20.28) | 103 (20.28) | 218 (42.91) | 18 (3.54) |
| K2. Assess the patient’s dietary calorie and other nutrient intake. (P) | 47 (9.25) | 106 (20.87) | 123 (24.21) | 130 (25.59) | 102 (20.08) |
| K3. Emphasize cooking methods such as steaming, boiling, and stir-frying, while minimizing the use of frying, deep-frying, and grilling. Reduce the use of condiments. (P) | 60 (11.81) | 287 (56.5) | 109 (21.46) | 51 (10.04) | 1 (0.2) |
| K4. Prepare a variety of foods known for their anti-cancer properties. (P) | 44 (8.66) | 165 (32.48) | 227 (44.69) | 68 (13.39) | 4 (0.79) |
| K5. When the patient’s appetite is diminished, encourage them to eat small, frequent meals or to eat whenever they feel hungry. (P) | 56 (11.02) | 231 (45.47) | 160 (31.5) | 60 (11.81) | 1 (0.2) |
| K6. Tailor the preparation of healthy foods based on the patient’s dietary preferences, creating a comfortable eating environment. (P) | 60 (11.81) | 241 (47.44) | 146 (28.74) | 59 (11.61) | 2 (0.39) |
| K7. Encourage patients to voice any questions or concerns encountered during the dietary recovery process, providing explanations and interventions. (P) | 60 (11.81) | 211 (41.54) | 162 (31.89) | 75 (14.76) | / |
| K8. Motivate patients to participate in support group meetings, health lectures, and other activities to boost enthusiasm for dietary management. (P) | 53 (10.43) | 183 (36.02) | 177 (34.84) | 90 (17.72) | 5 (0.98) |
| K9. Mobilize family support by establishing a family-centered dietary care model, offering both emotional and material assistance to the patient. (P) | 61 (12.01) | 154 (30.31) | 173 (34.06) | 117 (23.03) | 3 (0.59) |
| K10. Stay informed about post-gastric cancer surgery healthy dietary practices by accessing various websites and platforms. (P) | 57 (11.22) | 118 (23.23) | 195 (38.39) | 133 (26.18) | 5 (0.98) |
Correlation analysis of KAP scores
As demonstrated in Table 5, correlation analysis revealed a weak positive correlation between knowledge and attitude scores (r = 0.150, P < 0.001), while link to practice scores was negative (r=−0.228, P < 0.001). Additionally, attitude scores were positively correlated with practice scores (r = 0.117, P = 0.008).
Table 5.
Results of correlation analysis
| Knowledge | Attitude | Practice | |
|---|---|---|---|
| Knowledge | 1 | ||
| Attitude | 0.150 (P = 0.001) | 1 | |
| Practice | −0.228 (P < 0.001) | 0.117 (P = 0.008) | 1 |
Univariate and multivariate analyses of practice dimensions
To further investigate the associations between practice scores, knowledge, attitude and demographic characteristics of participants, logistic regression model was applied (Table 6). It was found that higher attitude scores were independently associated with higher practice scores (OR = 1.360; 95%CI, 1.223–1.513), P < 0.001), while higher knowledge scores (OR = 0.684; 95%CI, 0.575–0.815), P < 0.001), older age (OR = 0.951; 95%CI, 0.918–0.985), P = 0.005), duration of caregiving > 3 months (3–6 months (OR = 0.415; 95%CI, 0.193–0.894, P = 0.025); 6 months-1 year (OR = 0.269; 95%CI, 0.120–0.606), P = 0.002); >1 year (OR = 0.290; 95%CI, 0.120–0.705), P = 0.006), and follow-up location after patient’s surgery (OR = 0.072 (0.033–0.160), P < 0.001) were independently associated with lower practice scores.
Table 6.
Univariable and multivariable logistic regression analysis of factors influencing practice scores
| Practice (total score above 70% as positive) | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Knowledge score | 0.682 (0.612–0.761) | < 0.001 | 0.684 (0.575–0.815) | < 0.001 |
| Attitude score | 1.104 (1.032–1.181) | 0.004 | 1.360 (1.223–1.513) | < 0.001 |
| Gender | ||||
| Male | 1.252 (0.861–1.821) | 0.239 | ||
| Female | ref | |||
| Age | 0.951 (0.935–0.966) | < 0.001 | 0.951 (0.918–0.985) | 0.005 |
| Marital Status | ||||
| Married | 0.111 (0.064–0.192) | < 0.001 | 0.329 (0.139–0.783) | 0.012 |
| Unmarried/ divorced/ widowed | ref | ref | ||
| Education | ||||
| Junior high school and below | ref | ref | ||
| High school/ technical school | 0.478 (0.291–0.784) | 0.003 | 0.382 (0.169–0.864) | 0.021 |
| Associate/ bachelor’s degree and above | 2.357 (1.482–3.747) | < 0.001 | 1.048 (0.435–2.525) | 0.917 |
| Residence | ||||
| Urban | 1.362 (0.852–2.177) | 0.197 | ||
| Rural | ref | |||
| Occupation | ||||
| Enterprise employee | 0.633 (0.319–1.259) | 0.192 | 1.333 (0.479–3.711) | 0.582 |
| Farmer | 0.916 (0.484–1.732) | 0.788 | 0.382 (0.131–1.119) | 0.079 |
| Public official | 3.273 (1.651–6.489) | 0.001 | 4.585 (1.594–13.192) | 0.005 |
| Worker | 1.796 (0.940–3.430) | 0.076 | 1.684 (0.613–4.627) | 0.312 |
| Self-employed/ businessperson | 1.710 (0.844–3.466) | 0.137 | 1.736 (0.595–5.071) | 0.313 |
| Other | ref | ref | ||
| Monthly per capita income | ||||
| 4000 | ref | |||
| 4000–6000 | 0.759 (0.433–1.329) | 0.335 | ||
| 6000 | 0.930 (0.502–1.724) | 0.818 | ||
| Relationship with gastric cancer patient | ||||
| Spouse | ref | ref | ||
| Parent | 7.389 (3.895–14.019) | < 0.001 | 1.659 (0.589–4.676) | 0.338 |
| Only child | 5.921 (3.290–10.655) | < 0.001 | 0.911 (0.338–2.457) | 0.854 |
| Non-only child | 4.241 (2.604–6.908) | < 0.001 | 1.982 (0.936–4.200) | 0.074 |
| Other | 12.236 (3.038–49.279) | < 0.001 | 4.235 (0.559–32.054) | 0.162 |
| Duration of caregiving | ||||
| < 3 months | ref | ref | ||
| 3–6 months | 0.332 (0.195–0.566) | < 0.001 | 0.415 (0.193–0.894) | 0.025 |
| 6 months-1 year | 0.112 (0.062–0.201) | < 0.001 | 0.269 (0.120–0.606) | 0.002 |
| > 1 year | 0.189 (0.099–0.361) | < 0.001 | 0.290 (0.120–0.705) | 0.006 |
| Assistance in caregiving | ||||
| Yes | 1.410 (0.893–2.227) | 0.140 | ||
| No | ref | |||
| Living with the patient | ||||
| Yes | 1.572 (0.970–2.548) | 0.067 | ||
| No | ref | |||
| Follow-up location after patient’s surgery | ||||
| Country and below | ref | ref | ||
| City and above | 0.078 (0.048–0.127) | < 0.001 | 0.072 (0.033–0.160) | < 0.001 |
| Strick adherence to 3-month follow-up frequency | ||||
| Yes | 0.632 (0.398–1.002) | 0.051 | ||
| No | ref | |||
Discussion
This study have found moderate knowledge and practice, but positive attitude towards implementing the principals of postoperative dietary management among primary caregivers of GC patients after gastrectomy. Gaps in knowledge were identified, such as lack of understanding of liquid diet and some dietary components and principles that might be addressed by engaging educational help from nutrition specialists more often. In some sub-populations, especially older caregivers and those performing caregiving duty for a longer time period, lower practice scores were demonstrated; those categories might benefit from the special attention, including additional help from nurses and/or post-discharge consultations with patients and their caregivers using modern methods of communication.
To the best of our knowledge, this was a first study assessing KAP towards clinical nutrition after gastrectomy undertaken among primary caregivers of GC patients, however some of the obtained results are in line with the KAP assessment in other populations. In particular, the mean knowledge scale score in this study was barely over 50% of maximum, indicating limited knowledge – similar to the poor knowledge of GC related dietary management among medical personnel demonstrated in the study by Durán-Poveda et al. [21]. Moreover, previous study by Qu et al. [25] showed that caregivers of cancer patients have insufficient nutrition knowledge and a strong demand for education in this specific field. Although the importance of the oral nutritional supplements [11, 28] in recovering the deficiency caused by cancer treatment was known to the majority of responders (81.69%), some specific gaps in knowledge were identified in this study. For GC patients after gastrectomy early oral feeding is recommended with the transfer from liquid diet to regular diet [28, 29], however in this study the contents and specifications of liquid/semi-liquid diet were not clear for > 80% of participants. According to the recent sources including American Institute for Cancer Research [5] high salt diet may act synergistically with H. pylori infection and has been associated with a higher GC risk, which was unknown to 55.53% of responders in the present study. Points discussed above should be taken into account during the future educational interventions in the similar populations; the lack of specific knowledge might be addressed by engaging educational help from nutrition specialists more often to improve post-discharge nutritional outcomes and quality of life [11].
Despite moderate knowledge, almost all participants in this study demonstrated positive attitude, with the mean attitude scores being 82.18% of maximum, in line with other GC studies conducted in China [20, 25, 30]. Neutral or negative attitude was demonstrated in less direct questions such as the capability of dietary management to reduce the tumor recurrence rate, extend survival or reduce the risk of adverse drug reactions, also noted before and understandable in the context of the statistical probability [30]. One of the less expected findings of this study is that despite attitude scores directly correlated with knowledge (r = 0.150, P < 0.001) and practice scores (r = 0.117, P = 0.008), the correlation between knowledge and practice scores was negative (r=−0.228, P < 0.001). It partly contradicts the results reported in the previous study by Qu et al. [25] conducted in the population of the family caregivers of cancer patients, which demonstrated a significant positive correlation between nutrition knowledge and attitude(r = 0.88, P < 0.05), knowledge and practice (r = 0.766, P < 0.01), attitude and practice (r = 0.186, P < 0.01). The difference suggests that other factors might play prominent role in forming practice patterns after gastrectomy – for instance present study found that participants who performed caregiving duty for more than 1 year, and those not living with the patient had both significantly higher knowledge scores and lower practice scores. Conversely, GC patients themselves might have lesser control of their practices, as was discussed in the study by Tian et al. [20], which did not find significant correlation links between the weight loss after gastrectomy and higher nutrition knowledge. It might suggest that sub-population of caregivers, although having sufficient knowledge and positive attitude, might benefit from special help in maintaining the GC patients’ dietary support for a longer periods of time.
The mean practice score in this study was only 66.48% of maximum, which might be described as moderate, and some questions demonstrated notably lower scores. It seems slightly better than results reported by Qu et al. [25], where only 78 of 208 family caregivers of GC patients (37.5%) carried out sufficient nutrition practice. Study by Jiang et al. [31], which evaluated the adherence to the prescribed oral nutrient supplements in GC patients during preoperative and adjuvant chemotherapy periods, also reported very low compliance (24.7%), citing low motivation as the main barrier. The notable difference in the post-operative period is the even lower ability of patient to tend to their own needs and adhere to the dietary prescriptions. As the result, responsibility for the nutrition practice is at least partly shared with the nurse before discharge and the family caregiver after discharge. While the guidance of nurses was shown to be essential in improving the nutritional status of GC patients after gastrectomy [23, 32], the transfer of dietary management to the caregiver is less studied. Results obtained in the present study might provide some context to the situation around GC patients after discharge and help to plan educational interventions based on the KAP model in order to improve the self-management ability of GC patients.
Thai study has some limitations. Firstly it was a single-center study and, although the sample was comparatively big, results should be interpreted with caution to specific regional and other peculiarities. Secondly, only subjective features were accessed, and in the future the comparison of KAP with the objective measurements, such as body weight loss of GC patient after discharge, is necessary. And finally, KAP study is subjected to the inherited biases, as all questions are answered by the participants themselves, with a possibility to guess more socially accepted answer.
Conclusions
In conclusion, family caregivers of GC patients that participated in this study demonstrated moderate knowledge and practice, but positive attitude towards dietary management after gastrectomy. The lack of knowledge might be addressed by engaging educational help from nutrition specialists, while some of caregivers, although having sufficient knowledge and positive attitude, might benefit from special help in maintaining the GC patients’ dietary support for a longer periods of time.
Acknowledgements
The researchers would like to express gratitude to all participants who spend their valuable time to complete this questionnaire and contribute to the study.
Abbreviations
- GC
Gastric cancer
- KAP
Knowledge, Attitude and Practice
- KMO
Kaiser-Meyer-Olkin
Authors’ contributions
DSL and HTX carried out the studies, participated in collecting data, and drafted the manuscript. DSL, HTX, CJ and JJZ performed the statistical analysis and participated in its design. DSL, HTX, CJ, JJZ, DW, LZT, HFQ and ZS participated in acquisition, analysis, or interpretation of data and draft the manuscript. DSL, HTX, CJ, JJZ, DW, LZT, HFQ and ZS read and approved the final manuscript.
Funding
Impact and significance of different digestive tract reconstruction methods on pancreatic exocrine function after laparoscopic early proximal gastric cancer resection. [2022KY1429].
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The research received ethical approval from the Ethics Committee of Lishui Central Hospital [No: 2023(525)], and informed consent was obtained from all participants. I confirm that all methods were performed in accordance with the relevant guidelines. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Desheng Luo, Email: yiyunfei@126.com.
Hongtao Xu, Email: 38784503@qq.com.
References
- 1.Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39(4):1179–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC. Lordick F gastric cancer. Lancet. 2020;396(10251):635–48. [DOI] [PubMed] [Google Scholar]
- 3.Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. 2022;28(12):1187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thrift AP, El-Serag HB. Burden of gastric Cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [DOI] [PubMed] [Google Scholar]
- 7.Johnston FM, Beckman M. Updates on management of gastric Cancer. Curr Oncol Rep. 2019;21(8):67. [DOI] [PubMed] [Google Scholar]
- 8.Tan Z. Recent advances in the Surgical treatment of Advanced Gastric Cancer: a review. Med Sci Monit. 2019;25:3537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrillo Lozano E, Osés Zárate V. Campos Del Portillo R Nutritional management of gastric cancer. Endocrinol Diabetes Nutr (Engl Ed). 2021;68(6):428–38. [DOI] [PubMed] [Google Scholar]
- 10.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr. 2021;40(1):40–6. [DOI] [PubMed] [Google Scholar]
- 12.Wang HM, Wang TJ, Huang CS, Liang SY, Yu CH, Lin TR, et al. Nutritional status and related factors in patients with gastric Cancer after gastrectomy: a cross-sectional study. Nutrients. 2022;14(13):2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Ortega AJ, Piñar-Gutiérrez A, Serrano-Aguayo P, González-Navarro I, Remón-Ruíz PJ, Pereira-Cunill JL et al. Perioperative Nutritional support: a review of current literature. Nutrients. 2022;14(8):1601. [DOI] [PMC free article] [PubMed]
- 14.Gamble LA, Lopez R, Rajasimhan S, Samaranayake SG, Bowden C, Famiglietti AL, et al. Micronutrient Supplementation and Bone Health after prophylactic total gastrectomy in patients with CDH1 variants. J Clin Endocrinol Metab. 2023;108(10):2635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namikawa T, Shimizu S, Yokota K, Tanioka N, Iwabu J, Munekage M, et al. Serum zinc deficiency in patients after gastrectomy for gastric cancer. Int J Clin Oncol. 2021;26(10):1864–70. [DOI] [PubMed] [Google Scholar]
- 16.Mulazzani GEG, Corti F, Della Valle S, Di Bartolomeo M. Nutritional support indications in Gastroesophageal Cancer patients: from perioperative to Palliative systemic therapy. A Comprehensive Review of the last decade. Nutrients. 2021;13(8):2766. [DOI] [PMC free article] [PubMed]
- 17.Kasiri K, Amin-Shokravi F, Shahnazi H. Feeding behavior associated with gastric cancer. Iran J Health Educ Health Promotion. 2015;3(2):83–94. [Google Scholar]
- 18.van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripodi SI, Bergami E, Panigari A, Caissutti V, Brovia C, De Cicco M, et al. The role of nutrition in children with cancer. Tumori. 2023;109(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Q, Qin L, Zhu W, Xiong S, Wu B. Analysis of factors contributing to postoperative body weight change in patients with gastric cancer: based on generalized estimation equation. PeerJ. 2020;8:e9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durán-Poveda M, Suárez-de-la-Rica A, Cancer Minchot E, Ocón-Bretón J, Sánchez-Pernaute A. Rodríguez-Caravaca G Knowledge and practices of digestive surgeons concerning specialized nutritional support in cancer patients: a survey study. Nutrients. 2022;14(22):4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naffouje SA, De La Cruz K, Berard D, Guy S, Salti GI. Knowledge, attitudes and practice of surgeons regarding nutritional support in CRS and HIPEC patients: are we missing something? Eur J Cancer Care. 2019;28(1):e12930. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Zhu NN, Jiang HJ, Tao XB, Lu WH, Shen HC, et al. Prevention of underfeeding during enteral nutrition after gastrectomy in adult patients with gastric cancer: an evidence utilization project. JBI Evid Implement. 2020;19(2):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furness K, Huggins C, Croagh D, Haines T. Exploring the attitudes of health professionals providing care to patients undergoing treatment for upper gastrointestinal cancers to different models of nutrition care delivery: a qualitative investigation. Nutrients. 2021;13(3):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.QU Q, Shi P, Yang C, XU Y. Nutritional knowledge and needs of caregivers of patients with gastric cancer. Chin J Practical Nurs. 2018,34(22):1730–4.
- 26.Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(2):167–92. [DOI] [PubMed] [Google Scholar]
- 27.Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20. [DOI] [PubMed] [Google Scholar]
- 28.Hsu PI, Chuah SK, Lin JT, Huang SW, Lo JC, Rau KM, et al. Taiwan nutritional consensus on the nutrition management for gastric cancer patients receiving gastrectomy. J Formos Med Assoc. 2021;120(1 Pt 1):25–33. [DOI] [PubMed] [Google Scholar]
- 29.Bae M, Cassilly CD, Liu X, Park SM, Tusi BK, Chen X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature. 2022;608(7921):168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Zeng X, Wang W, Huang R-l, Huang Y-j, Liu S, et al. Awareness of risk factors and warning symptoms and attitude towards gastric cancer screening among the general public in China: a cross-sectional study. BMJ open. 2019;9(7):e029638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X-h, Chen X-j, Chen S, Chen J-m, Yuan X-h, Lin Y-j, et al. Compliance with oral nutritional supplementation among gastric cancer patients at nutritional risk: a cross-sectional study. Nutr Cancer. 2022;74(9):3312–21. [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Zhang W, Liu L, Guo L, Guo M, He X, et al. Application of nursing intervention based on the IKAP model in self-management of patients with gastric cancer. Am J Transl Res. 2022;14(9):6389–98. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.