Abstract
Functional investigation of genetic variants found in long QT syndrome can provide evidence that is needed to confirm the genetic diagnosis and establish the cause of the condition. We performed functional assessment to determine the z-score, using a clinically calibrated automated patch clamp assay, for 2 missense KCNH2 variants found in 2 families that have been diagnosed with long QT syndrome. These variants are currently classified as variant of uncertain significance in ClinVar. The z-scores for homozygous and heterozygous NM_000238.4:c.1819A>T p.(Ile607Phe) from family 1 were –5.16 and –3.97, respectively, and for heterozygous NM_000238.4:c.1832A>G p.(Tyr611Cys) from family 2 was –6.63. The z-scores for these variants are consistent with severe loss-of-function phenotypes. We have established the genetic cause of the long QT syndrome in these 2 families by providing validated functional evidence that supports the pathogenicity of p.(Ile607Phe) and p.(Tyr611Cys). Clinical diagnosis of long QT syndrome has been very successful in providing adequate clinical management for patients. However, functional assessment of variants found in these patients by using variant-specific z-scores can further enhance the current clinical management of patients with long QT syndrome through shared decision making based on validated experimental evidence.
Keywords: Automated Patch Clamp, Channelopathies, KCNH2, Long QT syndrome, Variant of uncertain significance
Introduction
Variants in 11 genes have been implicated in long QT syndrome (LQTS).1 Specifically, LQTS type 2 results from pathogenic variants found in KCNH2 (HGNC:6251), which encodes for the pore-forming a-subunit of the cardiac K+ channel (KV11.1).
LQTS is challenging to diagnose because of incomplete penetrance, dynamic changes in QT interval, and a high rate of concealed QT prolongation.2,3 Once suspected, genetic testing is the standard of care for confirming the diagnosis.4 Factors such as phenotype strength, rarity among ancestries, segregation analysis, and data from previous reports or cases can help to classify a specific variant.5 However, most missense variants identified in genetic testing are classified as variants of uncertain significance (VUS)6 because of insufficient evidence. Recently, functional data obtained using a calibrated automatic patch clamp (APC) assay can enhance the clinical interpretation of KCNH2 variants to aid VUS reclassification.7,8
Here, we present clinical details for 2 unique KCNH2 missense variants with 1 of them also present as homozygous because of a consanguineous marriage. The functional assessment based on z-score7 provides validated functional evidence for variant reclassification to improve the clinical management of these patients through shared decision making.
Materials and Methods
Patient cohort and genetic testing
The index cases were referred to the Chest Diseases Hospital in Kuwait for clinical management of their LQTS. Targeted gene panel testing for LQTS was performed by clinical testing laboratories using Sanger sequencing.
APC assay
A detailed protocol describing the generation of the KCNH2 DNA plasmid, Flp-In HEK293 cell and procedure to perform APC experiment has been published.9 Briefly, the plasmids carrying both the variant and wild-type genes were synthesized by GenScript Inc (Pistcataway). The heterozygous KCNH2 cell lines were generated by stable transfection of plasmids into the Flp-In T-Rex HEK 293 cells (ThermoFisher, cat. #R78007). Ion channel expression was induced by 48-h incubation with doxycycline (Sigma Aldrich, cat. # D9891) and functional evaluation of variants undertaken using a Syncropatch 384PE (Nanion Technologies). The current density was calculated by normalizing the recorded current amplitude by cell capacitance and then transformed to a normal distribution using a square root function.7,8 The transformed current densities of VUS were reported as 10th to 90th percentiles, and the z-score was utilized to assess variant function.7
Functional interpretation of VUS
The determination of z-score for the KCNH2 VUS was performed according to the calibrated KCNH2 APC assay based on benign and pathogenic variant controls.7 In brief, a scaled functional evidence range is determined by calculating the average ± standard deviation (z-score) of the normalized current density from benign controls. Functional normal range is within a deviation of ± 2 z-score. A z-score below −2 is considered as a loss of function (LoF), with the severity increasing as the z-score becomes more negative.7
Results
Figure 1 shows the genetic diagnosis pathway integrated with functional investigation to enable the interpretation of VUS in KCNH2 and facilitate the reclassification of VUS to likely pathogenic if the VUS has an abnormal function. Below, we discuss the application of this pathway to 2 families.
Figure 1.
Integration of functional investigation to improve management of LQTS. A validated APC assay can enhance the interpretation of VUS and provide validated functional evidence for variant reclassification to likely pathogenic if the VUS is abnormal or to likely benign if the VUS has a normal function with allele frequency higher than expected for the disorder (BS1). Created with BioRender.com.
Family 1
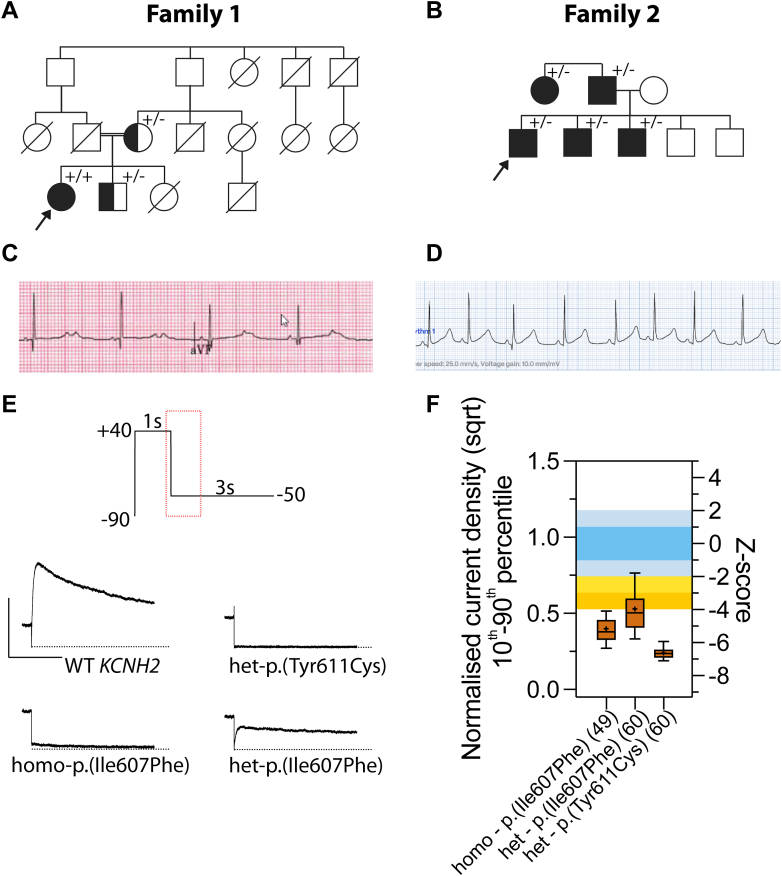
The index case was a product of a consanguineous marriage (Figure 2A), born with functional atrioventricular block due to very prolonged repolarization (>600 msec) and bifid T waves (Figure 2C). She had a rough course with frequent symptomatic Torsades de Pointes, especially during febrile episodes, despite pacing. She later received a dual chamber implantable cardiac defibrillator (ICD) with frequent appropriate shocks, left cardiac sympathetic denervation therapy, and combination medical therapy including propranolol (later bisoprolol), flecainide, labetalol, and spironolactone. Eventually, after completing bilateral sympathectomy, her symptoms could be controlled with nadolol therapy. Genetic testing found a homozygous variant in KCNH2 ((NC_000007.14:g.150951574T>A NM_000238.4:c.1819A>T p.(Ile607Phe)).
Figure 2.
Clinical and functional investigation of KCNH2 variants. A. Pedigree for family 1 with p.(Ile607Phe). Proband was detected to have homozygous p.(Ile607Phe). B. Pedigree for family 2 with heterozygous p.(Tyr611Cys). C. Electrocardiogram for index patient from family 1. D. Electrocardiogram for index patient from family 2. E. A section of –50mV peak tail current of KV11.1 channels encoded by different KCNH2 variants. F. The quantified function based on z-score for different KCNH2 variants. The evidence levels from Thomson et al7 were reproduced here with functionally normal range (z < ± 2), mild LoF (−2 < z < −3), moderate LoF (−3 < z < −4), and severe LoF (z < −4). Data are presented as 10th and 90th percentile, solid line indicates the median, and + indicates the mean. The normalized square root transformed current density with standard deviation for homo-p.(Ile607Phe), het-p.(Ile607Phe) and het-p.(Tyr611Cys) were 0.40 ± 0.12 (16% of wild type [WT]), 0.53 ± 0.18 (28.1% of WT) and 0.24 ± 0.06 (5.7% of WT), respectively. N values are shown in brackets.
Her family history is impressive. She has a sister who died soon after birth, after pacemaker implantation for 2:1 conduction and prolonged corrected QT (QTc) intervals (not genotyped). Her brother (asymptomatic and on nadolol) and mother (primary prevention ICD only) are both heterozygous for the same variant. Both were found to have borderline resting QTc intervals. The mother has a history of appropriate shock once while fasting after surgery. The father died suddenly at 37 years of age. Three aunts and 2 cousins died postpartum. Furthermore, a cousin died suddenly at 3 years with similar initial presentation as the proband and after a febrile illness. Moreover, 2 uncles died suddenly (1 preceded by a febrile illness). Unfortunately, all deceased cases were not genotyped.
The p.(Ile607Phe) is located in the pore domain and not present in gnomAD (v4.1), it is currently classified as VUS in ClinVar (Variation ID: 418245). Functional investigation using a validated APC assay shows both the homozygous and heterozygous p.(Ile607Phe) exhibiting severe LoF with z-scores of −5.16 and −3.97, respectively (Figure 2F). The functional evidence for this variant would be recommended to be applied at PS3_strong, which enables the reclassification of p.(Ile607Phe) to a likely pathogenic variant (Table 1) when using the ACMG/AMP framework.5
Table 1.
ACMG/AMP criteria and classification pre and post functional evidence (PS3)
| Family | KCNH2 | ACMG/AMP Criteria | Without PS3 | With PS3 |
|---|---|---|---|---|
| Family 1 | c.1819A>T p.(Ile607Phe) | PM1, PM2_supporting, PP3, PS3 | VUS | Likely pathogenic |
| Family 2 | c.1823A>G p.(Tyr611Cys) | PM1, PM2_supporting, PM5_supporting, PP3, PP1, PS3 | Likely pathogenic | Pathogenic |
VUS, variants of uncertain significance.
Family 2
The proband is a 9-year-old male who presented with 2 episodes of fainting associated with seizures. The electrocardiogram showed sinus bradycardia with 550 msec QTc (Figure 2D). He was started on nadolol without further symptoms. Genetic testing found a heterozygous variant in KCNH2 ((NC_000007.14:g.150951561T>C NM_000238.4:c.1832A>G p.(Tyr611Cys)). Similarly, p.(Tyr611Cys) is located in the pore domain and not present in gnomAD (v4.1). It is also currently classified as VUS in ClinVar (variation ID: 1019935).
In terms of family history, the patient’s paternal aunt was diagnosed with LQTS after presenting with sudden cardiac arrest. The father electrocardiogram showed abnormal repolarization but with QTc measuring at 460 msec. However, the aunt has not undergone genetic testing. Cascade screening confirmed the diagnosis in the father and 2 asymptomatic siblings with similar abnormal repolarization.
There were 2 missense variants in ClinVar at this position, one with pathogenic classification [NM_000238.4:c.1831T>C p.(Tyr611His)] and the other with likely pathogenic classification [NM_000238.4:c.1831T>G p.(Tyr611Asp)]. These ClinVar submissions were submitted as research and literature curations and more critically, they were submitted before the 2015 ACMG/AMP framework. However, we have previously reclassified p.(Tyr611His) as a likely pathogenic variant.7 As a result, PM5 criteria at supporting level would be applicable for p.(Tyr611Cys). Functional investigation shows that it exhibits a dominant-negative effect, consistent with other severe pore domain in KCNH2 variants.10 The z-score for p.(Tyr611Cys) was determined to be −6.63, and our functional evidence is recommended to be applied at PS3_strong, which enables reclassification of p.(Tyr611Cys) from likely pathogenic to pathogenic (Table 1).
Discussion
From the clinical perspective, both the KCNH2 p.(Ile607Phe) and p.(Tyr611Cys) missense variants were highly suspicious for causing the LQTS in these families because both were highly penetrant and were not observed in the latest release of gnomAD v4.1. Bifid T waves was a strong indication that they likely had KCNH2-related LQTS. Both variants were predicted to be deleterious with REVEL scores11 of 0.917 and 0.952, respectively. However, they are currently classified as VUS in ClinVar.12 Our functional assessment using a clinically calibrated patch clamp assay7 found both variants to be abnormal with z-scores ranging from −3.97 to −6.63.
The functional assessment of p.(Ile607Phe) (Figure 2E and F) is consistent with the observed clinical presentation in family 1 in which the homozygous p.(Ile607Phe) (z = −5.16) found in the index case has a more severe presentation than the heterozygous p.(Ile607Phe) (z = −3.97) found in the sibling and mother. This is consistent with our previous finding in which more negative z-score correlates to a more severe disease and more prolonged QTc.7 However, we do not have sufficient prospective data yet to determine whether a specific z-score value might inform therapy for such cases in the future and provide guidance in terms of nontherapy versus medical treatment, cardiac sympathetic denervation therapy, and ICD solo/combination therapies. It is also possible that functional assessment might help families further accept the diagnosis and be compliant with therapies.
One limitation of the in vitro functional testing is that it does not specifically interrogate the effect of environmental triggers. For example in family 1, members with heterozygous p.(Ile607Phe) often had episodes associated with febrile episodes.13
Conclusion
Incorporating functional assessment into the diagnosis pathway for LQTS will improve the clinical management of patients. Functional evidence established for missense variants can aid variant reclassification, especially for those with severe LoF when PS3 is applied at strong level.
Data Availability
The current traces from the APC assay that support the findings of this study are available as csv files upon request.
Conflict of Interest
The authors declare no conflicts of interests.
Acknowledgment
The authors acknowledge support from the Victor Chang Cardiac Research Institute Innovation Centre, funded by the NSW Government.
Funding
This work was funded by an NSW Cardiovascular Disease Senior Scientist grant (J.I.V.), a National Health and Medical Research Council Principal Research Fellowship (J.I.V.), and a Medical Research Future Fund: Genomics Health Futures Mission grant MRF2016760 (J.I.V. and C.-A.N.).
Author Contributions
Conceptualization: J.I.V., M.A.E., C.-A.N.; Data Curation: R.W.A., Q.S.; Formal Analysis: R.W.A., Q.S., C.-A.N.; Funding Acquisition: J.I.V., C.-A.N.; Investigation: R.W.A., Q.S., B.A., M.A.E., C.-A.N.; Methodology: R.W.A., Q.S., J.I.V., M.A.E., C.-A.N.; Supervision: J.I.V., M.A.E., C.-A.N.; Writing-original draft: R.W.A., Q.S., M.A.E., C.-A.N.; Writing-review and editing: J.I.V., M.A.E., C.-A.N.
ORCIDs
Chai-Ann Ng: http://orcid.org/0000-0002-3120-5450
Jamie I. Vandenberg: http://orcid.org/0000-0002-3859-3716
Ethics Declaration
The research included in this report was conducted in a manner consistent with the principles of research ethics, such as those described in the Declaration of Helsinki and/or the Belmont Report. In particular, this research was conducted with the voluntary, informed consent of any research participants, free of coercion or coercive circumstances, and received Research Ethics Committee (REC) approval consistent with the principles of research ethics and the legal requirements of the lead authors’ jurisdiction(s).
Footnotes
The Article Publishing Charge (APC) for this article was paid by Jamie Vandenberg.
Reema W. Aljassar and Qianyi Shen are co-first authors.
Additional Information
The online version of this article (https://doi.org/10.1016/j.gimo.2024.101868) contains supplemental material, which is available to authorized users.
Contributor Information
Jamie I. Vandenberg, Email: j.vandenberg@victorchang.edu.au.
Mohammad A. Ebrahim, Email: mohammad.ebrahim@ku.edu.kw.
Chai-Ann Ng, Email: c.ng@victorchang.edu.au.
Supplementary Material
References
- 1.Wilde A.A.M., Semsarian C., Márquez M.F., et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace. 2022;24(8):1307–1367. doi: 10.1093/europace/euac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copier J.S., Bootsma M., Ng C.A., et al. Reclassification of a likely pathogenic Dutch founder variant in KCNH2; implications of reduced penetrance. Hum Mol Genet. 2023;32(7):1072–1082. doi: 10.1093/hmg/ddac261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taggart N.W., Haglund C.M., Tester D.J., Ackerman M.J. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115(20):2613–2620. doi: 10.1161/CIRCULATIONAHA.106.661082. [DOI] [PubMed] [Google Scholar]
- 4.Musunuru K., Hershberger R.E., Day S.M., et al. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2020;13(4) doi: 10.1161/HCG.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 5.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fayer S., Horton C., Dines J.N., et al. Closing the gap: systematic integration of multiplexed functional data resolves variants of uncertain significance in BRCA1, TP53, and PTEN. Am J Hum Genet. 2021;108(12):2248–2258. doi: 10.1016/j.ajhg.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson K.L., Jiang C., Richardson E., et al. Clinical interpretation of KCNH2 variants using a robust PS3/BS3 functional patch-clamp assay. HGG Adv. 2024;5(2) doi: 10.1016/j.xhgg.2024.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang C., Richardson E., Farr J., et al. A calibrated functional patch-clamp assay to enhance clinical variant interpretation in KCNH2-related long QT syndrome. Am J Hum Genet. 2022;109(7):1199–1207. doi: 10.1016/j.ajhg.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng C.A., Farr J., Young P., et al. Heterozygous KCNH2 variant phenotyping using Flp-In HEK293 and high-throughput automated patch clamp electrophysiology. Biol Methods Protoc. 2021;6(1) doi: 10.1093/biomethods/bpab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss A.J., Zareba W., Kaufman E.S., et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105(7):794–799. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis N.M., Rothstein J.H., Pejaver V., et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877–885. doi: 10.1016/j.ajhg.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landrum M.J., Lee J.M., Benson M., et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46(D1):D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin A.S., Herfst L.J., Delisle B.P., et al. Fever-induced QTc prolongation and ventricular arrhythmias in individuals with type 2 congenital long QT syndrome. J Clin Invest. 2008;118(7):2552–2561. doi: 10.1172/JCI35337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The current traces from the APC assay that support the findings of this study are available as csv files upon request.