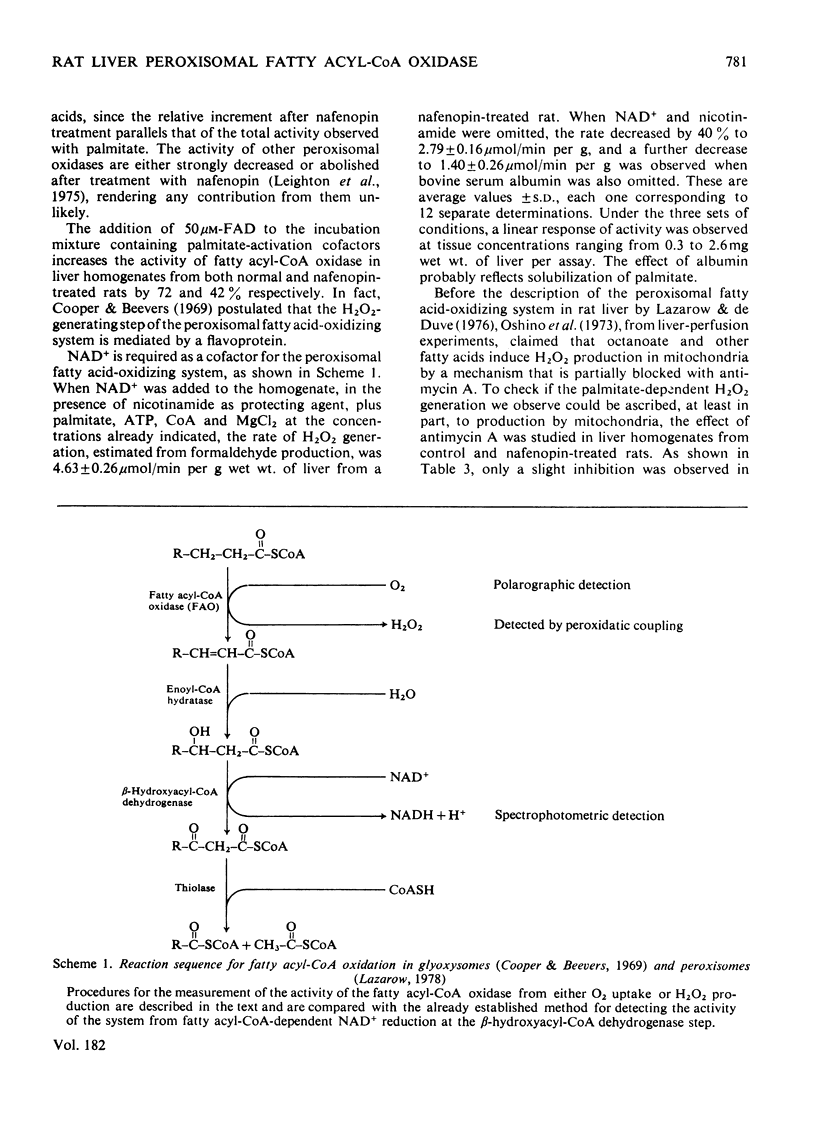
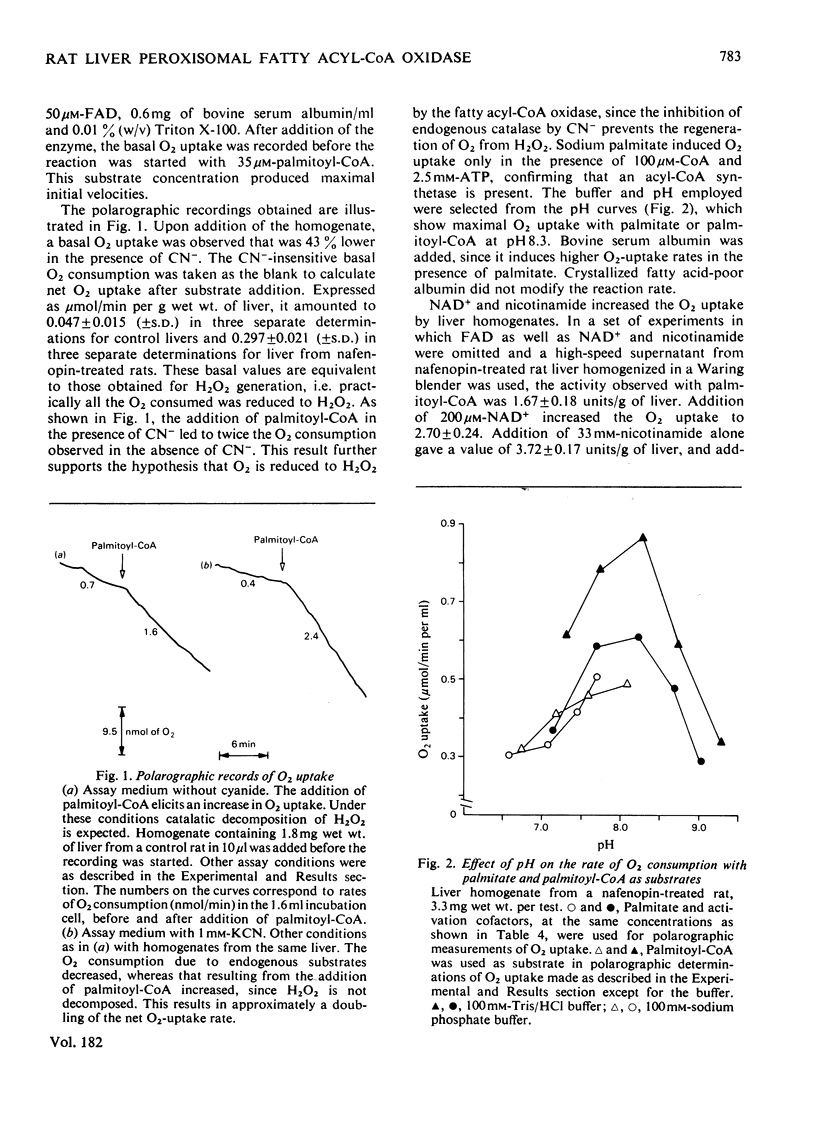
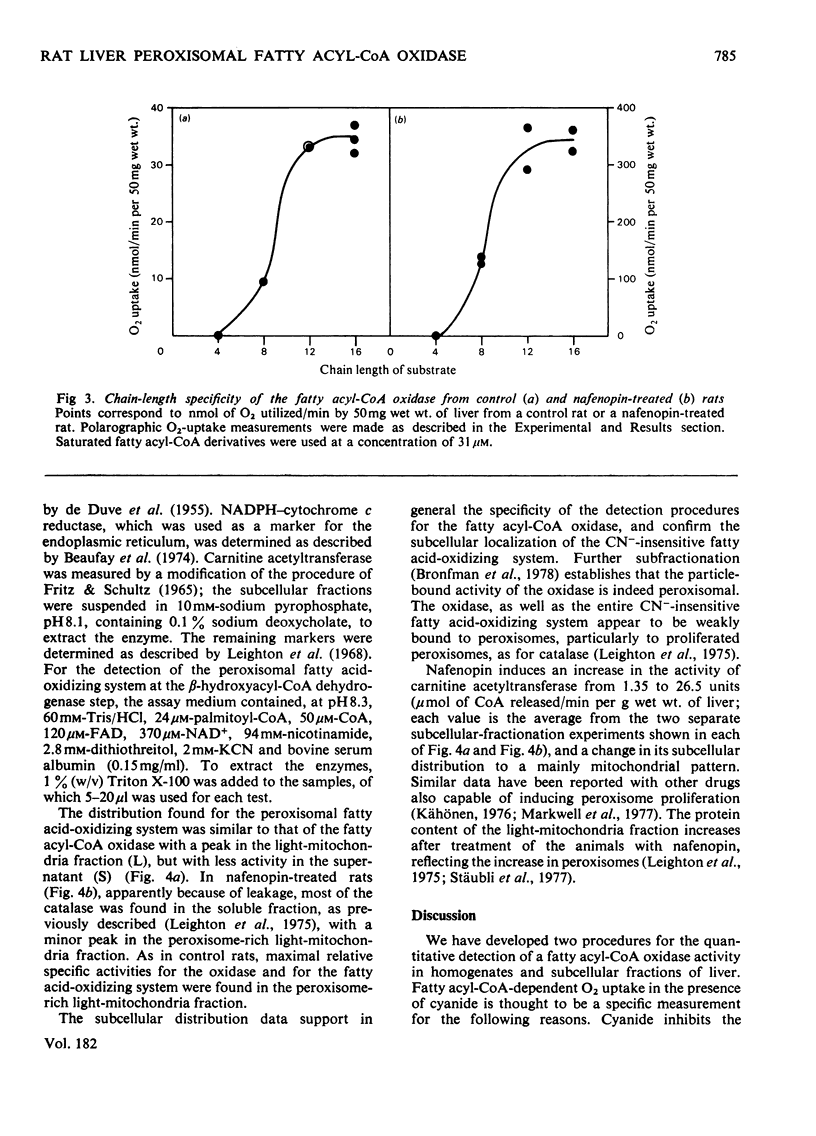
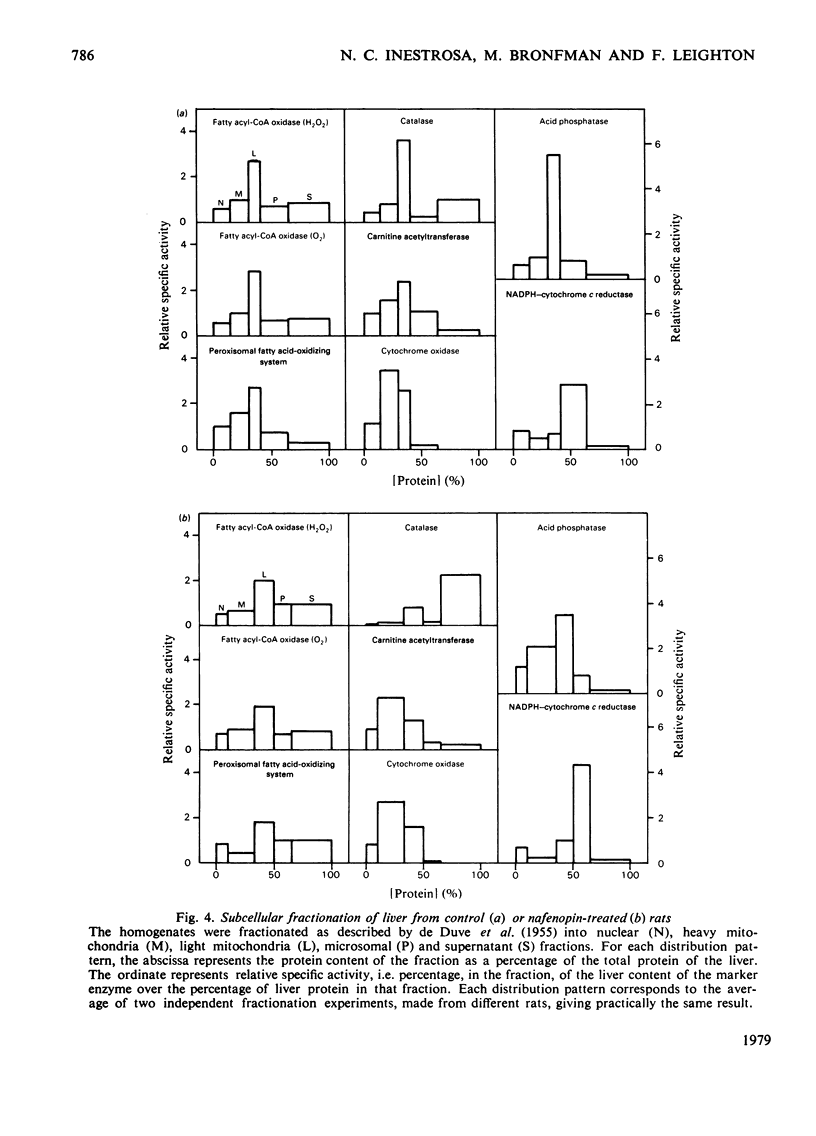
Abstract
It has been postulated that the peroxisomal fatty acid-oxidizing system [Lazarow & de Duve (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 2043--2046; Lazarow (1978) J. Biol. Chem. 253, 1522--1528] resembles that of mitochondria, except for the first oxidative reaction. In this step, O2 would be directly reduced to H2O2 by an oxidase. Two specific procedures developed to detect the activity of the characteristic enzyme fatty acyl-CoA oxidase are presented, namely polarographic detection of palmitoyl-CoA-dependent cyanide-insensitive O2 consumption and palmitoyl-CoA-dependent H2O2 generation coupled to the peroxidation of methanol in an antimycin A-insensitive reaction. Fatty acyl-CoA oxidase activity is stimulated by FAD, which supports the flavoprotein nature postulated for this enzyme. Its activity increases 7-fold per g wet wt. of liver in rats treated with nafenopin, a hypolipidaemic drug. Subcellular fractionation of livers from normal and nafenopin-treated animals provides evidence for its peroxisomal localization. The stoicheiometry for palmitoyl-CoA-dependent O2 consumption, H2O2 generation and NAD+ reduction is 1 : 1 : 1. This suggests that fatty acyl-CoA oxidase is the rate-limiting enzyme of the peroxisomal fatty acid-oxidizing system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H. Evidence for an intermediate in the oxidation-reduction of flavoproteins. J Biol Chem. 1957 Mar;225(1):465–478. [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Moldeus P. W., Schulz R. A., Schenkman J. B., Keyes S. R., Cinti D. L. Hepatic organelle interaction. IV. Mechanism of succinate enhancement of formaldehyde accumulation from endoplasmic reticulum N-dealkylations. J Cell Biol. 1976 Jun;69(3):589–598. doi: 10.1083/jcb.69.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K. CARNITINE ACETYLTRANSFERASE. II. INHIBIITON BY CARNITINE ANALOGUES AND BY SULFHYDRYL REAGENTS. J Biol Chem. 1965 May;240:2188–2192. [PubMed] [Google Scholar]
- Fiecchi A., Galli-Kienle M., Scala A., Galli G., Paoletti R. The beta-oxidative cleavage of long-chain fatty acids in rat-liver cytoplasm. Eur J Biochem. 1973 Oct 18;38(3):516–528. doi: 10.1111/j.1432-1033.1973.tb03087.x. [DOI] [PubMed] [Google Scholar]
- Freebrey P. D., White J. T. A multi channel recorder of oxygen concentration. Phys Med Biol. 1966 Jul;11(3):471–473. doi: 10.1088/0031-9155/11/3/412. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Effects of the free acids and their carnitine esters on coenzyme A-dependent oxidations in rat liver mitochondria. Biochem J. 1973 Sep;136(1):157–171. doi: 10.1042/bj1360157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi V. C., Wilson A. C., Wakil S. J. Assay for the terminal enzyme of the stearoyl coenzyme A desaturase system using chick embryo liver microsomes. J Lipid Res. 1977 Jan;18(1):32–36. [PubMed] [Google Scholar]
- Kahonen M. T. Effect of clofibrate treatment on carnitine acyltransferases in different subcellular fractions of rat liver. Biochim Biophys Acta. 1976 May 28;428(3):690–701. doi: 10.1016/0304-4165(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Nozaki C., Tanaka A., Fukui S. Fatty acid beta-oxidation system in microbodies of n-alkane-grown Candida tropicalis. Eur J Biochem. 1978 Feb;83(2):609–613. doi: 10.1111/j.1432-1033.1978.tb12130.x. [DOI] [PubMed] [Google Scholar]
- Krahling J. B., Gee R., Murphy P. A., Kirk J. R., Tolbert N. E. Comparison of fatty acid oxidation in mitochondria and peroxisomes from rat liver. Biochem Biophys Res Commun. 1978 May 15;82(1):136–141. doi: 10.1016/0006-291x(78)90587-9. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Lazarow P. B. Three hypolipidemic drugs increase hepatic palmitoyl-coenzyme A oxidation in the rat. Science. 1977 Aug 5;197(4303):580–581. doi: 10.1126/science.195342. [DOI] [PubMed] [Google Scholar]
- Leighton F., Coloma L., Koenig C. Structure, composition, physical properties, and turnover of proliferated peroxisomes. A study of the trophic effects of Su-13437 on rat liver. J Cell Biol. 1975 Nov;67(2PT1):281–309. doi: 10.1083/jcb.67.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerer C. R. Effect of sub-acute administration of clofibrate on the oxidation of fatty acids by liver mitochondria. Biochem Pharmacol. 1977 Dec 1;26(23):2225–2230. doi: 10.1016/0006-2952(77)90283-0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Bieber L. L., Tolbert N. E. Differential increase of hepatic peroxisomal, mitochondrial and microsomal carnitine acyltransferases in clofibrate-fed rats. Biochem Pharmacol. 1977 Sep 15;26(18):1697–1702. doi: 10.1016/0006-2952(77)90147-2. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Chance B., Sies H., Bücher T. The role of H 2 O 2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys. 1973 Jan;154(1):117–131. doi: 10.1016/0003-9861(73)90040-4. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Bremer J. A spectrophotometric procedure for rapid and sensitive measurements of beta-oxidation. Demonstration of factors that can be rate-limiting for beta-oxidation. Biochem J. 1977 Jun 15;164(3):621–633. doi: 10.1042/bj1640621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem Biophys Res Commun. 1978 Jul 28;83(2):479–485. doi: 10.1016/0006-291x(78)91015-x. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Enhancement of fatty acyl-CoA oxidizing activity in rat liver peroxisomes by di-(i-ethylhexyl)phthalate. J Biochem. 1978 May;83(5):1361–1365. doi: 10.1093/oxfordjournals.jbchem.a132044. [DOI] [PubMed] [Google Scholar]
- Stäubli W., Schweizer W., Suter J., Weibel E. R. The proliferative response of hepatic peroxidomes of neonatal rats to treatment with SU-13 437 (nafenopin). J Cell Biol. 1977 Sep;74(3):665–689. doi: 10.1083/jcb.74.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]


