Abstract
Objectives
Propofol administration is associated with pain, mediated by the activation of vascular endothelium. Hydroxyethyl starch (HES) inhibits endothelial membrane activation by various nociceptive substances. Thus, this study hypothesised that pre-administration of HES can reduce pain on propofol administration. This study aimed to compare the proportion of patients with no pain on propofol administration and to compare the severity of pain and any change in pre- and post-induction haemodynamic variables.
Methods
This prospective, randomised, placebo-controlled clinical trial was conducted at Chirayu Medical College & Hospital, Bhopal, India, between August 2023 and December 2023 and included patients undergoing elective surgery under general anaesthesia. Patients were randomly assigned to 3 groups to receive either 100 mL of 6% HES followed by propofol (Group HES), 100 mL normal saline (NS) followed by propofol premixed with 2% lidocaine (Group L) or 100 mL NS followed by propofol induction (Group P).
Results
A total of 339 patients were included. The proportion of patients with no pain on propofol injection was significantly higher in the HES group (n = 75) than in the lignocaine (n = 33) and placebo (n = 13) groups (P <0.0001 each). The median pain scores were 0 (interquartile range [IQR]: 0–1), 1 (IQR: 0–1) and 2 (IQR: 2–3) in the HES, lignocaine and placebo groups, respectively. The proportion of patients with moderate (n = 44) and severe (n = 48) pain scores was significantly higher in the placebo group than in the HES and lignocaine groups (P <0.0001 each).
Conclusion
The proportion of patients experiencing pain on propofol injection was found to be significantly less with the pre-administration of 100 mL 6% HES compared to the pre-administration of lidocaine.
Keywords: Hydroxyethyl Starches, Lidocaine, Pain, Propofol
Advances in Knowledge
- The present study compares the efficacy of 6% hydroxyethyl starch (HES) and 2% lidocaine in reducing the pain of propofol injection in patients undergoing elective surgery under general anaesthesia.
- The proportion of patients with no pain on propofol injection was significantly higher in the HES group than in the lignocaine and placebo groups. The median (interquartile range) pain scores were 0 (0–1), 1 (0–1) and 2 (2–3) in the HES, lignocaine and placebo groups, respectively.
- This study suggests that pre-administration of 100 mL 6% HES significantly reduces the pain on propofol injection.
Application to Patient Care
- Pain on propofol injection is distressing to patients undergoing surgery under general anaesthesia, leading to discomfort and anxiety. Pre-administration of 2% lignocaine with propofol is a commonly practised method for the reduction of pain on propofol injection, although the pain persists.
- In this prospective, randomised, placebo-controlled clinical trial, pre-administration of 100 mL 6% HES to propofol induction was associated with a significant reduction in the proportion of patients with pain on propofol injection as compared to pre-administration of 2% lignocaine.
Propofol, a commonly used intravenous anaesthetic agent, is known for its rapid induction of anaesthesia and short recovery time, which makes it a preferred choice for various medical procedures requiring anaesthesia. However, the pain associated with propofol injection, often described as making people feel like their skin is burning or stinging, can be distressing to patients, leading to discomfort and anxiety. The incidence of pain on propofol injection varies between 28% and 90% and is ranked seventh among the postoperative problems after anaesthesia.1 Although the aetiology of pain on propofol injection (POPI) remains obscure, it has been ascribed to the release of a kininogen from the vein wall, which strikes a regional kinin cascade.1,2 Pre-administration of 2% lignocaine with propofol is a commonly practised method for the reduction of POPI, although the pain persists.3,4 Pre-administration of 6% hydroxyethyl starch (HES) with propofol inhibits the activation of the endothelial membrane by various substances and molecules by modifying endothelial cell junctions and the permeability of the vascular endothelium, and it can be used to reduce the POPI by preventing contact activation of vascular endothelium.5–11 However, no previous study has compared the efficacy of pre-administration of 2% lignocaine and 6% HES in the reduction of POPI. Therefore, this study aimed to investigate the efficacy of the pre-administration of 2% lignocaine and 6% HES compared to the placebo in the prevention of POPI during induction of general anaesthesia with propofol.
Methods
This prospective, randomised placebo-controlled clinical trial was conducted at Chirayu Medical College & Hospital, Bhopal, India, between August 2023 and December 2023 on patients undergoing elective surgery under general anaesthesia.
Patients aged 18–70 years of either gender and belonging to the American Society of Anesthesiology – Physical status I or II, posted for elective surgery under general anaesthesia, were included in this study. Patients with a known history of allergy to propofol or HES or patients undergoing emergency surgery were excluded.
Standard fasting guidelines were followed. All the patients were given tablets of alprazolam 0.25 mg in the night and morning before surgery. After wheeling the patient into the operation theatre, standard monitoring (non-invasive blood pressure, electrocardiogram and pulse oximetry) was established. An 18 G cannula was secured in the dorsum of the hand.
Patients were randomised to receive either 100 mL of 6% HES followed by an induction dose of 1% propofol (group HES), or 100 mL of normal saline (NS) followed by an induction dose of 1% propofol pre-mixed with 2 mL 2% lidocaine (180 mg of propofol in 20 mL syringe mixed with 2 mL of 2% lidocaine; group L) or 100 mL of NS followed by an induction dose of 1% propofol (group P). Randomisation was carried on by a computer-generated random number table with a block size of 3. Sealed envelopes were used for the concealment of study group allocation until the preparation of the study drugs. The pre-administration drugs, i.e. 100mL of 6% HES (Voluven® 6%, Fresenius Kabi, India) or 100 mL of NS and 20 mL of 1% propofol (long chain triglycerides propofol, Neorof®, Neon Laboratories, India) with or without 2% lidocaine (lignocaine hydrochloride, Loxicard®, Neon Laboratories, India) were prepared by an assistant who was not involved in anaesthesia induction and assessment of pain. Both the patient and the investigators were blinded to the randomised group allocation and the study drugs. All drugs were prepared and stored at room temperature and used within 10 minutes.
Over a period of 5 minutes, 100 mL of HES or NS was administered. Propofol was injected 5 minutes after the study fluid administration at 2.5 mL/5 seconds till the loss of verbal response in the patient. After induction and confirmation of mask ventilation, intravenous fentanyl and atracurium were administered subsequently for tracheal intubation and surgery.
POPI was assessed by a blinded investigator before the loss of verbal contact wherein 0 is no pain, 1 is mild pain evident only on questioning after 10 seconds without any obvious discomfort, 2 is moderate pain self-reported by patients within 10 seconds with some discomfort and 3 is severe pain accompanied by withdrawing of hand, facial grimace/wincing and/or howling/crying. Any haemodynamic instability after propofol administration was also noted. The primary outcome of this study was to compare the proportion of patients with no pain on propofol injection, while the secondary outcomes were to compare the pain scores, the proportion of patients with mild, moderate and severe pain and observe any change in pre- and post-induction hemodynamic variables in the study groups.
Misra et al., who studied the effect of 6% HES pre-administration for the reduction of POPI, found that the incidence of POPI in the NS group was 53%, whereas only 28% of patients in the HES group had POPI.11 Using the G*Power 3 software for Windows (University of Kiel, Kiel, Germany) for calculation of the sample size, and based on the differences in the incidence of POPI between the NS and HES groups in the study by Misra et al., a sample size of 103 participants in each group was required to achieve an alpha error of 0.05 and with 95% as the power of the study. Accounting for potential loss to follow-up (10%), a sample size of 339 patients (113 in each group) was chosen.
Data were entered, cleaned and coded into Microsoft Excel, Version 2021 (Microsoft Corp., Redmond, Washington, USA). Data analysis was done using Statistical Package for the Social Sciences (SPSS), Version 24 (IBM Corp., Armonk, New York, USA). Quantitative data were expressed in terms of means and standard deviation and compared using ANOVA. Categorical data was expressed as numbers and frequencies and analysed using the Chi-squared test. Variables between the groups and within each group were compared by using a repeated measures analysis of variance. A P value of <0.05 was considered statistically significant.
This study was conducted after obtaining Institutional Ethics Committee approval (approval number CMCH/EC/2023/68) and registration of the trial with the Clinical Trial Registry India (CTRI/2023/07/055821). Written informed consent was obtained from all the participants to use their data for research and educational purposes. The study was conducted in accordance with the declaration of Helsinki 2013 and adherence to the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Results
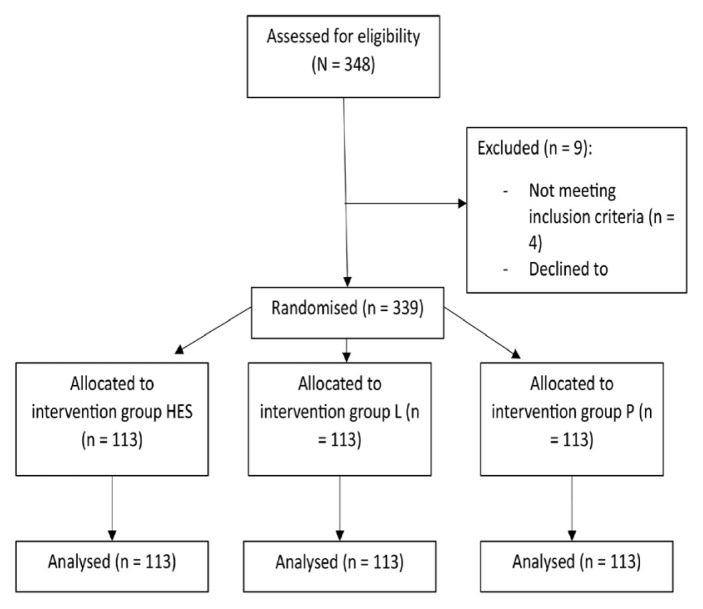
A total of 345 patients were assessed for eligibility in the study; 9 patients were excluded and 113 patients were included in each treatment arm (N = 339) [Figure 1]. The demographics and surgical characteristics of the study participants were comparable between the groups [Table 1].
Figure 1.
Flowchart showing this study’s selection process.
HES = hydroxyethyl starch; L = lignocaine; P = placebo.
Table 1.
Characteristics of the participants in study groups (N = 339)
| Characteristic | Number of patients | P value | ||
|---|---|---|---|---|
| HES | Lignocaine | Placebo | ||
| Mean age in years ± SD | 42.32 ± 10.96 | 41.13 ± 9.31 | 42.68 ± 9.26 | 0.467 |
| Gender | 0.064 | |||
| Male | 56 | 61 | 73 | |
| Female | 57 | 52 | 40 | |
| ASA-PS | 0.09 | |||
| I | 84 | 84 | 71 | |
| II | 29 | 29 | 42 | |
| Surgery type | 0.376 | |||
| Spine | 15 | 12 | 24 | |
| ENT | 77 | 76 | 65 | |
| Gynaecology | 13 | 15 | 16 | |
| Urology | 8 | 10 | 8 | |
HES = hydroxyethyl starch; ASA-PS = American Society of Anesthesiology–Physical Status; ENT = ear, nose and throat.
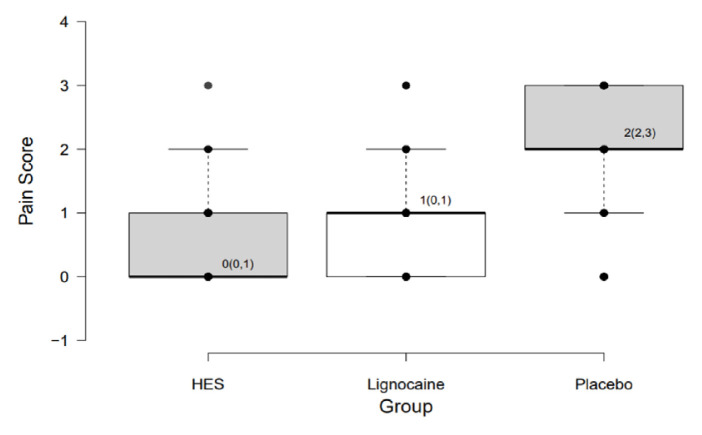
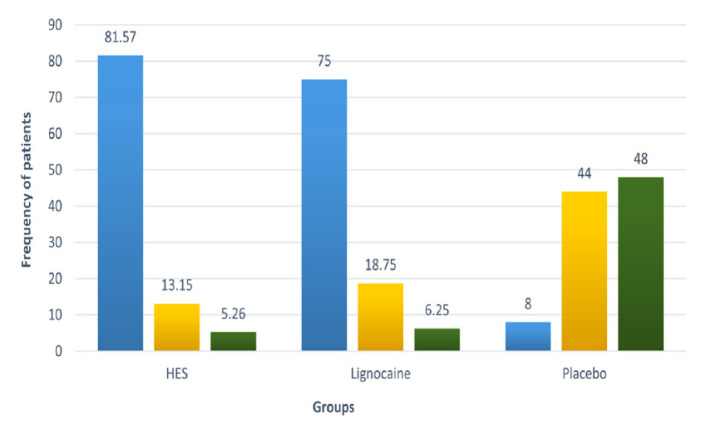
The proportion of patients with no pain on propofol injection was significantly higher in the HES group (66.37%) compared to the lignocaine (29.2%) and placebo (11.5%) groups (P <0.0001 each) [Table 2]. The median (interquartile range [IQR]) pain scores were 0 (0–1), 1 (0–1) and 2 (2–3) in the HES, lignocaine and placebo groups, respectively (P <0.00001) [Figure 2]. The overall incidence of pain in HES and lignocaine group was 33.63% and 70.8% respectively. The proportion of participants with mild pain (81.57% versus 75.00%; P = 0.23), moderate pain (13.16% versus 18.75%; P = 0.25) and severe pain (5.26% versus 6.25%; P = 0.74) on propofol injection in HES and lignocaine groups, respectively, were comparable, whereas the proportion of patients with mild pain (8.00%; P <0.0001) in the placebo group was significantly less compared to the HES and lignocaine groups. Furthermore, the proportion of patients with moderate (44.00%) and severe (48.00%) pain scores were significantly higher in the placebo group compared to the HES and lignocaine groups (P <0.0001) [Table 2 and Figure 3].
Table 2.
Comparison of pain classification in the study groups
| Pain classification | Number of patients/total (%) | P value | ||
|---|---|---|---|---|
| HES | Lignocaine | Placebo | ||
| No Pain | 75/113 (66.37) | 33/113 (29.20) | 13/113 (11.5) | <0.0001 (HES and lignocaine) <0.0001 (HES and placebo) <0.0001 (Lignocaine and placebo) |
| Mild Pain | 31/38 (81.57) | 60/80 (75) | 8/100 (8) | 0.23 (HES and lignocaine) <0.0001 (HES and placebo) <0.0001 (Lignocaine and placebo) |
| Moderate Pain | 5/38 (13.15) | 15/80 (18.75) | 44/100 (44) | 0.25 (HES and lignocaine) <0.0001 (HES and placebo) <0.0001 (Lignocaine and placebo) |
| Severe Pain | 2/38 (5.26) | 5/80 (6.25) | 48/100 (48) | 0.74 (HES and lignocaine) <0.0001 (HES and placebo) <0.0001 (Lignocaine and placebo) |
HES = hydroxyethyl starch.
Figure 2.
Box-plot diagram for distribution of pain scores in the study groups.
Values are presented as median (IQR). HES = hydroxyethyl starch.
Figure 3.
Proportion of patients with mild, moderate and severe pain in study groups.
HES = hydroxyethyl starch.
A significant rise in post-induction mean heart rate was observed in the placebo group as compared to the HES and lignocaine groups (P <0.0001). No significant change in pre- and post-induction systolic or diastolic blood pressures was observed in the study groups [Table 3].
Table 3.
Comparison of pre- and post-induction haemodynamic variables between the study groups
| Variable | Mean ± SD (95% CI) | P value | ||
|---|---|---|---|---|
| HES | Lignocaine | Placebo | ||
| ΔSBP in mmHg | 14.55 ± 13.34 (12.06 to 17.04) | 17.34 ± 16.27 (14.31 to 20.37) | 17.23 ± 23.22 (12.9 to 21.56) | 0.423 |
| ΔDBP in mmHg | 9.17 ± 10.05 (7.3 to 11.05) | 7.26 ± 10.49 (5.30 to 9.22) | 10.95 ± 14.99 (8.16 to 13.75) | 0.072 |
| ΔHR | −1.17 ± 11.45 (−3.31 to 0.95) | −4.68 ± 9.39 (−6.43 to −2.93) | −9.69 ± 14.64 (−12.42 to −6.96) | <0.0001 |
SD= standard deviation; CI = confidence interval; HES = hydroxyethyl starch; ΔSBP = difference in systolic blood pressure; ΔDBP = difference in diastolic blood pressure; ΔHR = difference in heart rates.
Discussion
One of the goals of anaesthesia is to provide adequate analgesia in the perioperative period. POPI, during induction, is a serious concern and has been ranked seventh among peri-operative problems related to anaesthesia.1
The present study demonstrated that the proportion of patients with POPI was significantly lower in the HES group compared to both the lignocaine and placebo groups. The distribution of patients was comparable in the HES and lignocaine groups in terms of severity score of pain, whereas the placebo group had a significantly higher proportion of patients with moderate to severe pain compared to the HES and lignocaine groups.
Kwak et al. reported that propofol acts on the venous endothelial tissue and stimulates the kallikrein–kinin system to produce bradykinin, which makes blood vessels dilate and increases its permeability, causing the free propofol to make contact with nerve endings on the inner wall of the blood vessel that cause pain.2 Fischer et al. performed a study on human and mouse transient receptor potential ankyrin 1 (TRPA1) channel with different formulations of propofol, revealing that propofol injection leads to the activation of TRPA1 and transient receptor potential vanilloid 1 (TRPV1). TRPA1 and TRPV1 upregulate the release of neuropeptides and induce vascular leakage and dilatation, contributing to neurogenic inflammation and central sensitisation in the spinal dorsal horn, thus resulting in pain on propofol injection.12
Lidocaine reduces POPI through local anaesthetic effects at the nerve endings on the inner wall of the blood vessel.3,4 Intravenous lidocaine may also have antinociceptive actions by inhibiting several inflammatory activities, including p38 MAPK and NF-kB, signalling pathways and toxic oxygen-free radical production.13
Margraf et al. investigated the effect of 6% HES on glycocalyx integrity and vascular permeability in mice and suggested that 6% HES exerts protective effects on glycocalyx integrity and attenuates the increase of vascular permeability during systemic inflammation.14 Zhao et al. observed the effect of HES on vascular permeability and its relationship to endothelial glycocalyx using a haemorrhagic shock rat model and hypoxia-treated vascular endothelial cells, finding HES-protected haemorrhagic shock-induced vascular leakage by protecting the endothelial glycocalyx and intercellular junction proteins. HES down-regulates the expression of endothelial glycocalyx degradation enzyme heparinase, hyaluronidase and neuraminidase, thus preventing vascular leakage.15 This protective effect of HES on venous endothelium via the downregulation of endothelial glycocalyx degradation neuropeptides prevents the contact activation of the various nociceptive receptors by propofol. This modulation of the endothelium by HES has been demonstrated in many in-vivo and in-vitro experimental models.5–11 The overall incidence of POPI in the present study was significantly less in the HES group than in the lignocaine and placebo groups, which can be explained by the protective effect of HES on vascular endothelium via the downregulation of the release of neuropeptides and subsequent central desensitisation in the spinal dorsal horn.
The incidence of POPI in adult individuals ranges from 28% to 90%, and the present study showed an overall incidence of pain of 33.63% and 70.8% in the HES and lidocaine groups, respectively, suggesting that HES is more efficacious in reducing POPI than lidocaine.1–4
Misra et al. compared the pre-administration of 100 mL HES and 100 mL of NS for the reduction in POPI and found that the overall incidence of pain in the HES group was significantly lower than in the NS group, which is consistent with the results of the present study.11 The incidence of severe and moderate pain was higher in the NS group compared to the HES group in the study conducted by Misra et al., whereas in the present study, the HES and lignocaine groups were comparable in terms of severity of pain. This difference in the severity of pain may be because Misra et al. had pre-administered either 100 mL of HES or NS followed by an induction dose of 1% propofol premixed with 2% lidocaine – i.e. they compared HES with lignocaine and lignocaine alone for the reduction of POPI.11
Other interventional modalities that are efficacious for reducing POPI are ketamine, opioids and non-steroidal anti-inflammatory drugs with the incidence of pain ranging from 43% to 67%.16 Even administration of steroids and 5-hydroxytryptamine-3 (5-HT3) antagonists have been studied for decreasing propofol injection pain.17,18 Recently, a combination of 2 drugs (opioids and 5-HT3 antagonists) has been found to be more efficacious in reducing propofol injection pain.11,17 In the present study, only 33.63% of the patients in the HES group had POPI, which is much less as compared to the above modalities. Thus, pre-administration of 100 mL of 6% HES may offer better pain relief than other routinely used modalities.
There was no significant difference in change in pre- and post-induction systolic and diastolic blood pressures in the study groups. However, a significant increase in post-induction heart rate was observed in the placebo group, which may be due to the POPI.
This study was subject to certain limitations. This study was a single-centre study with a small sample size. However, even with this small sample size, the authors were able to achieve a reduction in pain with pre-administration of an arbitrary volume of 100 mL of HES, which needs to be standardised. Furthermore, larger randomised controlled trials are required to validate these provocative observations.
Conclusion
Pain on propofol injection can be significantly reduced by pre-administration of 100 mL 6% HES. The proportion of patients experiencing pain on propofol injection was found to be significantly less with the pre-administration of 6% HES compared to that with the pre-administration of lidocaine.
Footnotes
AUTHORS’ CONTRIBUTION: TKS and ST conceptualised and designed the study. All authors performed the literature review, data collection and data analysis, along with drafting and revising the manuscript. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST: The authors declare no conflicts of interest.
FUNDING: This study did not receive any funding.
References
- 1.Desousa KA. Pain on propofol injection: Causes and remedies. Indian J Pharmacol. 2016;48:617–23. doi: 10.4103/0253-7613.194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwak KH, Ha J, Kim Y, Jeon Y. Efficacy of combination intravenous lidocaine and dexamethasone on propofol injection pain: A randomized, double-blind, prospective study in adult Korean surgical patients. Clin Ther. 2008;30:1113–19. doi: 10.1016/j.clinthera.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Kundra P, Vinayagam S. Perioperative intravenous lidocaine: Crossing local boundaries and reaching systemic horizons. Indian J Anaesth. 2020;64:363–5. doi: 10.4103/ija.IJA_431_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singla B, Malde AD. A prospective observational study of injection pain in children with medium plus long chain triglyceride and long chain triglyceride propofol premixed with lignocaine. Indian J Anaesth. 2018;62:214–18. doi: 10.4103/ija.IJA_506_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossaint J, Berger C, Kraft F, Van Aken H, Giesbrecht N, Zarbock A. Hydroxyethyl starch 130/0.4 decreases inflammation, neutrophil recruitment, and neutrophil extracellular trap formation. Br J Anaesth. 2015;114:509–19. doi: 10.1093/bja/aeu340. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Jacob M, Vicaut E, Guidet B, Van Aken H, Kurz A. Effect of waxy maize-derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology. 2013;118:387–94. doi: 10.1097/ALN.0b013e31827e5569. [DOI] [PubMed] [Google Scholar]
- 7.Wisselink W, Patetsios P, Panetta TF, Ramirez JA, Rodino W, Kirwin JD, et al. Medium molecular weight pentastarch reduces reperfusion injury by decreasing capillary leak in an animal model of spinal cord ischemia. J Vasc Surg. 1998;27:109–16. doi: 10.1016/S0741-5214(98)70297-6. [DOI] [PubMed] [Google Scholar]
- 8.Nishimoto R, Kashio M, Tominaga M. Propofol-induced pain sensation involves multiple mechanisms in sensory neurons. Pflugers Arch. 2015;467:2011–20. doi: 10.1007/s00424-014-1620-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Zhou L, Wu LX, Wang T, Zhang CB, Sun L. 5-HT3 Receptor antagonists for propofol injection pain: A meta-analysis of randomized controlled trials. Clin Drug Investig. 2016;36:243–53. doi: 10.1007/s40261-016-0375-1. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan SS, Park TS, Gonzales ER, Gidday JM. Hydroxyethyl starch reduces leucocyte adherence and vascular injury in the newborn pig cerebral circulation after asphyxia. Stroke. 2000;31:2218–23. doi: 10.1161/01.STR.31.9.2218. [DOI] [PubMed] [Google Scholar]
- 11.Misra S, Behera BK, Sahoo AK. Effect of 6% hydroxyethyl starch pre administration for reduction of pain on propofol injection: A placebo-controlled randomised study. Indian J Anaesth. 2022;66:107–11. doi: 10.4103/ija.ija_884_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer MJ, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, et al. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem. 2010;285:34781–92. doi: 10.1074/jbc.M110.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing J, Liang L, Zhou S, Luo C, Cai J, Hei Z. Intravenous lidocaine alleviates the pain of propofol injection by local anesthetic and central analgesic effects. Pain Medicine. 2018;19:598–607. doi: 10.1093/pm/pnx070. [DOI] [PubMed] [Google Scholar]
- 14.Margraf A, Herter JM, Kühne K, Stadtmann A, Ermert T, Wenk M, et al. 6% Hydroxyethyl starch (HES 130/0.4) diminishes glycocalyx degradation and decreases vascular permeability during systemic and pulmonary inflammation in mice. Crit Care. 2018;22:111. doi: 10.1186/s13054-017-1846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Zhu Y, Zhang J, Wu Y, Xiang X, Zhang Z, et al. The beneficial effect of HES on vascular permeability and its relationship with endothelial glycocalyx and intercellular junction after hemorrhagic shock. Front Pharmacol. 2020;11:597. doi: 10.3389/fphar.2020.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivanna S, Priye S, Singh D, Jagannath S, Mudassar S, Reddy DP. Efficacy of methylprednisolone and lignocaine on propofol injection pain: A randomised, double-blind, prospective study in adult cardiac surgical patients. Indian J Anaesth. 2016;60:848–51. doi: 10.4103/0019-5049.193683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumalatha GB, Dodawad RR, Pandarpurkar S, Jajee PR. A comparative study of attenuation of propofol-induced pain by lignocaine, ondansetron, and ramosetron. Indian J Anaesth. 2016;60:25–9. doi: 10.4103/0019-5049.174810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakhtiari E, Mousavi SH, Gharavi Fard M. Pharmacological control of pain during propofol injection: A systematic review and meta-analysis. Expert Rev Clin Pharmacol. 2021;14:889–99. doi: 10.1080/17512433.2021.1919084. [DOI] [PubMed] [Google Scholar]