Abstract
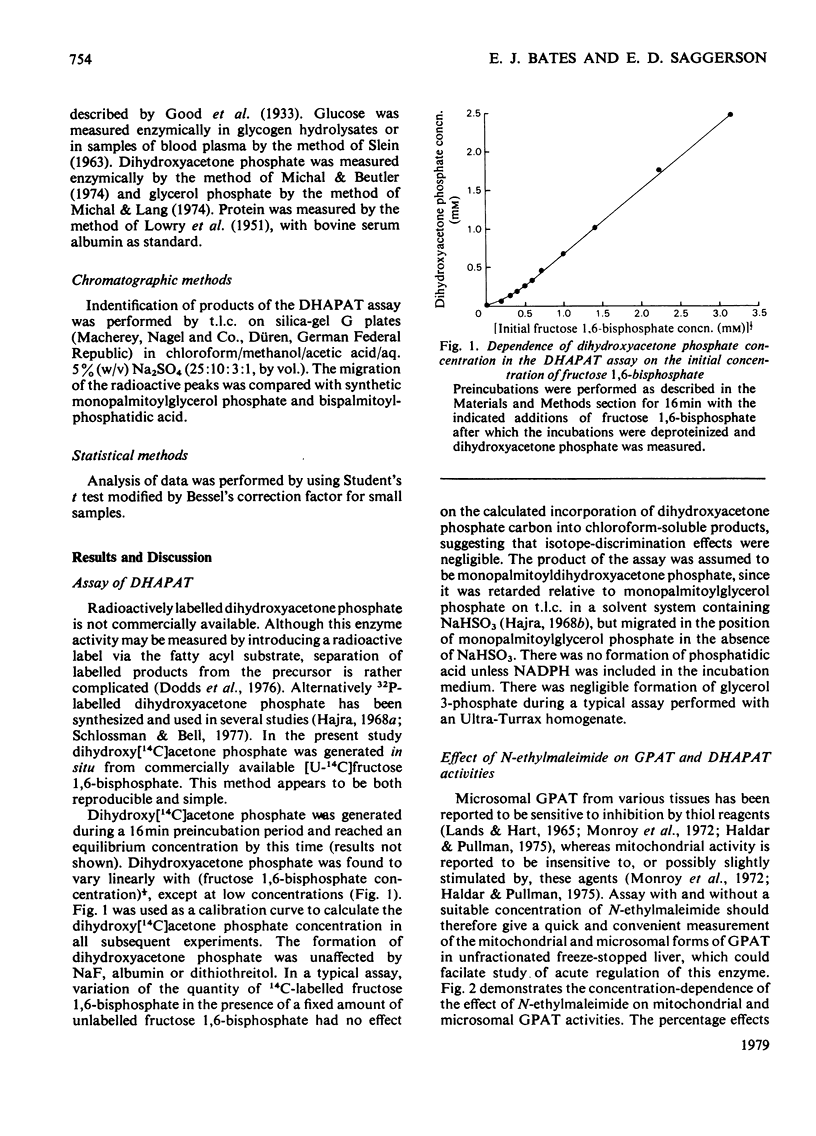
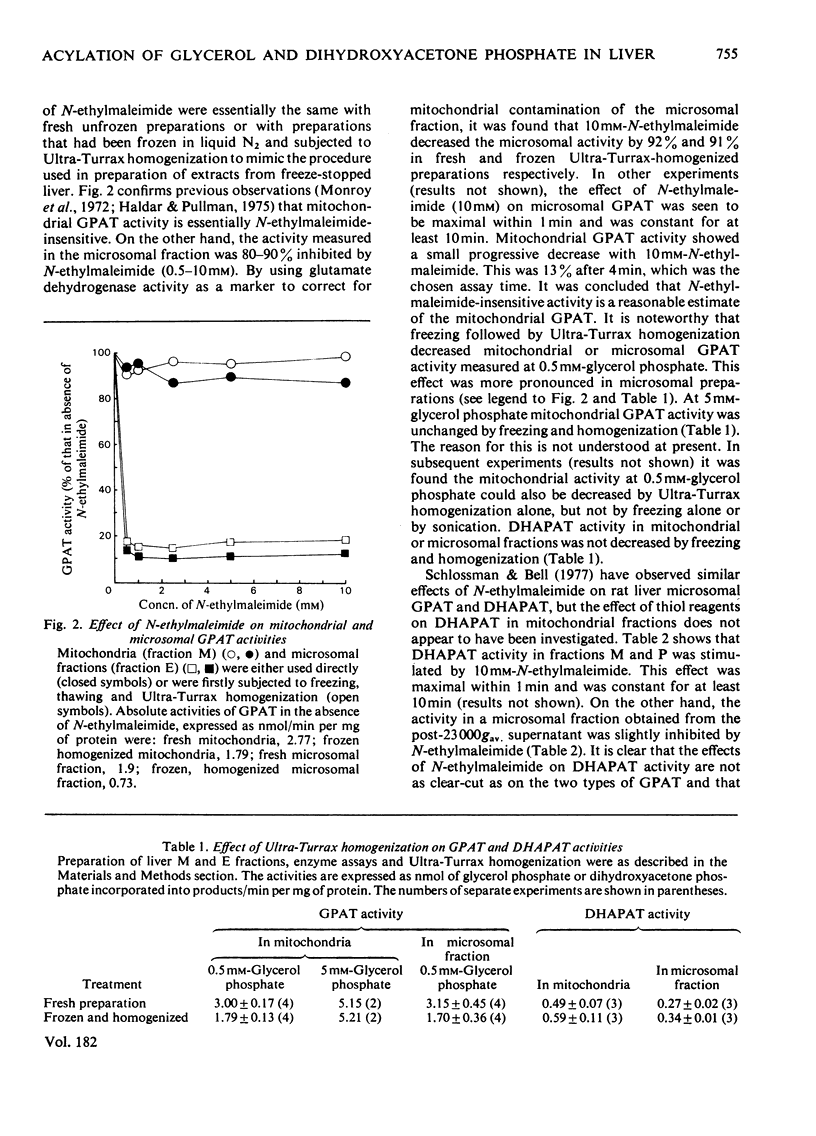
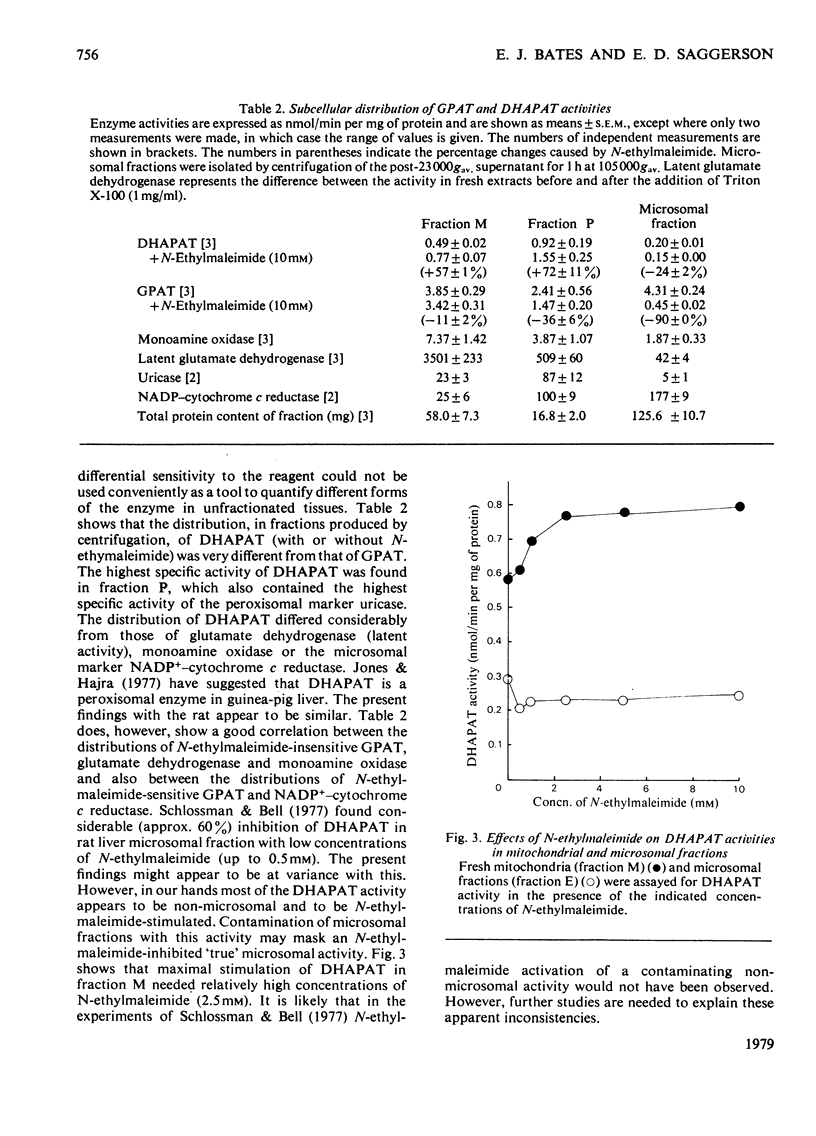
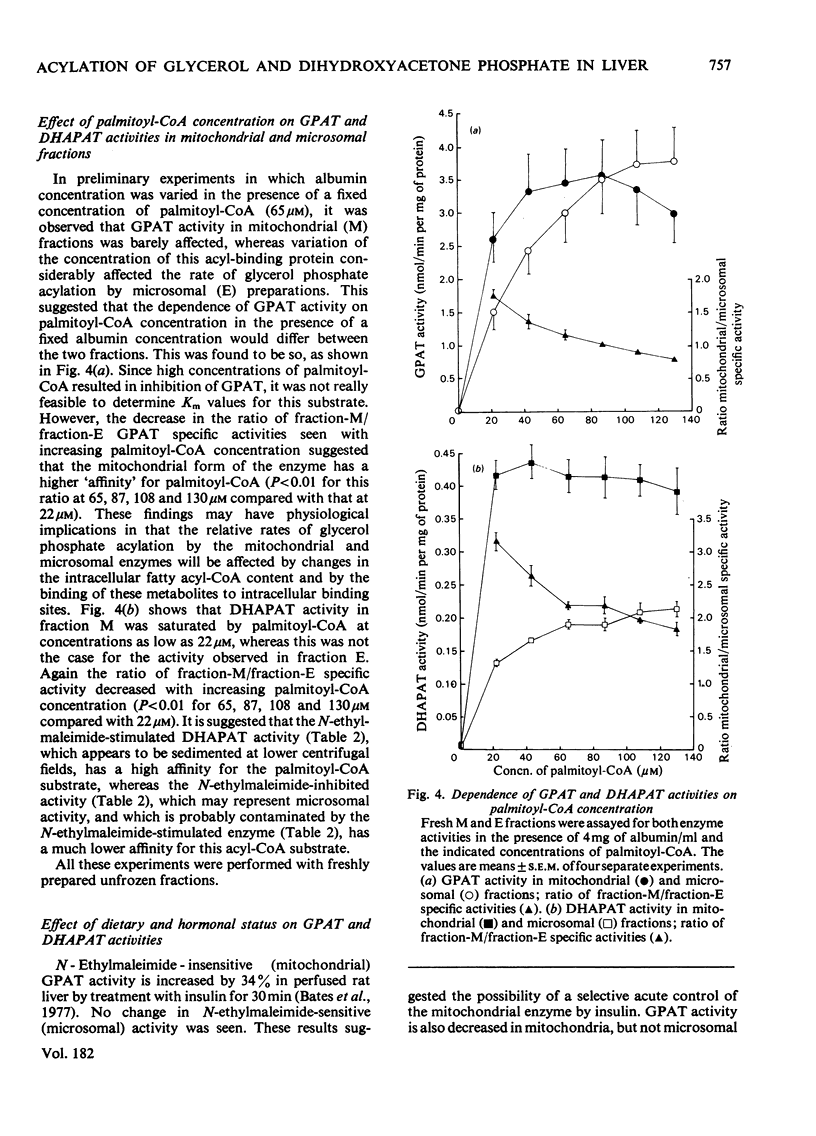
1. GPAT (glycerol phosphate acyltransferase) and DHAPAT (dihydroxyacetone phosphate acyltransferase) activities were measured both in subcellular fractions prepared from fed rat liver and in whole homogenates prepared from freeze-stopped pieces of liver. 2. GPAT activity in mitochondria differed from the microsomal activity in that it was insensitive to N-ethylmaleimide, had a higher affinity towards the palmitoyl-CoA substrate and showed a different response to changes in hormonal and dietary status. 3. Starvation (48 h) significantly decreased mitochondrial GPAT activity. The ratio of mitochondrial to microsomal activities was also significantly decreased. The microsomal activity was unaffected by starvation, except after adrenalectomy, when it was significantly decreased. Mitochondrial GPAT activity was decreased by adrenalectomy in both fed and starved animals. 4. Acute administration of anti-insulin serum significantly decreased mitochondrial GPAT activity after 60 min without affecting the microsomal activity. 5. A new assay is described for DHAPAT. The subcellular distribution of this enzyme differed from that of GPAT. The highest specific activity of DHAPAT was found in a 23 000 gav. pellet obtained by centrifugation of a post-mitochondrial supernatant. This fraction also contained the highest specific activity of the peroxisomal marker uricase. DHAPAT activity in mitochondrial fractions or in the 23 000 gav. pellet was stimulated by N-ethylmaleimide, whereas that in microsomal fractions was slightly inhibited by this reagent. The GPAT and DHAPAT activities in mitochondrial fractions had a considerably higher affinity for the palmitoyl-CoA substrate. 6. Total liver DHAPAT activity was significantly decreased by starvation (48 h), but was unaffected by administration of anti-insulin serum. 7. The specific activities of GPAT and DHAPAT were lower in non-parenchymal cells compared with parenchymal cells, but the GPAT/DHAPAT ratio was 5--6-fold higher in the parenchymal cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Daae L. N. Fatty acid activation and acyl transfer in organs from rats in different nutritional states. Biochim Biophys Acta. 1971 Jul 13;239(2):208–216. doi: 10.1016/0005-2760(71)90166-4. [DOI] [PubMed] [Google Scholar]
- Aas M. Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim Biophys Acta. 1971 Feb 2;231(1):32–47. doi: 10.1016/0005-2760(71)90253-0. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson D. A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett. 1977 Dec 15;84(2):229–232. doi: 10.1016/0014-5793(77)80694-7. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Topping D. L., Sooranna S. P., Saggerson D., Mayes P. A. Acute effects of insulin on glycerol phosphate acyl transferase activity, ketogenesis and serum free fatty acid concentration in perfused rat liver. FEBS Lett. 1977 Dec 15;84(2):225–228. doi: 10.1016/0014-5793(77)80693-5. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. Phosphatidic acid biosynthesis in rat liver mitochondria and microsomal fractions. Regulation of fatty acid positional specificity. Biochem J. 1976 Aug 15;158(2):249–254. doi: 10.1042/bj1580249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Daae L. N. The acylation of glycerol 3 -phosphate in different rat organs and in the liver of different species (including man). Biochim Biophys Acta. 1973 May 24;306(2):186–193. doi: 10.1016/0005-2760(73)90224-5. [DOI] [PubMed] [Google Scholar]
- Dodds P. F., Gurr M. I., Brindley D. N. The glycerol phosphate, dihydroxyacetone phosphate and monoacylglycerol pathways of glycerolipid synthesis in rat adipose-tissue homogenates. Biochem J. 1976 Dec 15;160(3):693–700. doi: 10.1042/bj1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon H. J., Kemp E. L. Effects of diet on hepatic triglyceride synthesis. J Clin Invest. 1968 Apr;47(4):712–719. doi: 10.1172/JCI105766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in guinea pig liver mitochondria. J Biol Chem. 1968 Jun 25;243(12):3458–3465. [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of glycerolipids via acyldihydroxyacetone phosphate. Biochem Soc Trans. 1977;5(1):34–36. doi: 10.1042/bst0050034. [DOI] [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of phosphatidic acid from dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1968 Dec 30;33(6):929–935. doi: 10.1016/0006-291x(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Hajra A. K. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974 Aug;9(8):502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Hajra A. K. The subcellular distribution of acyl CoA: dihydroxyacetone phosphate acyl transferase in guinea pig liver. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1138–1143. doi: 10.1016/0006-291x(77)90974-3. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Verrinder T. R., Hems D. A. Fatty acid synthesis in the perfused liver of adrenalectomized rats. Biochem J. 1976 Jun 15;156(3):593–602. doi: 10.1042/bj1560593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner H., Heimberg M. Effect of adrenalcortical hormones on release of triglycerides and glucose by liver. Am J Physiol. 1967 Jun;212(6):1236–1246. doi: 10.1152/ajplegacy.1967.212.6.1236. [DOI] [PubMed] [Google Scholar]
- LANDS W. E., HART P. METABOLISM OF GLYCEROLIPIDS. VI. SPECIFICITIES OF ACYL COENZYME A: PHOSPHOLIPID ACYLTRANSFERASES. J Biol Chem. 1965 May;240:1905–1911. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiapane E. H., Lloyd-Davies K. A., Brindley D. N. A study of some enzymes of glycerolipid biosynthesis in rat liver after subtotal hepatectomy. Biochem J. 1973 May;134(1):103–112. doi: 10.1042/bj1340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning R., Brindley D. N. Tritium isotope effects in the measurement of the glycerol phosphate and dihydroxyacetone phosphate pathways of glycerolipid biosynthesis in rat liver. Biochem J. 1972 Dec;130(4):1003–1012. doi: 10.1042/bj1301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. M., Zucker-Franklin D. Electron microscopic study of isolated Kupffer cells. Am J Pathol. 1969 Feb;54(2):147–166. [PMC free article] [PubMed] [Google Scholar]
- Monroy G., Rola F. H., Pullman M. E. A substrate- and position-specific acylation of sn-glycerol 3-phosphate by rat liver mitochondria. J Biol Chem. 1972 Nov 10;247(21):6884–6894. [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Rao G. A., Sorrels M. F., Reiser R. Dietary regulation of phosphatidic acid synthesis from dihydroxyacetone phosphate and fatty acid by rat liver microsomes. Lipids. 1971 Feb;6(2):88–92. doi: 10.1007/BF02531322. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Kolterman O. G., Reaven G. M. Ultrastructural and physiological evidence for corticosteroid-induced alterations in hepatic production of very low density lipoprotein particles. J Lipid Res. 1974 Jan;15(1):74–83. [PubMed] [Google Scholar]
- Rognstad R., Clark D. G., Katz J. Pathways of glyceride glycerol synthesis. Biochem J. 1974 May;140(2):249–251. doi: 10.1042/bj1400249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Microsomal sn-glycerol 3-phosphate and dihydroxyacetone phosphate acyltransferase activities from liver and other tissues. Evidence for a single enzyme catalizing both reactions. Arch Biochem Biophys. 1977 Aug;182(2):732–742. doi: 10.1016/0003-9861(77)90555-0. [DOI] [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Schiefer H. G. Biosynthesis and composition of phosphatides in outer and inner mitochondrial membranes. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1017–1026. doi: 10.1515/bchm2.1968.349.2.1017. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel T. J., Kruijt J. K. Different types of mitochondria in parenchymal and non-parenchymal rat-liver cells. Eur J Biochem. 1977 Feb 15;73(1):223–229. doi: 10.1111/j.1432-1033.1977.tb11310.x. [DOI] [PubMed] [Google Scholar]
- Van Lan V., Yamaguchi N., Garcia M. J., Ramey E. R., Penhos J. C. Effect of hypophysectomy and adrenalectomy on glucagon and insulin concentration. Endocrinology. 1974 Mar;94(3):671–675. doi: 10.1210/endo-94-3-671. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand R. D., Rao G. A., Reiser R. Dietary regulation of fatty acid synthetase and microsomal glycerophosphate acyltransferase activities in rat liver. J Nutr. 1973 Oct;103(10):1414–1424. doi: 10.1093/jn/103.10.1414. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]
- Wright P. H., Makulu D. R., Posey I. J. Guinea pig anti-insulin serum. Adjuvant effect of H. pertussis vaccine. Diabetes. 1968 Aug;17(8):513–516. doi: 10.2337/diab.17.8.513. [DOI] [PubMed] [Google Scholar]
- Wright P. H., Malaisse W. J. A simple method for the assay of guinea pig anti-insulin serum. Diabetologia. 1966 Nov;2(3):178–188. doi: 10.1007/BF01222068. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J. Difference spectra, catalase- and peroxidase activities of isolated parenchymal and non-parenchymal cells from rat liver. Biochem Biophys Res Commun. 1974 Nov 6;61(1):204–209. doi: 10.1016/0006-291x(74)90553-1. [DOI] [PubMed] [Google Scholar]
- van Tol A. The effect of fasting on the acylation of carnitine and glycerophosphate in rat liver subcellular fractions. Biochim Biophys Acta. 1974 Jul 25;357(1):14–23. doi: 10.1016/0005-2728(74)90107-8. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]


