Abstract
Previous studies from our laboratory demonstrated that PVC-211 murine leukemia virus (MuLV), a neuropathogenic variant of Friend MuLV (F-MuLV), had undergone genetic changes which allowed it to efficiently infect rat brain capillary endothelial cells (BCEC) in vivo and in vitro. Two amino acid changes from F-MuLV in the putative receptor binding domain (RBD) of the envelope surface protein of PVC-211 MuLV (Glu-116 to Gly and Glu-129 to Lys) were shown to be sufficient for conferring BCEC tropism on PVC-211 MuLV. Recent examination of the unique RBD of PVC-211 MuLV revealed that the substitution of Lys for Glu at position 129 created a new heparin-binding domain that overlapped a heparin-binding domain common to ecotropic MuLVs. In this study we used heparin-Sepharose columns to demonstrate that PVC-211 MuLV, but not F-MuLV, can bind efficiently to heparin and that one or both of the amino acids in the RBD of PVC-211 MuLV that are associated with BCEC tropism are responsible. We further showed that heparin can enhance or inhibit MuLV infection and that the mode of action is dependent on heparin concentration, sulfation of heparin, and the affinity of the virus for heparin. Our results suggest that the amino acid changes that occurred in the envelope surface protein of PVC-211 MuLV may allow the virus to bind strongly to the surface of BCEC via heparin-like molecules, increasing the probability that the virus will bind to its cell surface receptor and efficiently infect these cells.
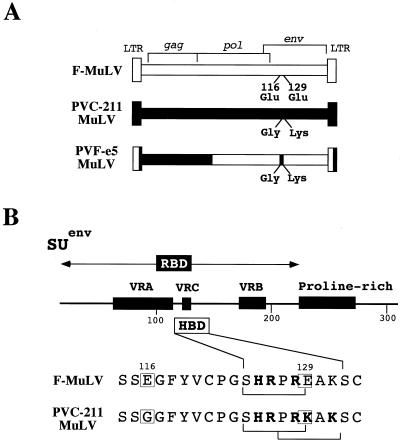
PVC-211 murine leukemia virus (MuLV), a neuropathogenic variant of the leukemia-inducing Friend MuLV (F-MuLV) (13), causes a progressive neurodegenerative disease in susceptible rodents (11, 19). The primary target of PVC-211 MuLV infection is the brain capillary endothelial cell (BCEC), with reactive astrocytes and degenerating neurons within the central nervous system showing no evidence of virus infection (11, 20, 22). Previous studies using chimeras between F-MuLV and PVC-211 MuLV have demonstrated a direct correlation between BCEC tropism and neuropathogenicity, with chimera PVF-e5 localizing the changes responsible for BCEC tropism to two amino acids (Glu-116 to Gly and Glu-129 to Lys) which lie within the putative receptor binding domain (RBD) of the envelope surface glycoprotein (21) (Fig. 1).
FIG. 1.
Genetic structures of MuLVs used in this study and location of putative HBDs in their envelope proteins. (A) Comparison of the genetic structures of F-MuLV, PVC-211 MuLV, and the chimera PVF-e5. The envelope protein of PVC-211 MuLV has undergone two amino acid changes from F-MuLV, Glu-116 to Gly and Glu-129 to Lys, that were shown by the chimera PVF-e5 to be responsible for its unique BCEC tropism. (B) Location of putative HBDs within the RBD of the MuLV surface envelope protein (SU). Positions of variable regions and a proline-rich domain similar to the immunoglobulin hinge region are shown (7). A comparison of F-MuLV and PVC-211 MuLV sequences within this region is shown below. Boxes indicate amino acid differences that are associated with BCEC tropism. Brackets indicate HBDs based on the consensus sequence XBBXBX, where X is any amino acid and B is a basic amino acid (4). The substitution of Lys at position 129 in PVC-211 MuLV creates an additional, overlapping HBD in the RBD of this virus.
It is not known how the genetic changes in the PVC-211 MuLV RBD result in BCEC tropism. Both PVC-211 MuLV and F-MuLV utilize the cationic amino acid transporter CAT-1 to enter cells (1), and it is possible that the changes that occurred in the RBD of PVC-211 MuLV allow this virus to bind more efficiently than F-MuLV to CAT-1 expressed on BCECs. Alternatively, the changes may allow PVC-211 virions to come in contact with the highly negative surfaces of BCECs (9, 30), increasing the probability that they will interact with CAT-1. In support of the latter idea, we observed that the changes in the PVC-211 MuLV RBD from Glu to Gly at position 116 and from Glu to Lys at position 129 decreased the net negative charge of the protein and created an additional heparin binding domain (HBD) in the RBD of the virus; both of these features could allow PVC-211 MuLV to bind efficiently to proteins containing heparin or heparan sulfate on the surfaces of BCECs.
To date, two mammalian consensus HBDs have been described (XBBXBX and XBBBXXBX, where X is any amino acid and B is a basic amino acid) (4). As shown in Fig. 1B, PVC-211 MuLV and F-MuLV each contain a linear HBD in the RBD. However, substitution of Lys at position 129 in PVC-211 MuLV creates an additional, overlapping putative HBD in the RBD of this virus, raising the possibility that PVC-211 MuLV may exhibit an increased affinity for heparin and that this may allow the virus to efficiently infect BCECs through interaction with heparin-like molecules on the surfaces of these cells. Therefore, we compared the affinities of PVC-211 MuLV, F-MuLV, and chimera PVF-e5 for heparin and tested whether exposure of virus or cells to heparin could enhance or inhibit the infectivities of these viruses for rat fibroblasts or BCECs.
Binding of virus to heparin.
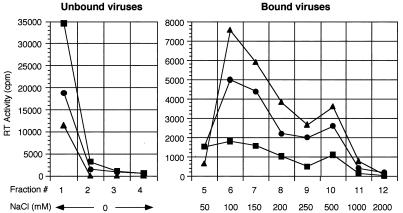
The ability of virus to bind to heparin was investigated by heparin-Sepharose chromatography of concentrated virus particles. For these studies, virus was quantified using a reverse transcriptase (RT) assay on cell supernatants, which correlates strongly with expression of viral envelope protein as well as with infectious virus titer as determined by a focal infectivity assay on NIH 3T3 cells (data not shown). As shown in Fig. 2 and summarized in Table 1, a large amount of F-MuLV quickly passed through the heparin column (unbound virus), while only a small amount (15%) was eluted with NaCl (bound virus). In contrast, a much smaller amount of PVC-211 MuLV passed through the column, while a large amount was eluted with NaCl (50%), indicating much stronger binding of PVC-211 MuLV than of F-MuLV to heparin. To determine if the additional putative HBD in the envelope protein of PVC-211 MuLV (Fig. 1) accounted for its increased heparin binding, we utilized chimera PVF-e5 MuLV, which contains this additional putative HBD on the background of F-MuLV envelope sequences. As shown in Fig. 2 and Table 1, PVF-e5 MuLV bound to heparin even more strongly than PVC-211 MuLV, with 71% of the virus retained on the column. Western blotting of each fraction with an anti-gp70 antiserum indicated that the envelope protein remained tightly associated with virions during the course of separation on heparin columns (data not shown). These results suggest that the change from Glu-129 to Lys, and probably that from Glu-116 to Gly, in the RBD of the envelope protein is critical in enhancing the affinity of viral particles for heparin.
FIG. 2.
Heparin-Sepharose chromatography of virus. Concentrated F-MuLV (▪), PVC-211 MuLV (●), and PVF-e5 (▴) virions were prepared by high-speed centrifugation and quantified using an RT assay as previously described (16). Equivalent amounts of virus (each corresponding to an RT activity of 2,500,000 cpm) were added to heparin-Sepharose columns (HiTrap, Amersham Pharmacia Biotech., Piscataway, N.J.) equilibrated with 10 mM phosphate buffer (pH 7.4). After collection of unbound viruses, bound viruses were eluted with increasing NaCl concentrations ranging from 50 mM to 2.0 M. The RT activity of each fraction was then determined.
TABLE 1.
Affinities of viruses for heparin-Sepharosea
| Virus | % Unbound virus
|
% Bound virus
|
||||
|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Mean | Expt 1 | Expt 2 | Mean | |
| F-MuLV | 83 | 86 | 85 | 17 | 14 | 15 |
| PVC-211 MuLV | 54 | 43 | 49 | 46 | 53 | 50 |
| PVF-e5 MuLV | 31 | 27 | 29 | 69 | 73 | 71 |
Viruses were separated by heparin-Sepharose chromatography. The ratio of the total amount of RT activity in the passthrough fractions (no NaCl) (unbound virus) to that of the eluted fractions (50 to 2,000 mM NaCl) (bound virus) was calculated. The results obtained were from two independent experiments.
Effect of heparin on virus infectivity.
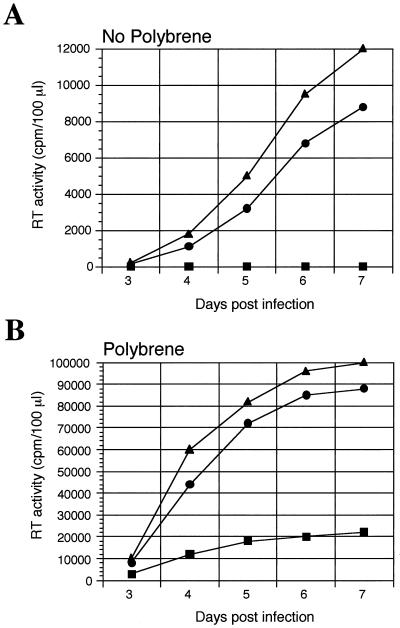
Anionic polymers such as heparin and dextran sulfate have been reported to inhibit retrovirus infection (2, 12, 15), while cationic polymers such as DEAE-dextran or hexadimethrine bromide (Polybrene) enhance infection of cells by most animal retroviruses (27, 29). Since PVC-211 and PVF-e5 MuLV can bind heparin significantly better than F-MuLV, we carried out studies to determine if the infectivities of these viruses for cells were modulated differently by heparin. Although MuLV infections are usually carried out in the presence of Polybrene to enhance infection, we carried out these experiments in its absence, since Polybrene has been reported to inhibit the activity of heparin (29). As shown in Fig. 3A, in the absence of Polybrene, PVC-211 and PVF-e5 MuLV, but not F-MuLV, can infect primary BCECs, while all three viruses were able to infect Rat-1 fibroblasts (data not shown). Addition of Polybrene to the medium (Fig. 3B) significantly enhanced infection of BCECs by F-MuLV as well as by PVC-211 and PVF-e5 MuLV. These data were confirmed by Western blotting with an antiserum to MuLV gp70 (data not shown).
FIG. 3.
Susceptibility of primary rat BCECs to viral infection in the presence and absence of Polybrene. Primary rat BCECs were isolated from the brains of 21-day-old F344 rats and grown as previously described (11). Cells were infected in the absence of Polybrene (A) or in the presence of 5 μg of Polybrene/ml (B) with equivalent amounts of F-MuLV (▪), PVC-211 MuLV (●), or PVF-e5 MuLV (▴) based on RT activity (10,000 cpm). At various times after infection, virion-associated RT activity in culture supernatants was determined. Each data point is the mean of values for duplicate samples.
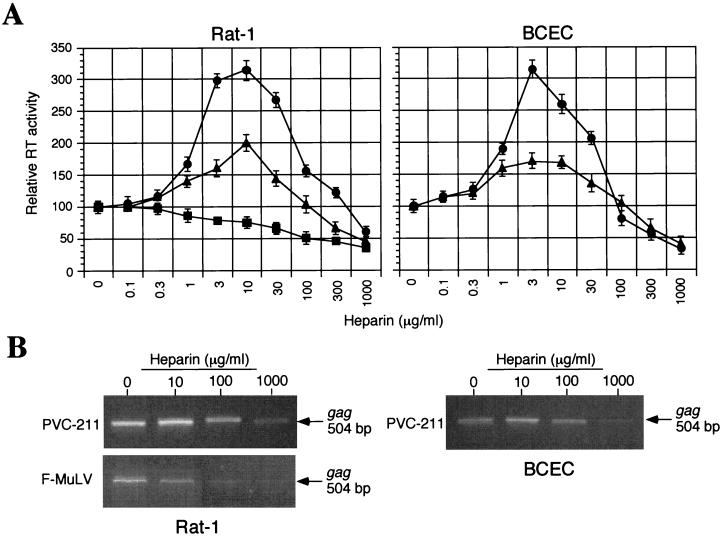
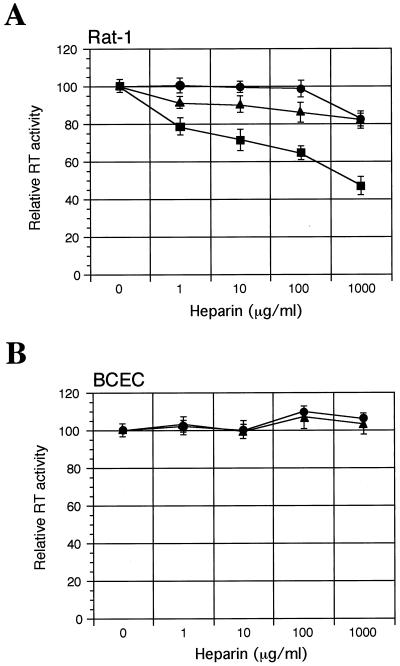
To determine the effects of heparin on virus infectivity in the absence of Polybrene, virions were incubated with or without various concentrations of soluble heparin (from porcine intestinal mucosa; Sigma, St. Louis, Mo.) for 1 h at 37°C. The virus-heparin mixture was incubated with cells for another hour, and then the cells were washed and cultured for 4 days (Rat-1 cells) or 7 days (BCECs). Replication of virus was evaluated by measurement of virion-associated RT activity in culture supernatants. Surprisingly, concentration-dependent dual effects (enhancement and inhibition) of heparin on the infectivities of PVC-211 and PVF-e5 MuLV for both Rat-1 fibroblasts and BCECs were found (Fig. 4A). Infection was inhibited at high heparin concentrations (>300 μg/ml) but enhanced at low heparin concentrations (<30 μg/ml). In contrast, only inhibitory effects of heparin on F-MuLV infection of Rat-1 fibroblasts were observed (Fig. 4A). F-MuLV is unable to infect BCECs when Polybrene is excluded from the medium (see Fig. 3A), so the effect of heparin on F-MuLV infection of these cells was not tested. The effects of heparin do not appear to be dependent on multiple rounds of infection but rather reflect an effect at the level of viral entry. Using PCR to detect integrated viral DNA at 24 h postinfection, we were able to demonstrate that the level of integrated viral DNA increases and decreases in a heparin-dependent manner and directly correlates with the level of virus detected by the RT assay at 4 to 7 days postinfection (Fig. 4B). This assay is specific for viral entry, since integrated viral DNA could not be detected when cells were infected with MuLV that had been preincubated with an anti-MuLV gp70 serum (data not shown).
FIG. 4.
Effect of treating virus with heparin on virus infectivity. Prior to infection, equivalent amounts (corresponding to an RT activity of 10,000 cpm) of F-MuLV (▪), PVC-211 MuLV (●), or PVF-e5 MuLV (▴) were incubated in the presence or absence of the indicated concentrations of heparin for 1 h at 37°C. Then the virus-heparin mixture was incubated with Rat-1 cells or primary rat BCECs in the absence of Polybrene for 1 h at 37°C. To stop the adsorption process, cells were washed and fresh culture medium was added. (A) After 4 days (Rat-1) or 7 days (primary rat BCECs), virion-associated RT activity in the culture supernatants was measured. Results are expressed as RT activity relative to that in the control (no heparin). Each data point is the mean ± standard error of the mean of triplicate samples. (B) After 24 h, cellular DNA was extracted and subjected to PCR analysis with primers specific for the viral gag region (sense, 5′-CTGGGAGACGTCCCAGGGACTTCG-3′; antisense, 5′-GACCTGATCCGGATGTCCATGTGG-3′). The effect of treating F-MuLV with heparin on BCEC infectivity was not determined because these cells are already resistant to F-MuLV.
It has been reported that heparin binds specifically to a number of cell types, including endothelial cells (8, 17, 23). To determine if heparin affects virus infection by binding to the target cells, Rat-1 fibroblasts or primary rat BCECs were incubated with increasing concentrations of heparin for 1 h at 37°C, washed to remove unbound heparin, and incubated with virus for 1 h at 37°C. After several days of culture, virus infection was determined by the RT assay. As shown in Fig. 5A, treatment of Rat-1 cells with heparin had little or no effect on infection by the BCEC-tropic viruses PVC-211 and PVF-e5 MuLV, whereas F-MuLV infection was inhibited in a dose-dependent manner. When primary rat BCECs were incubated with heparin before virus inoculation, heparin had no significant effect on PVC-211 or PVF-e5 MuLV infection (Fig. 5B). Heparin did not affect the infectivity of any of the viruses for Rat-1 cells or BCECs when it was added after virus inoculation (data not shown), indicating that its activity is not due to alteration of cell growth or inhibition of intracellular viral RT activity. These results suggest that heparin affects the initial steps of infection, such as the binding or fusion of virus, and that the mode of action of heparin on virus infectivity is strongly dependent on its affinity for the virus.
FIG. 5.
Effect of treating cells with heparin on virus infectivity. Rat-1 cells (A) or primary rat BCECs (B) were incubated for 1 h in fresh medium containing heparin (1 to 1,000 μg/ml), washed with medium, and then incubated with equivalent amounts of F-MuLV (▪), PVC-211 MuLV (●), or PVF-e5 virus (▴) (RT activity, 10,000 cpm) for 1 h in the absence of Polybrene. Then cells were washed, and fresh medium was added. After 4 days (Rat-1 cells) or 7 days (primary rat BCECs), virion-associated RT activity in the culture supernatants was measured. Results are expressed as RT activity relative to that in the control (no heparin). Each data point is the mean ± standard error of the mean of triplicate samples. Infection of Rat-1 cells by F-MuLV was significantly inhibited by heparin (P < 0.05 by Student's t test) at all concentrations tested. The effect of treating BCECs with heparin on F-MuLV infectivity was not determined because these cells are already resistant to F-MuLV.
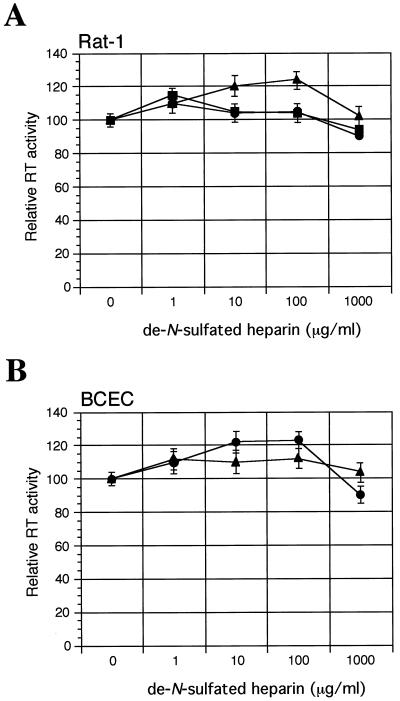
Heparin is known to be the most acidic polysaccharide in nature due to its high extent of sulfation. To examine the importance of the sulfate in heparin for its effect on virus infection, desulfated heparin was tested for its ability to alter viral infectivity. As shown in Fig. 6, de-N-sulfated heparin, which lacks N-sulfate, had little or no effect, compared to unmodified heparin, on virus infectivity (see Fig. 4). Thus, the N-sulfate of heparin is essential for its effect on virus infection.
FIG. 6.
Effect of de-N-sulfated heparin on virus infection. Prior to infection, equivalent amounts (corresponding to an RT activity of 10,000 cpm) of F-MuLV (▪), PVC-211 MuLV (●), or PVF-e5 MuLV (▴) were incubated in the presence or absence of indicated concentrations of de-N-sulfated heparin for 1 h at 37°C. Then the virus–de-N-sulfated heparin mixture was incubated with Rat-1 cells (A) or primary rat BCECs (B) in the absence of Polybrene for 1 h at 37°C. To stop the adsorption process, cells were washed and fresh culture medium was added. After 4 days (Rat-1 cells ) or 7 days (primary rat BCECs), virion-associated RT activity in culture supernatants was measured. Results are expressed as RT activity relative to that in the control (no de-N-sulfated heparin). Each data point is the mean ± standard error of the mean of triplicate samples. Heparin (Fig. 4) and de-N-sulfated heparin were significantly different (P < 0.01) in their effects on virus infection. The effect of treating F-MuLV with de-N-sulfated heparin on BCEC infectivity was not determined because these cells are already resistant to F-MuLV.
In conclusion, our results indicate that the level of binding of PVC-211 MuLV to heparin is significantly higher than that of F-MuLV and that this can be attributed to one or both of the amino acid changes in the RBD of PVC-211 MuLV, particularly Glu-129 to Lys, which creates a unique heparin binding site. Thus, the ability of PVC-211 MuLV to bind heparin, combined with its decreased negative charge, may increase its interaction with the surfaces of BCECs. Consistent with this idea, we were able to use heparin to modulate the infection of BCECs or rat fibroblasts by MuLV; the effect (enhancement or inhibition) was dependent on heparin concentration and sulfation and on the affinity of the virus for heparin.
Although the RBD of F-MuLV, as well as those of other ecotropic MuLVs, contains a putative HBD, the virus binds poorly to heparin. This may be due to the presence of a negatively charged glutamic acid residue in the middle of this domain, which is unusual among heparin binding sites of published proteins (3, 10) and may inhibit efficient binding to heparin. In contrast, the unique HBD present in the PVC-211 MuLV envelope protein contains residues commonly found in heparin binding proteins, and it is most likely responsible for the ability of this virus to bind to heparin efficiently. The second amino acid change in the PVC-211 MuLV RBD that is associated with BCEC tropism (Glu-116 to Gly) may also contribute to the increased affinity of the virus for heparin, because this mutation (negatively charged amino acid to nonpolar amino acid) would elevate the net positive charge of the RBD. Since our studies were carried out using intact virions, the unique HBD of PVC-211 MuLV must be exposed on the trimeric envelope glycoprotein structures known to exist on the surfaces of MuLV particles (5). Interestingly, PVF-e5 MuLV, which contains the RBD of PVC-211 MuLV on an F-MuLV background, has an even higher affinity than PVC-211 MuLV for heparin. Perhaps the F-MuLV-derived envelope gene sequences in PVF-e5 MuLV change the conformation of the envelope protein in the intact virion to better expose the PVC-211 MuLV-derived HBD to heparin.
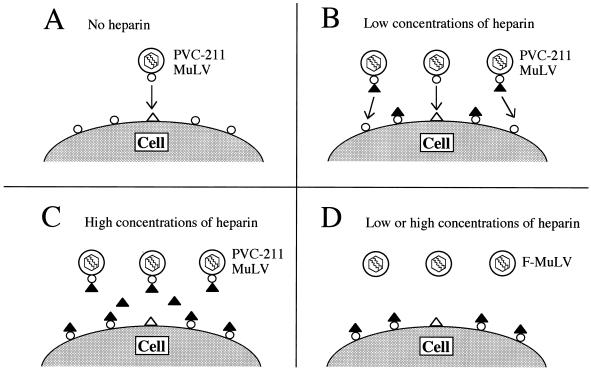
Previous studies have shown that heparin can inhibit retroviral infection, and our work extends those studies by showing that the effect of heparin on MuLV infection is strongly influenced by the affinity of the virus for heparin. Figure 7 is a schematic representation of the putative action of heparin on virus infection. PVC-211 is normally able to infect BCECs due to the presence in the viral envelope protein of an HBD which binds to heparin-like molecules on the cell surface (Fig. 7A). In addition, the decreased negative charge of the viral envelope protein, due to the change from Glu-116 to Gly, should also increase the probability that the virus will bind to the negatively charged cell surface. Infection of cells by PVC-211 MuLV is further enhanced by the treatment of the virus with low concentrations (1 to 30 μg/ml) of heparin (Fig. 7B). Since initial binding of MuLV particles to cells does not require specific envelope-receptor interactions (25), virus-bound heparin might promote nonspecific binding of virus to cells mediated by a heparin binding molecule(s) expressed on the cell surface. Alternatively, a conformational change in the envelope protein induced by heparin binding (24, 26) may increase the affinity of the virus for CAT-1, its cell surface receptor (not shown). The high affinity of PVC-211 MuLV for heparin is apparently required for this enhancement, because exposure of cells to heparin before infection with virus did not lead to enhancement of virus infection. Infection of cells with PVC-211 MuLV is inhibited by treatment of the virus with high concentrations (>300 μg/ml) of heparin (Fig. 7C). Increased amounts of virus-bound heparin, cell-bound heparin, and free heparin might inhibit attachment of the virus to the cell surface through electrostatic repulsions of negatively charged heparin. Infection of cells with F-MuLV is inhibited by treatment of cells with low or high concentrations of heparin (Fig. 7D). In addition to lacking an effective HBD, the envelope protein of F-MuLV is much more negatively charged than that of PVC-211 MuLV, causing it to repel the negatively charged heparin bound to the cell surface. Thus, the net effect of heparin on virus infection appears to be dependent on the concentration of heparin, the affinity of the virus for heparin, and probably the affinity and number of binding sites for heparin on the target cells.
FIG. 7.
Schematic representation of a putative model for the effects of heparin on virus infection. (A) PVC-211 MuLV infection of BCECs; (B and C) PVC-211 MuLV infection of BCECs or Rat-1 cells; (D) F-MuLV infection of Rat-1 cells. ▴, soluble heparin; ▵, heparin-like molecules on the cell surface; ○, heparin binding proteins on the virus or the cell. Other negatively charged molecules on the cell surface are not shown.
The heparin-binding ability of PVC-211 MuLV may be related to its BCEC tropism. We observed a direct correlation between the heparin-binding ability of MuLV and efficiency of replication in primary BCECs. Heparin-like molecules are expressed on the surfaces of endothelial cells, where they play a role in regulating blood coagulation (6, 14, 18). Due to the high negative charge density of heparin, BCECs would be expected to repel most negatively charged MuLVs, decreasing the probability that the virus will be able to enter these cells. However, creation of an efficient heparin binding site within the MuLV envelope protein would allow it to bind to heparin-like molecules on the surfaces of BCECs. Thus, the amino acid changes that occurred in the RBD of the envelope surface protein of PVC-211 MuLV may play an essential role in determining the BCEC tropism of the virus by allowing the viral envelope protein to interact with heparin-like molecules on the surfaces of BCECs, increasing the probability that the virus will bind the viral cell surface receptor, CAT-1, and enter these cells.
Further analysis will clarify the role of increased heparin binding activity of BCEC-tropic viruses in the initial step of infection. Interestingly, PVC-441 MuLV, which is closely related to PVC-211 MuLV but lacks the amino acid change at position 129 which creates the unique HBD, can still infect BCECs in vitro, albeit less efficiently than PVC-211 MuLV (28). PVC-441 MuLV, like PVC-211 MuLV, contains the Glu-116-to-Gly substitution, and this change, in conjunction with one or more of the 24 other amino acids substituted in its SU protein, may act to confer some level of BCEC tropism on the virus. The additional change of Glu-129 to Lys in the SU protein of PVC-211 MuLV not only enhances the efficiency of infection of BCECs by PVC-211 MuLV but also results in a virus that causes neurological disease after a much shorter latency period than that of PVC-441 MuLV (30 days versus 60 to 73 days) (28). Thus, the unique HBD in the SU protein of PVC-211 MuLV may play an important role not only in determining the efficiency of infection of BCECs but also in the development of neurological disease in vivo.
Acknowledgments
We thank Paul Hoffman and Natalie Dugger, Department of Veteran Affairs Medical Center, Baltimore, Md., for kindly preparing primary rat BCECs.
REFERENCES
- 1.Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Bagasra O, Lischner H W. Activity of dextran sulfate and other polysaccharides against human immunodeficiency virus. J Infect Dis. 1988;158:1084–1087. doi: 10.1093/infdis/158.5.1084. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell E E O, Nadkarni V D, Fromm J R, Linhardt R J, Weiler J M. Importance of specific amino acids in protein binding sites for heparin and heparan sulfate. Int J Biochem Cell Biol. 1996;28:203–216. doi: 10.1016/1357-2725(95)00123-9. [DOI] [PubMed] [Google Scholar]
- 4.Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 6.de Agostini A, Watkins S C, Slayter H S, Youssoufian H, Rosenberg R D. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J Cell Biol. 1990;111:1293–1304. doi: 10.1083/jcb.111.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fass D, Davey R A, Hamson C A, Kimm P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 Å resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 8.Glimelius B, Busch C, Hook M. Binding of heparin on the surface of cultured human endothelial cells. Thromb Res. 1978;12:773–782. doi: 10.1016/0049-3848(78)90271-2. [DOI] [PubMed] [Google Scholar]
- 9.Hardebo J E, Kahrstrom J. Endothelial negative surface charge areas and blood-brain barrier function. Acta Physiol Scand. 1985;125:495–499. doi: 10.1111/j.1748-1716.1985.tb07746.x. [DOI] [PubMed] [Google Scholar]
- 10.Hileman R E, Fromm J R, Weiler J M, Linhardt R J. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman P M, Cimino E H, Robbins D S, Broadwell R D, Powers J M, Ruscetti S K. Cellular tropism and localization in the rodent nervous system of a neuropathogenic variant of Friend murine leukemia virus. Lab Investig. 1992;67:314–321. [PubMed] [Google Scholar]
- 12.Hosoya M, Balzarini J, Shigeta S, Clercq E D. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob Agents Chemother. 1991;35:2515–2520. doi: 10.1128/aac.35.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kai K, Furuta T. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J Virol. 1984;50:970–973. doi: 10.1128/jvi.50.3.970-973.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima T, Leone C W, Marchildon G A, Marcum J A, Rosenberg R D. Isolation and characterization of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J Biol Chem. 1992;267:4859–4869. [PubMed] [Google Scholar]
- 15.Le Doux J M, Morgan J R, Yarmush M L. Differential inhibition of retrovirus transduction by proteoglycans and free glycosaminoglycans. Biotechnol Prog. 1999;15:397–406. doi: 10.1021/bp990049c. [DOI] [PubMed] [Google Scholar]
- 16.Leiber M M, Sherr C J, Todaro G J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974;13:587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- 17.Letourneur D, Caleb B L, Castellot J J. Heparin binding, internalization, and metabolism in vascular smooth muscle cells. I. Upregulation of heparin binding correlates with antiproliferative activity. J Cell Physiol. 1995;165:676–686. doi: 10.1002/jcp.1041650327. [DOI] [PubMed] [Google Scholar]
- 18.Marcum J A, Rosenberg R D. Heparin like molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985;126:365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- 19.Masuda M, Remington M P, Hoffman P M, Ruscetti S K. Molecular characterization of a neuropathogenic and nonerythroleukemic variant of Friend murine leukemia virus PVC-211. J Virol. 1992;66:2798–2806. doi: 10.1128/jvi.66.5.2798-2806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda M, Hoffman P M, Ruscetti S K. Viral determinants that control neuropathogenicity of PVC-211 murine leukemia virus in vivo determine brain capillary endothelial cell tropism of the virus in vitro. J Virol. 1993;67:4580–4587. doi: 10.1128/jvi.67.8.4580-4587.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda M, Hanson C A, Alvord W G, Hoffman P M, Ruscetti S K, Masuda M. Effect of subtle changes in the SU protein of ecotropic murine leukemia virus on its brain capillary endothelial cell tropism and interference properties. Virology. 1996;215:142–151. doi: 10.1006/viro.1996.0017. [DOI] [PubMed] [Google Scholar]
- 22.Masuda M, Hanson C A, Dugger N V, Robbins D S, Wilt S G, Ruscetti S K, Hoffman P M. Capillary endothelial cell tropism of PVC-211 murine leukemia virus and its application for gene transduction. J Virol. 1997;71:6168–6173. doi: 10.1128/jvi.71.8.6168-6173.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton W A, Granzow C A, Getts L A, Thomas S C, Zotter L M, Gunzel K A, Lowe-Krentz L J. Identification of a heparin-binding protein using monoclonal antibodies that block heparin binding to porcine aortic endothelial cells. Biochem J. 1995;311:461–469. doi: 10.1042/bj3110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petitou M, Lormeau J-C, Choay J. Interaction of heparin and antithrombin III. The role of O-sulfate groups. Eur J Biochem. 1988;176:637–640. doi: 10.1111/j.1432-1033.1988.tb14324.x. [DOI] [PubMed] [Google Scholar]
- 25.Pizzato M, Marlow S A, Blair E D, Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinay P, Jacquinet J-C, Petitou M, Duchaussory P, Lederman I, Choay J, Torri G. Total synthesis of heparin pentasaccharide fragment having high affinity for antithrombin III. Carbohydr Res. 1984;132:C5–C9. doi: 10.1016/0008-6215(87)80268-9. [DOI] [PubMed] [Google Scholar]
- 27.Somers K D, Kirsten W H. Focus formation by murine sarcoma virus: enhancement by DEAE-dextran. Virology. 1968;36:155–157. doi: 10.1016/0042-6822(68)90129-3. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka A, Oka K, Tanaka K, Jinno A, Ruscetti S K, Kai K. The entire nucleotide sequence of Friend-related and paralysis-inducing PVC-441 murine leukemia virus (MuLV) and its comparison with those of PVC-211 MuLV and Friend MuLV. J Virol. 1998;72:3423–3426. doi: 10.1128/jvi.72.4.3423-3426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyoshima K, Vogt P K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969;38:414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- 30.Vorbrodt A W. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J Neurocytol. 1989;18:359–368. doi: 10.1007/BF01190839. [DOI] [PubMed] [Google Scholar]