Abstract
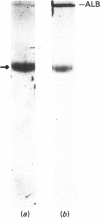
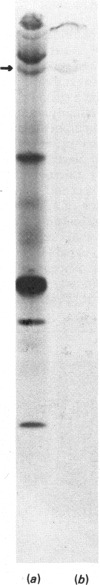
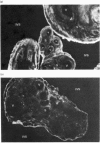
Ferritin has been purified from normal full-term human placentae and its antigenic and molecular characteristics compared with adult liver ferritin. Placental ferritin is composed predominantly of a single subunit type, co-migrating with a liver ferritin standard on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Comparison of dose-response curves in an immunoradiometric assay indicated some tissue-specific antigenicity for placental ferritin. This was supported by immunofluorescence studies on cryostat sections of human placentae by using antibodies to placental and spleen ferritin. Specific staining for placental ferritin was demonstrated within placental syncytiotrophoblast, particularly localized towards the microvillus plasma membrane. Ferritin has also been shown by electrophoretic and antigenic analysis to be present in protein fractions solubilized from isolated human syncytiotrophoblast microvillus plasma-membrane preparations, suggesting that ferritin may play an active role in the transfer of iron from maternal transferrin across the syncytiotrophoblast plasma membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison G. M., Beamish M. R., Hales C. N., Hodgkins M., Jacobs A., Llewellin P. An immunoradiometric assay for ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. J Clin Pathol. 1972 Apr;25(4):326–329. doi: 10.1136/jcp.25.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn H. Isolierung des Placenta-Proteins PP2 und seine Identifizierung als Ferritin. Arch Gynakol. 1973;215(3):263–275. doi: 10.1007/BF00672810. [DOI] [PubMed] [Google Scholar]
- Brown P. J., Leyland M. J., Keenan J. P., Dean P. D. Preparative electrophoretic desorption in the purification of human serum ferritin by immuno-adsorption. FEBS Lett. 1977 Nov 15;83(2):256–259. doi: 10.1016/0014-5793(77)81017-x. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Wada H. G., Sussman H. H. The plasma membrane of human placenta. Isolation of microvillus membrane and characterization of protein and glycoprotein subunits. J Biol Chem. 1976 Jul 10;251(13):4139–4146. [PubMed] [Google Scholar]
- Drysdale J. W., Adelman T. G., Arosio P., Casareale D., Fitzpatrick P., Harzard J. T., Yokota M. Human isoferritins in normal and disease states. Semin Hematol. 1977 Jan;14(1):71–88. [PubMed] [Google Scholar]
- Drysdale J. W., Singer R. M. Carcinofetal human isoferritins in placenta and Hela cells. Cancer Res. 1974 Dec;34(12):3352–3354. [PubMed] [Google Scholar]
- Faulk W. P., Johnson P. M. Immunological studies of human placentae: identification and distribution of proteins in mature chorionic villi. Clin Exp Immunol. 1977 Feb;27(2):365–375. [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Temple A. Distribution of beta2 microglobulin and HLA in chorionic villi of human placentae. Nature. 1976 Aug 26;262(5571):799–802. doi: 10.1038/262799a0. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Function of transferrin. Nature. 1968 Jun 29;218(5148):1211–1214. doi: 10.1038/2181211a0. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Suter P. E. The transport of iron by the human placenta. Clin Sci. 1969 Apr;36(2):209–220. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hourani B. T., Chace N. M., Pincus J. H. Plasma membrane glycoproteins from nucleated cells. I. Preparative techniques for isolation and partial characterization of a membrane glycoprotein extract from L1210 cells with lectin receptor activity. Biochim Biophys Acta. 1973 Dec 6;328(2):520–532. [PubMed] [Google Scholar]
- Johnson P. M., Faulk W. P. Immunological studies of human placentae: identification and distribution of proteins in immature chorionic villi. Immunology. 1978 Jun;34(6):1027–1035. [PMC free article] [PubMed] [Google Scholar]
- Johnson P. M., Natvig J. B., Ystehede U. A., Faulk W. P. Immunological studies of human placentae: the distribution and character of immunoglobulins in chorionic villi. Clin Exp Immunol. 1977 Oct;30(1):145–153. [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mansour M. M., Schulert A. R., Glasser S. R. Mechanism of placental iron transfer in the rat. Am J Physiol. 1972 Jun;222(6):1628–1633. doi: 10.1152/ajplegacy.1972.222.6.1628. [DOI] [PubMed] [Google Scholar]
- Miles L. E., Hales C. N. The preparation and properties of purified 125-I-labelled antibodies to insulin. Biochem J. 1968 Jul;108(4):611–618. doi: 10.1042/bj1080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez M. T., Glass J., Robinson S. H. Mobilization of iron from the plasma membrane of the murine reticulocyte. The role of ferritin. Biochim Biophys Acta. 1978 May 4;509(1):170–180. doi: 10.1016/0005-2736(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Ockleford C. D., Menon G. Differentiated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: a new organelle and the binding of iron. J Cell Sci. 1977 Jun;25:279–291. doi: 10.1242/jcs.25.1.279. [DOI] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]
- Stauffer C. E., Greenham C. C. Sources of error in measuring ferritin. Clin Chem. 1976 Oct;22(10):1755–1755. [PubMed] [Google Scholar]