Abstract
Background:
Human papillomavirus (HPV) vaccination was introduced in 2006 for females and in 2011 for males.
Objective:
To estimate vaccine impact and effectiveness against quadrivalent HPV vaccine (4vHPV)–type prevalent infection among sexually experienced U.S. females and vaccine effectiveness for sexually experienced U.S. males.
Design:
NHANES (National Health and Nutrition Examination Survey) conducted in 2003 to 2006 (prevaccine era) and in 2007 to 2010, 2011 to 2014, and 2015 to 2018 (vaccine eras).
Setting:
Nationally representative U.S. surveys.
Participants:
Sexually experienced participants aged 14 to 24 years.
Intervention:
U.S. HPV vaccination program.
Measurements:
Participant-collected cervicovaginal and penile specimens were tested for HPV DNA. The prevalences of 4vHPV and non-4vHPV types were estimated in each era for females and in 2013 to 2016 for males. Prevalences among the female population overall, vaccinated females, and unvaccinated females were compared in vaccine eras versus the prevaccine era (vaccine impact). Within each vaccine era, prevalence among vaccinated females was compared with that among unvaccinated females (vaccine effectiveness). Vaccine impact and effectiveness were estimated as (1 – prevalence ratio) · 100.
Results:
Among sexually experienced females aged 14 to 24 years, the impact on 4vHPV-type prevalence in 2015 to 2018 was 85% overall, 90% among vaccinated females, and 74% among unvaccinated females. No significant declines were found in non–4vHPV-type prevalence. Vaccine effectiveness ranged from 60% to 84% during vaccine eras for females and was 51% during 2013 to 2016 for males.
Limitation:
Self- or parent-reported vaccination history and small numbers in certain subgroups limited precision.
Conclusion:
Nationally representative data show increasing impact of the vaccination program and herd protection. Vaccine effectiveness estimates will be increasingly affected by herd effects.
Primary Funding Source:
Centers for Disease Control and Prevention.
Monitoring the real-world impact and effectiveness of vaccination programs is a key component of postlicensure vaccine evaluation. Human papillomavirus (HPV) vaccination was introduced in the United States in 2006. A quadrivalent vaccine (4vHPV) was first recommended for routine vaccination of females at age 11 to 12 years and for catch-up through age 26 years. Males were included in the vaccination program starting in 2011. The 4vHPV vaccine targets 2 oncogenic types, HPV 16 and 18, and 2 nononcogenic types, HPV 6 and 11. This vaccine was mainly used through 2015, when a 9-valent vaccine (9vHPV) targeting 5 additional oncogenic types (HPV 31, 33, 45, 52, and 58) was introduced (1). Coverage with HPV vaccination in the United States has been increasing but is lower than that with other adolescent vaccinations (2).
Human papillomavirus is the most common sexually transmitted infection in the United States (3). Persistent HPV infection can cause cervical, other anogenital, and oropharyngeal cancer and anogenital warts. Vaccination aims to prevent these types of cancer, which can develop years or decades after HPV infection (4). Prevalence monitoring for HPV infection is used to assess the early impact of HPV vaccination programs and can provide measurements of vaccine impact on vaccine-targeted types (5). Determining the prevalence of non–vaccine-targeted types allows evaluation of possible type replacement, cross-protection, and comparability of exposure by vaccination status and time period.
Monitoring national HPV prevalence in the United States is possible through NHANES (National Health and Nutrition Examination Survey), which incorporated testing for female genital HPV before the HPV vaccination program began (1, 6). Declines in the prevalence of vaccine-targeted types were observed first in 2007 to 2010 among females aged 14 to 19 years (5) and later among females aged 20 to 24 years (7, 8). The most recent NHANES data, through 2018, show continued declines among females in these age groups (9). Male genital HPV testing was incorporated into NHANES during 2013 to 2014, after the HPV vaccination program started. The first data available in males suggested that declines in 4vHPV-type prevalence had already occurred in males aged 14 to 19 and 20 to 24 years, likely attributable to indirect effects of vaccinating females (10, 11). NHANES data have also been used to estimate vaccine effectiveness by comparing prevalence in vaccinated and unvaccinated persons (5, 7, 8). We used the most recent NHANES data to estimate HPV vaccine impact in sexually experienced young females and vaccine effectiveness in sexually experienced females and males aged 14 to 24 years.
Methods
Design of Study
NHANES is an ongoing, cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics to monitor the health and nutrition of the population of noninstitutionalized U.S. civilians (12). It is designed to be nationally representative; data are released in 2-year cycles. Certain subpopulations are oversampled to increase the reliability and precision of estimates. Data collection for NHANES was approved by the Research Ethics Review Board of the National Center for Health Statistics.
Data Collection
Demographic and HPV vaccination information was obtained during in-home interviews. Vaccination history was collected beginning in 2007 for females and 2011 for males; collection of information on age at vaccination began in 2011. Individuals aged 16 years or older were directly interviewed; parents or guardians reported vaccination information for those younger than 16 years. Sexual behavior information via audio computer-assisted self-interview and self-collected cervicovaginal and, starting in 2013, penile specimens were obtained in mobile examination centers. The CDC determined HPV DNA types from specimens using the Research Use Only Linear Array HPV Genotyping Test (Roche Molecular Diagnostics), as described previously (8). This assay uses L1 consensus polymerase chain reaction followed by type-specific hybridization to detect 37 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52[XR], 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39) and β-globin. Samples negative for both HPV and β-globin were considered inadequate and were excluded.
Statistical Analysis
Self-reported race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Mexican American, or other races/ethnicities. Self- or parent-reported vaccination history was analyzed as ever vaccinated (receipt of ≥1 dose) versus unvaccinated; age at vaccination (first dose) was analyzed as younger than 15 years versus 15 years or older among those vaccinated. Sexual behavior information analyzed included sexually experienced (ever having had anal, oral, or vaginal sex) and number of lifetime sex partners (<3 or ≥3 same- or opposite-sex partners). Below poverty was defined as a poverty income ratio less than 1 (13). The following HPV type categories were analyzed: 4vHPV types (HPV 6, 11, 16, and 18); HPV 31, 33, and 45 (3 types for which there may be cross-protection) (14), and non-4vHPV types (33 types detected using Linear Array that are not HPV 6, 11, 16, or 18).
Females
For females, demographic and HPV prevalence data were analyzed in 4-year eras, including the prevaccine era (2003 to 2006) and 3 vaccine eras (2007 to 2010, 2011 to 2014, and 2015 to 2018). Vaccination coverage of at least 1 HPV dose was estimated for the 3 vaccine eras. Analyses were limited to sexually experienced participants to ensure that all those included had an opportunity for HPV exposure and to participants aged 14 to 24 years with adequate self-collected cervicovaginal specimens, resulting in a sample size of 3197 (Supplement Figure 1, available at Annals.org).
The impact and effectiveness of the HPV vaccine were estimated using a modified framework from Halloran and colleagues (15) (Figure 1). Distributions of characteristics and behaviors were estimated overall and by vaccination status to identify differences that might influence HPV prevalence comparisons. Prevalences of 4vHPV types, non-4vHPV types, and HPV 31, 33, and 45 in each 4-year period were estimated overall and among vaccinated and unvaccinated persons. Prevalence ratios (PRs) for each vaccine era compared with the prevaccine era were used to estimate overall population impact (comparison of vaccine era overall vs. prevaccine era overall), impact among vaccinated persons (comparison of vaccinated persons in the vaccine era vs. prevaccine era overall), and impact among unvaccinated persons (comparison of unvaccinated persons in the vaccine era vs. prevaccine era overall). Within each era, vaccine effectiveness was estimated by comparing prevalence among vaccinated versus unvaccinated persons. Impact and effectiveness were calculated as (1 — PR) · 100. We present unadjusted estimates; because of low prevalences of 4vHPV types and HPV 31, 33, and 45 and smaller sample sizes for groups stratified by vaccination status, adjusted models would not converge.
Figure 1.
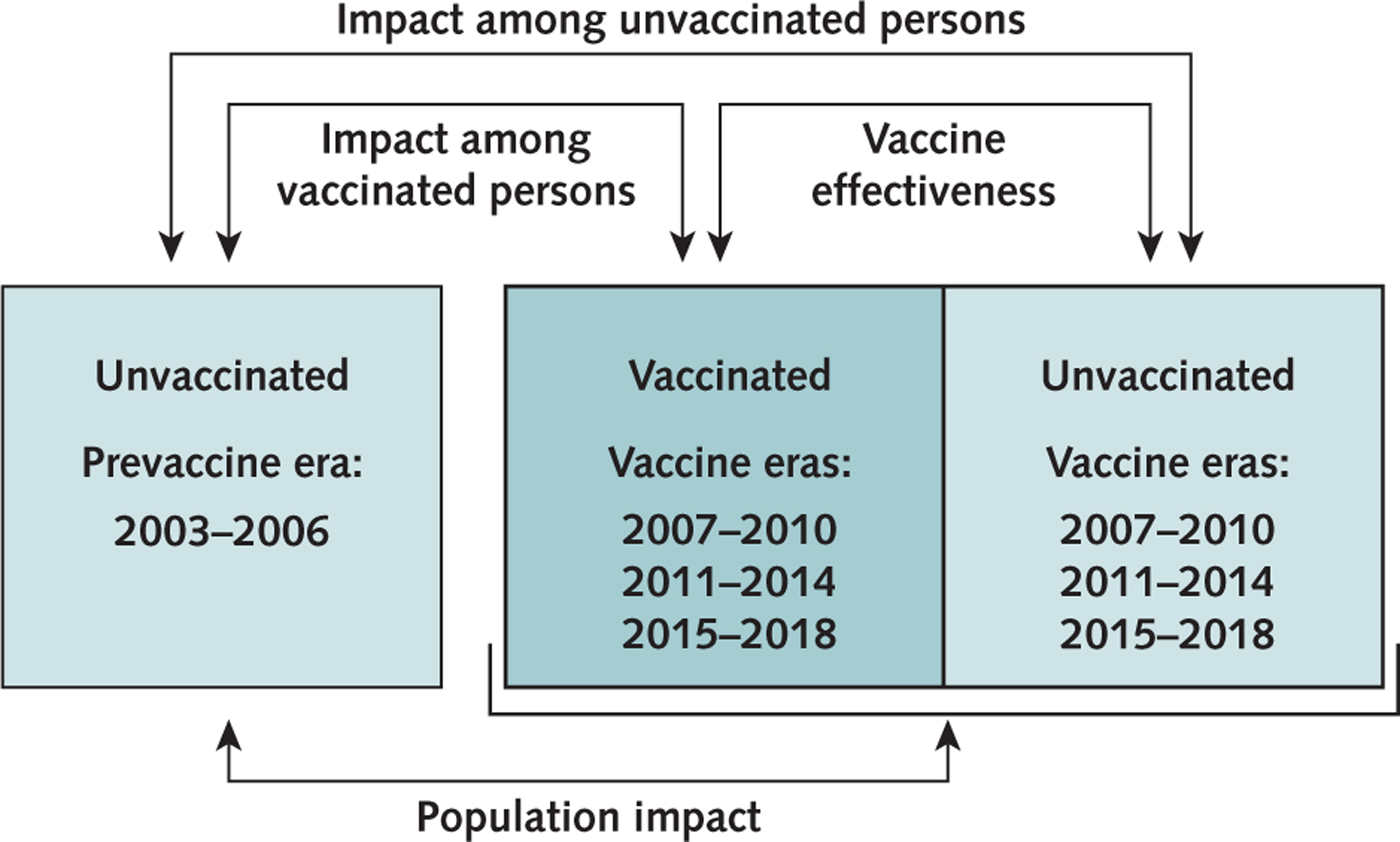
Vaccine impact and vaccine effectiveness framework.
Males
For males, demographic and HPV prevalence data were analyzed in 1 vaccine era, 2013 to 2016, the only years for which male HPV typing data are available in NHANES. Analyses were limited to sexually experienced participants aged 14 to 24 years with adequate self-collected penile specimens, resulting in a sample size of 661 for estimates of male prevalence and vaccine effectiveness (Supplement Figure 2, available at Annals.org). Vaccination coverage of at least 1 dose was estimated for 2 vaccine eras, 2011 to 2014 and 2015 to 2018. Coverage analyses were limited to sexually experienced participants aged 14 to 24 years who attended the mobile examination center, regardless of whether data were available from a swab, resulting in a sample size of 1475.
Using data from 2013 to 2016, we estimated distributions of characteristics and behaviors overall and by vaccination status. Prevalences of 4vHPV types, non-4vHPV types, and HPV 31, 33, and 45 were estimated overall and among vaccinated and unvaccinated persons. Vaccine effectiveness was estimated as described in the previous section.
Complex survey analytic methods were used to account for the survey design; all analyses used NHANES examination weights (16). Prevalence estimates with relative SE greater than 30% were noted as unstable and should be interpreted with caution. Statistical significance was defined as 95% CIs around PRs that did not include 1. Analyses were done using SAS, version 9.4 (SAS Institute) and SUDAAN, version 11.0 (RTI International). Because the analyses included restricted-use data, data were accessed through CDC’s Research Data Center.
Role of the Funding Source
The funding organization, CDC, conducted the study, collected and managed data, and prepared and reviewed the manuscript.
Results
HPV Vaccination Coverage: Females and Males
The percentage of females reporting receipt of at least 1 HPV vaccine dose was 25.2% in 2007 to 2010 and greater in the later vaccine eras, reaching 59.0% in 2015 to 2018 (Table 1 and Figure 2). In 2011 to 2014, vaccination before age 15 years was reported in 27.2% of vaccinated females aged 14 to 24 years, compared with 48.6% in 2015 to 2018. The percentage of males reporting receipt of at least 1 HPV vaccine dose was greater in 2015 to 2018 (29.5% [95% CI, 25.0% to 34.5%]) than in 2011 to 2014 (14.1% [CI, 11.7% to 16.9%]) (Figure 2). Vaccination before age 15 years was reported in 18.6% (CI, 11.7% to 28.3%) of vaccinated males in 2011 to 2014 and 48.7% (CI, 31.3% to 66.5%) in 2015 to 2018.
Table 1.
Characteristics of Sexually Experienced Females Aged 14–24 Years in the Prevaccine Era and in 3 Vaccine Eras, Overall and by HPV Vaccination History*
| Characteristic | Prevaccine Era 2003–2006 Overall (n = 1095) |
Vaccine Eras |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2007–2010 | 2011–2014 | 2015–2018 | ||||||||
|
| ||||||||||
| Overall (n = 740) |
Vaccinated (n = 171) |
Unvaccinated (n = 553) |
Overall (n = 748) |
Vaccinated (n = 347) |
Unvaccinated (n = 360) |
Overall (n = 614) |
Vaccinated (n = 316) |
Unvaccinated (n = 233) |
||
| HPV vaccination history | ||||||||||
| ≥1 dose | † | 25.2 (19.7–31.7) | – | – | 48.6 (42.9–54.3) | – | – | 59.0 (53.9–63.9) | – | – |
| Age at vaccination <15 y‡ | † | – | § | – | – | 27.2 (21.3–34.0) | – | – | 48.6 (41.1–56.2) | – |
| ≥3 lifetime sex partners | 58.4 (54.9–61.7) | 64.9 (61.1–68.5) | 67.3 (58.2–75.3) | 64.3 (58.8–69.5) | 65.3 (60.9–69.5) | 63.9 (56.8–70.4) | 67.0 (59.3–73.9) | 60.3 (55.7–64.7) | 62.7 (55.6–69.3) | 54.7 (46.3–62.8) |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 64.0 (56.7–70.7) | 58.0 (52.0–63.7) | 68.5 (58.1–77.3) | 55.2 (48.1–62.1) | 56.6 (48.9–64.0) | 63.2 (55.5–70.3) | 52.1 (42.3–61.8) | 55.8 (48.3–63.0) | 57.6 (49.3–65.5) | 53.1 (44.6–61.3) |
| Non-Hispanic Black | 15.8 (11.4–21.4) | 16.2 (13.1–19.9) | 11.5 (7.4–17.5) | 18.0 (14.2–22.6) | 17.0 (11.7–24.1) | 16.0 (11.0–22.7) | 18.0 (11.8–26.4) | 12.5 (9.1–17.1) | 12.2 (8.7–16.9) | 13.1 (9.1–18.6) |
| Mexican American | 10.9 (7.9–14.9) | 12.2 (8.8–16.7) | 5.6 (2.7–11.1)|| | 13.8 (10.0–18.9) | 10.4 (7.9–13.6) | 7.7 (5.3–11.0) | 12.0 (8.2–17.3) | 14.0 (9.6–19.8) | 12.7 (8.4–18.8) | 13.8 (8.6–21.3) |
| Other races/ethnicities | 9.3 (6.7–12.7) | 13.6 (10.3–17.9) | 14.4 (9.7–20.8) | 13.0 (9.1–18.1) | 16.1 (12.6–20.2) | 13.1 (9.3–18.1) | 17.9 (13.7–23.2) | 17.7 (14.0–22.1) | 17.5 (13.2–22.8) | 20.0 (14.5–27.0) |
| Below poverty level | 27.5 (22.3–33.3) | 29.9 (25.6–34.6) | 21.8 (16.1–28.9) | 31.8 (26.6–37.6) | 33.0 (25.6–41.4) | 27.4 (19.6–36.7) | 37.6 (28.9–47.1) | 23.7 (19.5–28.6) | 21.4 (15.8–28.3) | 23.7 (18.4–30.0) |
HPV = human papillomavirus.
Values are percentages (95% CIs). All estimates are weighted using NHANES (National Health and Nutrition Examination Survey) examination weights.
No HPV vaccine was administered during this time.
Among those vaccinated; refers to age at first dose.
Not asked.
Relative SE, 30%–50%.
Figure 2.
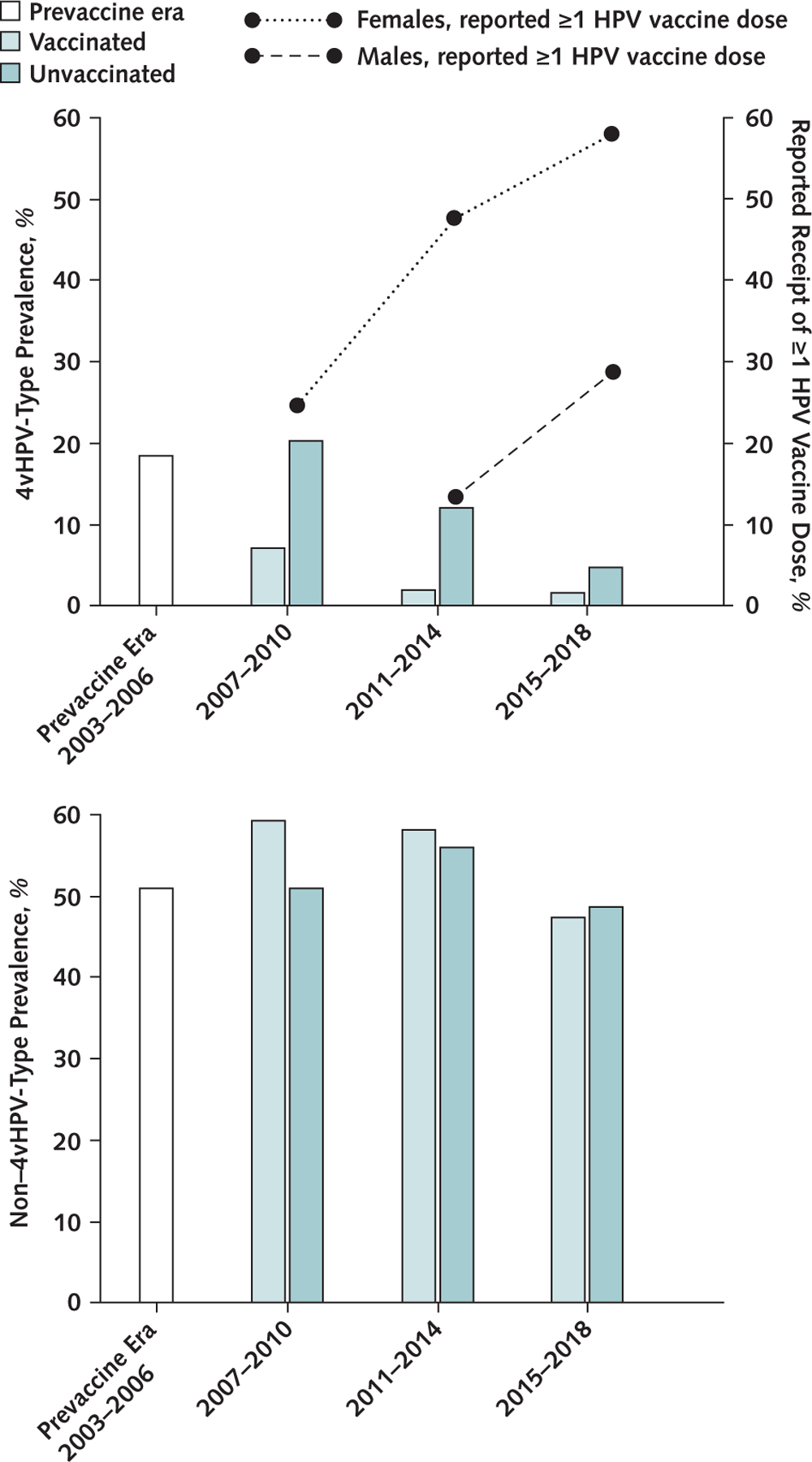
HPV vaccination coverage and 4vHPV-type (top) and non–4vHPV-type (bottom) prevalence among sexually experienced 14- to 24-year-old females, by vaccination era and history.
The reported receipt of ≥1 HPV vaccine dose (%) among 14- to 24-year-old female and male participants is represented by the lines. 4vHPV-type indicates quadrivalent HPV vaccine types: HPV 6, 11, 16, and 18. Non–4vHPV-type indicates 33 types detected using Linear Array that are not HPV 6, 11, 16, or 18. All estimates are weighted using NHANES (National Health and Nutrition Examination Survey) examination weights. 4vHPV = quadrivalent HPV vaccine; HPV = human papillomavirus.
Characteristics, HPV Prevalence, Vaccine Impact, and Vaccine Effectiveness: Females
Compared with the prevaccine era, percentages of females with 3 or more lifetime partners were slightly higher (by 5 to 7 percentage points) in the first 2 vaccine eras but similar in the most recent era (Table 1). There were small differences in the percentages with 3 or more lifetime partners between vaccinated and unvaccinated females in all eras (3 to 8 percentage points). The distribution of race/ethnicity varied over time; the percentage of females who identified as non-Hispanic White was lower in the vaccine eras (by 6 to 8 percentage points), with gradually increasing percentages identifying as other races/ethnicities (4 to 8 percentage points). In vaccine eras, a higher percentage (by 4 to 13 percentage points) of vaccinated than unvaccinated females identified as non-Hispanic White. A higher percentage of unvaccinated than vaccinated females were below the poverty level (by 10 percentage points in the earlier eras and 2 percentage points in the most recent period).
Human papillomavirus vaccine impact for females overall, among vaccinated females, and among unvaccinated females was determined by comparison of those populations in vaccine eras versus the prevaccine era (Figure 1). The prevalence of 4vHPV types decreased from 18.5% to 16.8% during 2007 to 2010; the overall population impact on 4vHPV-type prevalence was 9% (PR, 0.91 [CI, 0.70 to 1.18]) (Table 2 and Figure 3). By 2015 to 2018, the prevalence was 2.8% (PR, 0.15 [CI, 0.08 to 0.28]), for an overall impact of 85% (Figure 2). The impact among vaccinated females was 61% (PR, 0.39 [CI, 0.21 to 0.75]) by 2007 to 2010 and 90% (PR, 0.10 [CI, 0.04 to 0.28]) by 2015 to 2018. No impact was seen among unvaccinated females in 2007 to 2010; by 2015 to 2018, an impact of 74% (PR, 0.26 [CI, 0.12 to 0.57]) was observed.
Table 2.
HPV Prevalence and Prevalence Ratios for Sexually Experienced Females Aged 14–24 Years in the Prevaccine Era and 3 Vaccine Eras, Overall and by Vaccination History*
| HPV Types and Vaccination History | Prevalence (95% CI), % |
Prevalence Ratio (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccine Era 2003–2006 |
Vaccine Eras |
2007–2010 |
2011–2014 |
2015–2018 |
||||||
| 2007–2010 | 2011–2014 | 2015–2018 | Comparison of 2007–2010 With Prevaccine Era | Comparison Within 2007–2010 | Comparison of 2011–2014 With Prevaccine Era | Comparison Within 2011–2014 | Comparison of 2015–2018 With Prevaccine Era | Comparison Within 2015–2018 | ||
| 4vHPV † | ||||||||||
| Overall | 18.5 (16.1–21.2) | 16.8 (13.4–21.0) | 7.1 (4.7–10.5) | 2.8 (1.5–5.2)‡ | 0.91 (0.70–1.18) | – | 0.38 (0.25–0.58) | – | 0.15 (0.08–0.28) | – |
| Vaccinated | || | 7.3 (3.8–13.5)‡ | 2.0 (1.0–3.9)‡ | 1.9 (0.7–5.1)‡ | 0.39 (0.21–0.75) | 0.36 (0.19–0.67) | 0.11 (0.06–0.21) | 0.16 (0.07–0.39) | 0.10 (0.04–0.28) | 0.40 (0.11–1.41) |
| Unvaccinated | || | 20.4 (16.3–25.1) | 12.2 (7.6–19.2) | 4.8 (2.1–10.5)‡ | 1.10 (0.85–1.42) | Reference | 0.66 (0.41–1.07) | Reference | 0.26 (0.12–0.57) | Reference |
| Non-4vHPV § | ||||||||||
| Overall | 51.1 (46.4–55.8) | 53.1 (48.3–57.8) | 56.4 (51.5–61.2) | 47.6 (42.0–53.2) | 1.04 (0.92–1.18) | – | 1.10 (0.98–1.25) | – | 0.93 (0.80–1.08) | – |
| Vaccinated | || | 59.4 (51.7–66.6) | 58.2 (51.3–64.8) | 47.3 (40.8–53.9) | 1.16 (1.00–1.35) | 1.16 (0.99–1.35) | 1.14 (0.99–1.32) | 1.04 (0.88–1.23) | 0.93 (0.79–1.09) | 0.97 (0.82–1.14) |
| Unvaccinated | || | 51.2 (45.6–56.9) | 56.1 (48.9–63.0) | 48.8 (42.0–55.6) | 1.00 (0.87–1.15) | Reference | 1.10 (0.94–1.28) | Reference | 0.95 (0.81–1.13) | Reference |
| HPV 31, 33, 45 | ||||||||||
| Overall | 6.6 (4.7–9.2) | 5.3 (3.8–7.4) | 4.9 (3.1–7.6) | 3.6 (2.1–6.0) | 0.81 (0.51–1.28) | – | 0.74 (0.43–1.28) | – | 0.55 (0.30–1.00) | – |
| Vaccinated | || | 6.7 (3.7–12.0) | 4.9 (2.9–8.2) | 4.8 (2.6–8.9)‡ | 1.02 (0.53–1.98) | 1.38 (0.65–2.92) | 0.74 (0.40–1.36) | 0.97 (0.49–1.93) | 0.73 (0.36–1.47) | 1.80 (0.79–4.11) |
| Unvaccinated | || | 4.9 (3.2–7.4) | 5.0 (2.7–9.1) | 2.7 (1.4–5.2)‡ | 0.74 (0.44–1.26) | Reference | 0.76 (0.38–1.50) | Reference | 0.41 (0.20–0.84) | Reference |
4vHPV = quadrivalent HPV vaccine; HPV = human papillomavirus.
All estimates are weighted using NHANES (National Health and Nutrition Examination Survey) examination weights. We use HPV nomenclature to be consistent with our past publications; however, nomenclature has evolved since the Roche product was launched. IS39 is a subtype of HPV 82, HPV 55 was reclassified as HPV 44, and HPV 64 was reclassified as HPV 34.
Quadrivalent HPV vaccine types: HPV 6, 11, 16, and 18.
Relative SE, 30%–50%.
33 types detected using Linear Array that are not HPV 6, 11, 16, or 18.
No HPV vaccine was administered in this period.
Figure 3.
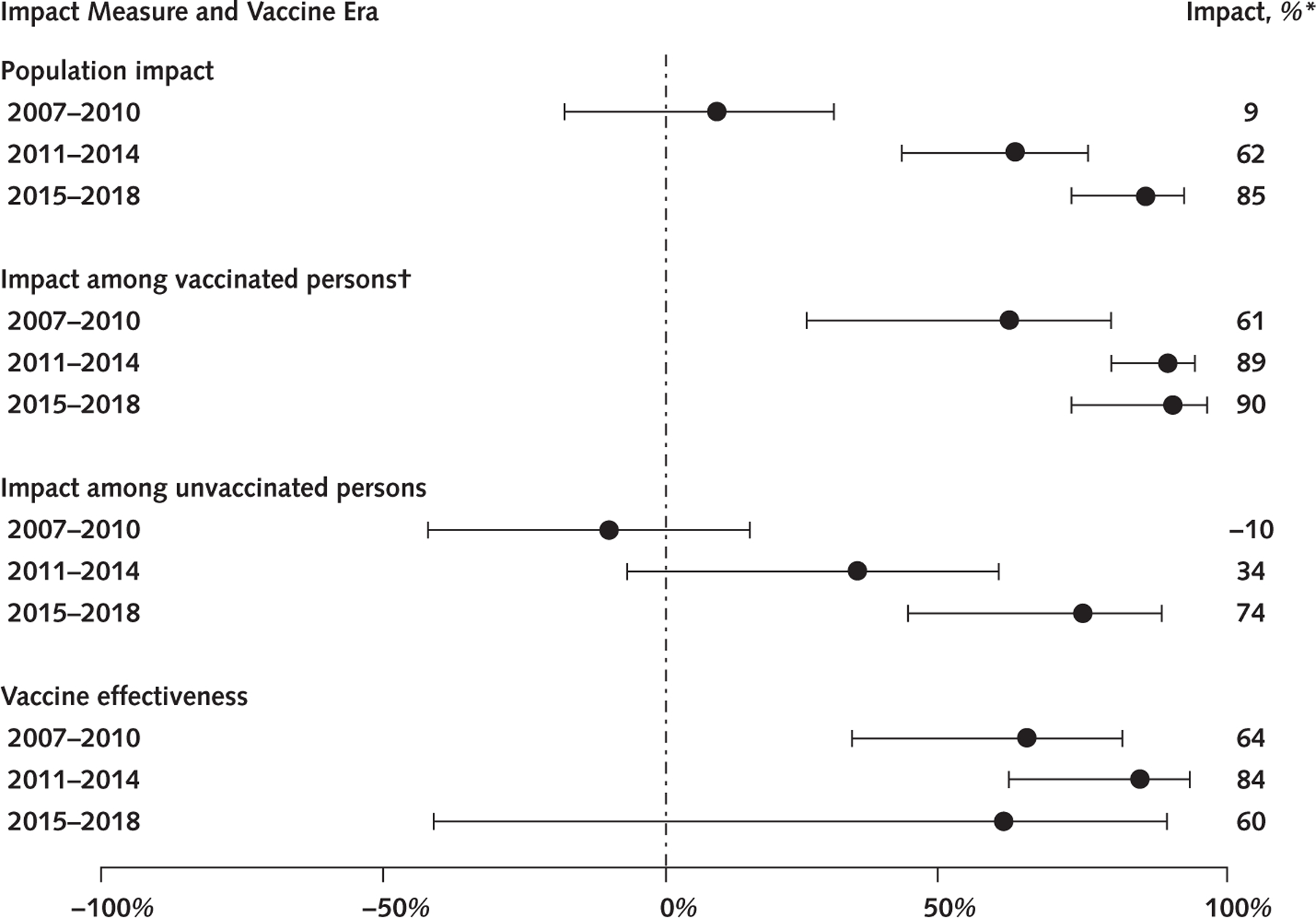
Vaccine impact measures and vaccine effectiveness for 4vHPV-type prevalence among sexually experienced 14- to 24-year-old females, in 3 vaccine eras.
4vHPV-type indicates quadrivalent HPV vaccine types: HPV 6, 11, 16, and 18. All estimates are weighted using NHANES (National Health and Nutrition Examination Survey) examination weights.
* Impact = (1 — prevalence ratio) · 100. Bars represent 95% CIs. The following impact estimates were calculated using prevalence estimates with relative SE >30% and should be interpreted with caution: population impact 2015–2018; impact among vaccinated persons in 2007–2010, 2011–2014, and 2015–2018; impact among unvaccinated persons in 2015–2018; and vaccine effectiveness in 2007–2010, 2011–2014, and 2015–2018.
† Vaccinated indicates self-/parent-reported receipt of ≥1 dose. 4vHPV = quadrivalent HPV vaccine; HPV = human papillomavirus.
In the earliest vaccine era, 2007 to 2010, 4vHPV-type prevalence was 7.3% among vaccinated females, compared with 20.4% among unvaccinated females (PR, 0.36 [CI, 0.19 to 0.67]), corresponding to a 64% vaccine effectiveness (Figures 1 and 3 and Table 2). During 2011 to 2014, 4vHPV-type prevalence was 2.0% among vaccinated and 12.2% among unvaccinated females, for an 84% vaccine effectiveness (PR, 0.16 [CI, 0.07 to 0.39]). During 2015 to 2018, 4vHPV-type prevalence was 1.9% among vaccinated and 4.8% among unvaccinated females, corresponding to a 60% vaccine effectiveness (PR, 0.40 [CI, 0.11 to 1.41]).
Non–4vHPV-type prevalence was similar in the prevaccine era (51.1%) and in 2015 to 2018 (47.6%) (PR, 0.93 [CI, 0.80 to 1.08]) (Table 2 and Figure 2). Non–4vHPV-type prevalence was similar between the prevaccine era and each vaccine era among vaccinated and unvaccinated females, and between vaccinated and unvaccinated females within each era. The prevalence of HPV 31, 33, and 45 decreased, but not statistically significantly, from the prevaccine era (6.6%) to 2015 to 2018 (3.6%). There was no evidence of vaccine effectiveness against HPV 31, 33, and 45 types in any era.
Characteristics, HPV Prevalence, and Vaccine Effectiveness: Males
Among sexually experienced males in 2013 to 2016, there were small differences in characteristics between vaccinated and unvaccinated participants (Supplement Table 1, available at Annals.org). The prevalence of 4vHPV types was 1.8% among vaccinated and 3.5% among unvaccinated males (PR, 0.49 [CI, 0.11 to 2.20]), resulting in an estimated 51% vaccine effectiveness (Supplement Table 2, available at Annals.org). Non–4vHPV-type prevalence was not statistically significantly different between vaccinated (30.7%) and unvaccinated (34.3%) males. The prevalence of HPV 31, 33, and 45 was higher among vaccinated than unvaccinated males, but differences were not statistically significant.
Discussion
This article presents the most recent national data from NHANES through 12 years after HPV vaccine introduction. As HPV vaccination coverage increased among females and vaccination was introduced for males, the observed impact of the vaccination program increased. By 2015 to 2018, compared with the prevaccine era, 4vHPV-type prevalence declined 85% overall among sexually experienced 14- to 24-year-old females, 90% among vaccinated females, and 74% among unvaccinated females. This analysis expands our previous report (9) about population impact by reporting on direct and indirect HPV vaccine impact among young, sexually experienced females and vaccine effectiveness for females and males.
Among females, population impact, which comprises both direct and indirect impact, increased from 62% in 2011 to 2014 to 85% in 2015 to 2018. The direct impact among vaccinated females remained high at 90% in 2015 to 2018. The decline in 4vHPV-type prevalence among unvaccinated females suggests strong herd effects or indirect protection. Herd effects in NHANES were first noted among sexually experienced, unvaccinated females aged 14 to 24 years in 2011 to 2014, with a 34% decrease in 4vHPV-type prevalence, when coverage with at least 1 HPV vaccine dose was 51% (7). The 74% decrease in 4vHPV-type prevalence in unvaccinated females was observed in 2015 to 2018, when 59% of females and 30% of males reported receiving at least 1 dose. Reports from other countries, some with similarly low coverage, also suggest herd protection (17–19), and modeling studies indicate that some herd effects are expected from vaccinating young females only, even with coverage as low as 20% (20). We previously showed likely herd effects for males from the U.S. female vaccination program (10, 11). The herd effects observed in this analysis are probably attributable to both female and male vaccination (1, 2). Of note, national survey data using provider-verified records show higher coverage with at least 1 HPV vaccine dose than NHANES: 63% to 70% among females and 50% to 66% among males aged 13 to 17 years in 2015 to 2018 (2).
Estimates of vaccine effectiveness and impact among vaccinated females were similar during 2007 to 2010 (64% and 61%, respectively) and 2011 to 2014 (84% and 89%, respectively), and both measures increased between these 2 vaccine eras. However, in 2015 to 2018, vaccine effectiveness and impact diverged (60% and 90%, respectively). Because vaccine effectiveness is estimated by comparing HPV prevalence among vaccinated versus unvaccinated individuals, as herd protection increases and prevalence among unvaccinated persons decreases, vaccine effectiveness might be difficult to estimate. Our effectiveness estimates are lower than the efficacy estimates (>95%) in the HPV vaccine clinical trials in per protocol analyses (limited to participants who had not had the relevant vaccine-type HPV infection) (21, 22). Most females in the NHANES sample from 2007 to 2010 received the vaccine at older ages than routinely recommended and might have been infected before vaccination. The increases in estimated effectiveness and impact among vaccinated females between 2007 to 2010 and 2011 to 2014 are likely due to more vaccinated females having received the vaccine at younger ages (2). Estimates of 4vHPV-type prevalence among females for 2015 to 2018 were low and unstable, and the vaccine effectiveness estimate was lower than that observed in 2011 to 2014, with a wide CI. We do not believe that these findings raise concerns about waning immunity; multiple studies show long-lasting protection after HPV vaccination (23–25).
Among males, the vaccine effectiveness estimate from 2013 to 2016 was also lower than that observed in clinical trials, where efficacy against HPV vaccine-type infection and external genital lesions was close to 90% in per protocol analyses (26). As with females, most vaccinated males in this NHANES analysis received the vaccine at older ages than recommended and were likely affected by herd effects by the time collection of male HPV data began (10). We do not have data from the prevaccine era on male HPV prevalence and cannot provide population impact estimates for males.
Although some randomized controlled trials and observational studies of 4vHPV found efficacy or effectiveness against HPV 31, 33, and 45, suggesting cross-protection, evidence is not consistent (14, 27–29). We found no statistically significant decrease in the prevalence of HPV 31, 33, and 45 among females between the prevaccine era and 2015 to 2018 and no evidence of vaccine effectiveness for these types in females or males. Of note, very few participants surveyed during 2015 to 2018 would have received 9vHPV. As coverage with 9vHPV increases, evaluating cross-protection may not be possible.
This study has several limitations. First, the self- or parent-reported vaccination history in NHANES may have been misclassified, resulting in over- or underestimation of vaccine impact and vaccine effectiveness. Vaccination coverage reported in NHANES is historically lower (30) than that from provider-verified records in the National Immunization Survey–Teen (2) but higher than that from self-reports in the National Health Interview Survey (31). Second, we estimated vaccine impact and effectiveness on the basis of history of at least 1 dose, although the recommended number of doses is 2 or 3, depending on age at initiation. Although NHANES collects number of doses received, previous work suggests that these data are not accurate enough for analyses by dose number (30). Last, small sample sizes precluded adjustment for potential confounders (such as ≥3 lifetime partners) and limited precision for certain subgroup analyses, resulting in several statistically unstable estimates, particularly for 4vHPV-type prevalence. Participant characteristics indicated some differences over time and by vaccination status; however, most of these were likely not large enough to meaningfully affect our conclusions about vaccine impact and effectiveness. Of note, non–4vHPV-type prevalence, a marker of sexual risk behavior, was similar among vaccinated and unvaccinated persons within each era.
Twelve years into the United States’ HPV vaccination program, national data demonstrate an increasing impact among females, strong herd effects among unvaccinated females in the context of increasing HPV vaccination coverage among both males and females, and greater percentages of persons receiving the HPV vaccine in early adolescence before HPV exposure through sexual contact. Although this NHANES report and others evaluated vaccine-type HPV prevalence (32, 33), additional studies have shown an impact of vaccination on anogenital warts (34–36) and cervical precancer (37–39). Other countries with national vaccination programs have also seen large impacts of HPV vaccination (18, 40–45).
The COVID-19 pandemic disrupted immunization programs worldwide. Data from the United States indicate that the pandemic may reverse trends of increasing HPV vaccination coverage (46, 47). To maintain the progress made (48), strong efforts to increase vaccination coverage are critical. Future monitoring will allow further evaluation of the public health impact of the HPV vaccination program, including 9vHPV, the 2-dose schedule, and duration of protection.
Supplementary Material
Acknowledgment:
The authors thank Carolyn Neal (National Center for Health Statistics, Research Data Center, CDC) and Juanita M. Onyekwuluje, Sonya Patel, and Krystle L. Love (Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC).
Financial Support:
By the CDC.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-3798.
Reproducible Research Statement: Study protocol: Available online (wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx). Statistical code: Not available. Data set: Publicly available data can be accessed on the NHANES website (wwwn.cdc.gov/nchs/nhanes/Default.aspx). Restricted data can be accessed through the Research Data Center via the National Center for Health Statistics (www.cdc.gov/rdc/index.htm).
For author, article, and disclosure information, see end of text.
Contributor Information
Hannah G. Rosenblum, Epidemic Intelligence Service and Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Rayleen M. Lewis, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, and Synergy America, Duluth, Georgia.
Julia W. Gargano, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Troy D. Querec, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Elizabeth R. Unger, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Lauri E. Markowitz, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- 1.Markowitz LE, Gee J, Chesson H, et al. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr 2018;18: S3–S10. doi: 10.1016/j.acap.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:1109–1116. doi: 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis 2021;48:208–214. doi: 10.1097/OLQ.0000000000001355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuschieri K, Unger ER. Human papillomaviruses. In: Carroll KC, Pfaller MA, Landry ML, et al. , eds. Manual of Clinical Microbiology. 12th ed. J Wiley; 2019:1847–62. [Google Scholar]
- 5.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013;208:385–93. doi: 10.1093/infdis/jit192 [DOI] [PubMed] [Google Scholar]
- 6.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis 2011;204:566–73. doi: 10.1093/infdis/jir341 [DOI] [PubMed] [Google Scholar]
- 7.Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017;216:594–603. doi: 10.1093/infdis/jix244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz LE, Liu G, Hariri S, et al. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016; 137:e20151968. doi: 10.1542/peds.2015-1968 [DOI] [PubMed] [Google Scholar]
- 9.Rosenblum HG, Lewis RM, Gargano JW, et al. Declines in prevalence of human papillomavirus vaccine-type infection among females after introduction of vaccine — United States, 2003–2018. MMWR Morb Mortal Wkly Rep 2021;70:415–420. doi: 10.15585/mmwr.mm7012a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013–2014. J Infect Dis 2017;215:1070–1079. doi: 10.1093/infdis/jix057 [DOI] [PubMed] [Google Scholar]
- 11.Lewis RM, Markowitz LE, Gargano JW, et al. Prevalence of genital human papillomavirus among sexually experienced males and females aged 14–59 years, United States, 2013–2014. J Infect Dis 2018;217:869–877. doi: 10.1093/infdis/jix655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. Updated 15 September 2017. Accessed at www.cdc.gov/nchs/nhanes/about_nhanes.htm on 17 September 2021. [Google Scholar]
- 13.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: 2017–2018 Data Documentation, Codebook, and Frequencies. February 2020. Accessed at wwwn.cdc.gov/Nchs/Nhanes/2017-2018/DEMO_J.htm on 19 September 2021. [Google Scholar]
- 14.Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:781–9. doi: 10.1016/S1473-3099(12)70187-1 [DOI] [PubMed] [Google Scholar]
- 15.Halloran ME, Struchiner CJ, Longini IM Jr. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol 1997;146:789–803. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. NHANES Survey Methods and Analytic Guidelines. Accessed at wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx on 7 September 2021. [Google Scholar]
- 17.Drolet M, Bénard É, Pérez N, et al. ; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi: 10.1016/S0140-6736(19)30298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017;17:1293–1302. doi: 10.1016/S1473-3099(17)30468-1 [DOI] [PubMed] [Google Scholar]
- 19.Hoes J, Woestenberg PJ, Bogaards JA, et al. ; Medical Microbiological Laboratories and Public Health Services. Population impact of girls-only human papillomavirus 16/18 vaccination in the Netherlands: cross-protective and second-order herd effects. Clin Infect Dis 2021;72: e103–e111. doi: 10.1093/cid/ciaa1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1:e8–e17. doi: 10.1016/S2468-2667(16)30001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garland SM, Hernandez-Avila M, Wheeler CM, et al. ; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928–43. [DOI] [PubMed] [Google Scholar]
- 22.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915–27. [DOI] [PubMed] [Google Scholar]
- 23.Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi: 10.1016/j.eclinm.2020.100401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstone SE, Giuliano AR, Palefsky JM, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2022;22:413–425. doi: 10.1016/S1473-3099(21)00327-3 [DOI] [PubMed] [Google Scholar]
- 26.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 2011;364:401–11. doi: 10.1056/NEJMoa0909537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown DR, Joura EA, Yen GP, et al. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine. 2021;39:2224–2236. doi: 10.1016/j.vaccine.2020.11.076 [DOI] [PubMed] [Google Scholar]
- 28.Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic non-vaccine HPV types in sexually active women aged 16–26 years. J Infect Dis 2009;199:936–44. doi: 10.1086/597309 [DOI] [PubMed] [Google Scholar]
- 29.Basu P, Malvi SG, Joshi S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol 2021;22:1518–1529. doi: 10.1016/S1470-2045(21)00453-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis RM, Markowitz LE. Human papillomavirus vaccination coverage among females and males, National Health and Nutrition Examination Survey, United States, 2007–2016. Vaccine. 2018;36:2567–2573. doi: 10.1016/j.vaccine.2018.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu PJ, Hung MC, Srivastav A, et al. Surveillance of vaccination coverage among adult populations — United States, 2018. MMWR Surveill Summ 2021;70:1–26. doi: 10.15585/mmwr.ss7003a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subasinghe AK, Wark JD, Phillips S, et al. Quadrivalent human papillomavirus vaccination successfully reduces the prevalence of vaccine-targeted genotypes in a young, vaccine-eligible-age sample of Australian females. Sex Health. 2020;17:510–516. doi: 10.1071/SH20033 [DOI] [PubMed] [Google Scholar]
- 33.Kahn JA, Widdice LE, Ding L, et al. Substantial decline in vaccine-type human papillomavirus (HPV) among vaccinated young women during the first 8 years after HPV vaccine introduction in a community. Clin Infect Dis 2016;63:1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health. 2018;108: 112–119. doi: 10.2105/AJPH.2017.304119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukac CD, Donken R, Otterstatter M, et al. Impacts of human papillomavirus immunization programs on rates of anogenital warts in British Columbia, Canada, 2000 to 2017. Sex Transm Dis 2020;47:691–697. doi: 10.1097/OLQ.0000000000001235 [DOI] [PubMed] [Google Scholar]
- 36.Tyros G, Mastraftsi S, Gregoriou S, et al. Incidence of anogenital warts: epidemiological risk factors and real-life impact of human papillomavirus vaccination. Int J STD AIDS 2021;32:4–13. doi: 10.1177/0956462420958577 [DOI] [PubMed] [Google Scholar]
- 37.Gargano JW, Park IU, Griffin MR, et al. ; HPV-IMPACT Working Group. Trends in high-grade cervical lesions and cervical cancer screening in 5 states, 2008–2015. Clin Infect Dis 2019;68:1282–1291. doi: 10.1093/cid/ciy707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClung NM, Gargano JW, Park IU, et al. ; HPV-IMPACT Working Group. Estimated number of cases of high-grade cervical lesions diagnosed among women — United States, 2008 and 2016. MMWR Morb Mortal Wkly Rep 2019;68:337–343. doi: 10.15585/mmwr.mm6815a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverberg MJ, Leyden WA, Lam JO, et al. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: a population-based case-control study. Lancet Child Adolesc Health. 2018;2:707–714. doi: 10.1016/S2352-4642(18)30220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leval A, Herweijer E, Ploner A, et al. Quadrivalent human papillomavirus vaccine effectiveness: a Swedish national cohort study. J Natl Cancer Inst 2013;105:469–74. doi: 10.1093/jnci/djt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majed L, Bresse X, El Mouaddin N, et al. Public health impact and cost-effectiveness of a nine-valent gender-neutral HPV vaccination program in France. Vaccine. 2021;39:438–446. doi: 10.1016/j.vaccine.2020.10.089 [DOI] [PubMed] [Google Scholar]
- 42.Kjaer SK, Dehlendorff C, Belmonte F, et al. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J Natl Cancer Inst 2021;113:1329–1335. doi: 10.1093/jnci/djab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338 [DOI] [PubMed] [Google Scholar]
- 44.Tabrizi SN, Brotherton JM, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 2014;14:958–66. doi: 10.1016/S1473-3099(14)70841-2 [DOI] [PubMed] [Google Scholar]
- 45.Baussano I, Sayinzoga F, Tshomo U, et al. Impact of human papillomavirus vaccination, Rwanda and Bhutan. Emerg Infect Dis 2021;27:1–9. doi: 10.3201/eid2701.191364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration — United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:591–593. doi: 10.15585/mmwr.mm6919e2 [DOI] [PubMed] [Google Scholar]
- 47.Patel Murthy B, Zell E, Kirtland K, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations — 10 U.S. jurisdictions, March–September 2020. MMWR Morb Mortal Wkly Rep 2021;70:840–845. doi: 10.15585/mmwr.mm7023a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Reduce infections of HPV types prevented by the vaccine in young adults — IID-07. Accessed at https://health.gov/healthypeople/objectives-and-data/browse-objectives/infectious-disease/reduce-infections-hpv-types-prevented-vaccine-young-adults-iid-07 on 15 July 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


