Abstract
Platelet-rich plasma (PRP) shows promise as a regenerative modality for mild-to-moderate erectile dysfunction (ED). However, its efficacy in treating severe ED remains unknown. Blood samples from 8-week-old male rats were used to prepare PRP through a two-step centrifugation procedure, followed by chitosan activation and freeze thaw cycle. A hyperhomocysteinemia (HHcy)-related ED model was established using a methionine-enriched diet, and an apomorphine (APO) test was conducted during the 4th week. APO-negative rats were divided into two groups and were injected with PRP or saline every 2 weeks. Erectile function and histological analyses of the corpus cavernosum were performed during the 16th week. The results revealed that erectile function was significantly impaired in rats with HHcy-related ED compared to that in age-matched rats but was improved by repeated PRP injections. Immunofluorescence staining revealed a reduction in reactive oxygen species and additional benefits on the recovery of structures within the corpus cavernosum in rats that received PRP treatment compared to those in the saline-injected control group. Therefore, PRP could enhance functional and structural recovery in a severe HHcy-related ED model. A notable strength of the present study lies in the use of a repeated intracavernous injection method, mirroring protocols used in human studies, which offers more reliable results for translating the findings to humans.
Keywords: erectile dysfunction, growth factor, hyperhomocysteinemia, platelet-rich plasma
INTRODUCTION
Erectile dysfunction (ED) is characterized by the inability to achieve and sustain sufficient rigidity for satisfactory sexual intercourse. Conditions that cause endothelial dysfunction (EDys), such as aging, diabetes, hypertension, and cardiovascular disease, are closely associated with ED. Epidemiological data have indicated that the risk of ED is sharply increased in individuals with these comorbidities,1,2 severely decreasing quality of life. Notably, hyperhomocysteinemia (HHcy), which is characterized by an increase in total plasma homocysteine (Hcy), has emerged as a novel independent risk factor for ED.3 Patients with HHcy exhibit three times higher odds of developing ED than control subjects.4
Plasma Hcy originates from methionine (Met), and HHcy contributes to EDys in vitro and in vivo. The mechanisms involve a decrease in the bioavailability of nitric oxide,5,6 an increase in oxidative stress,7,8 endoplasmic reticulum stress, and the unfolded protein response;9,10,11,12 exploring novel methods to address the underlying pathology of ED is essential.
Platelet-rich plasma (PRP) is obtained through the centrifugation and separation of whole blood and contains abundant and diverse signaling proteins (including growth factors and chemokines), adhesion proteins, and proteases that play key roles in tissue regeneration processes, including angiogenesis, cellular growth, and the immune response.13,14 However, PRP treatment in andrology is still in its infancy, with only a limited number of preclinical studies and clinical trials have suggested that its regenerative properties may benefit ED.15 While various animal models have been developed,16,17,18,19 no authenticated studies have reported the efficacy of PRP treatment in reversing HHcy-related ED.
Common ED conditions, such as advanced age, diabetes, and hyperlipidemia, may not necessarily cause ED. We noted that previous related studies did not perform an apomorphine (APO) screen.17,18,19 The rationale for APO exclusion was founded on established evidence, indicating that only rats that tested APO-negative exhibited severely impaired erectile function, whereas those that tested APO-positive exhibited mild-to-moderate ED.20 Although PRP shows promise as a regenerative agent for mild-to-moderate ED, its efficacy in treating APO-negative rats remains unknown. For the purpose of this study, only APO-negative rats were used for further experiments.
Regarding the PRP injection protocol, high-quality randomized controlled trials (RCTs) used two sessions of PRP injections separated by 1 month in patients with ED, and postinjection follow-up lasted 6 months.21,22 However, published studies have independently assessed short-term (2 weeks) or medium-term (4 weeks) in vivo experiments involving a single intracavernous injection of PRP. Several studies reported a more positive outcome with better regeneration potential than that in the control group after a single administration of PRP.23,24 It remained unclear whether repeated PRP injections yielded favorable outcomes in improving erectile function in a severe ED model. Therefore, the objective of this study was to assess the long-term effects (12 weeks) of repeated PRP injections on an HHcy rat model with severely impaired erectile function.
MATERIALS AND METHODS
All animal experiments were approved by the Institutional Animal Care and Use Committee of Fujian Medical University (Quanzhou, China; Approval No. 2023-FYFE-591).
Preparation of PRP
Twelve 8-week-old male Sprague-Dawley rats were sacrificed, and the blood was obtained for two-step preparation of PRP. In brief, the blood was transferred to tubes containing the anticoagulant citrate dextrose solution A and centrifuged at 500g for 30 min (5425R, Eppendorf SE, Hamburg, Germany). After the sublayer was discarded, the sample was subjected to a second centrifugation at 1500g for 15 min, after which the top layer was collected. The platelet concentration was adjusted to 1.5 × 106 platelets per μl in the supernatant. Subsequently, 175 mg ml−1 chitosan was used to activate the PRP solution, which was then stored at −20°C until further use. According to a previous study, the combination of a two-step centrifugation process and freeze-thawing yields the highest quantities of growth factors.25
Determination of growth factor concentrations
The levels of growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and insulin-like growth factor 1 (IGF-1), were analyzed in the PRP samples. Enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Xitang Biotechnology Co., Ltd., Shanghai, China) was used for this purpose. The concentrations of growth factors were determined by referencing a standard curve.
Animals and experimental design
After PRP was harvested, 42 rats were randomly divided into three groups (Figure 1). Six rats were fed standard rodent chow and served as the age-matched control (Con group). The remaining 36 rats were fed with the diet enriched in Met (4%) for 4 weeks, which was previously shown to induce impaired penile hemodynamics and erectile function.26,27
Figure 1.

Flowchart showing the experimental schedule. SD: Sprague–Dawley; Met: methionine; PRP: platelet-rich plasma; ICI: intracavernosal injection.
After the HHcy rat model was established, APO testing was performed to confirm the presence of severe ED. In brief, APO was injected into the loose skin of the cervical vertebra subcutaneously (80 mg kg−1; Sigma-Aldrich, St. Louis, MO, USA) after a 10-min habituation period. The erectile response was observed for 30 min.20
A total of twelve APO-negative rats were acquired and anesthetized by an intraperitoneal sodium pentobarbital injection (40 mg kg−1). The rats were placed in a supine position, and the penile base was temporarily ligated. Following blunt dissection and a 1 mm incision at the prepuce, a 25-G syringe was used to administer 200 μl of saline (Met + saline group) or PRP (Met + PRP group) into the corpus cavernosum (CC). The needle was kept in place for 5 min to facilitate substance diffusion. PRP injections were administered every 2 weeks for 12 consecutive weeks, for a total of 6 injections.
Erectile function measurement
Erectile function was evaluated during the 16th week through electrical stimulation of the cavernous nerve using a bipolar electrode. The stimulation parameters were set at 2.5 V and 5.0 V, with a frequency of 15 Hz and a pulse width of 1.2 ms for a duration of 60 s. Intracavernosal pressure (ICP) and real-time arterial pressure were recorded using a pressure transducer (AD Instruments Powerlab/4SP, Bella Vista, Australia).
Tissue preparation
After the in vivo experiments were concluded, the rats were euthanized with an overdose of sodium pentobarbital by intraperitoneal injection (200 mg kg−1). The CC was extracted, and the central region was fixed in 4% paraformaldehyde overnight and embedded for subsequent histological examination. The remaining CC samples were frozen in liquid nitrogen and stored at −80°C for further molecular analyses.
Measurement of plasma Hcy levels
Plasma Hcy levels were measured using a Hitachi Model 7600 Series Automatic Analyzer and an enzymatic cycling assay (Hitachi Corporation, Tokyo, Japan).
Determination of oxidative stress
Frozen tissues were sliced into 8 μm-thick sections and fixed to glass slides. Reactive oxygen species (ROS) levels in the CC were determined by staining with 1 mmol l−1 dihydroethidium (Beyotime Biotechnology, Wuhan, China) for 30 min. A BX51 microscope (Olympus Corporation, Tokyo, Japan) was used for image acquisition, while image analysis was performed using Image-Pro Plus 6.0 software (Media Cybernetics Inc., Bethesda, MD, USA).
Malondialdehyde (MDA; Beyotime Biotechnology) and superoxide dismutase (SOD; Beyotime Biotechnology) levels in the CC were measured according to the manufacturer’s protocols.
Histological assessment
For immunofluorescence analysis, 5 μm-thick slices of the CC were blocked and then incubated with primary antibodies against endothelial cell antigen (RECA-1, 1:200; Bio-Rad Laboratories, Hercules, CA, USA) and von Willebrand factor (vWF; 1:400; Affinity Bioscience, Wuhan, China) to label endothelial cells. Additionally, α-smooth muscle actin (α-SMA; 1:200; Thermo Fisher Scientific, Waltham, MA, USA) and desmin (1:200; Abcam, Cambridge, MA, USA) were used to label smooth muscle cells, followed by detection with fluorescein isothiocyanate-conjugated secondary antibodies.
Quantitative analysis of the smooth muscle-to-collagen ratio was performed using Masson’s trichrome staining.
Western blot analyses
Protein extraction and quantification were followed by electrophoretic separation of the protein samples using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were subsequently transferred to polyvinylidene fluoride membranes and blocked in Tris-buffered saline Tween 20 (TBST) containing 5% bovine serum albumin. The membranes were then incubated with primary antibodies against endothelial nitric oxide synthase (eNOS; 1:100; Cell Signaling Technology, Framingham, MA, USA) and β-actin (1:500; Boster, Wuhan, China) at 4°C overnight. Then, the membranes were incubated with secondary antibodies (1:5000; Proteintech, Wuhan, China), after which the protein bands were visualized and analyzed using an enhanced chemiluminescence detection system (Pierce; Thermo Fisher Scientific).
Statistical analysis
The data were presented as the mean ± standard deviation (s.d.) and were analyzed using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by the Tukey–Kramer test was performed for post hoc comparisons between groups. P < 0.05 indicated statistical significance.
RESULTS
High levels of growth factors were present in PRP
The concentrations of key platelet growth factors (PDGF, VEGF, bFGF, and IGF-1) within PRP were assessed before injection (Table 1). These representative growth factors were shown to have the greatest capacity to enhance tissue regeneration.28
Table 1.
Concentration of growth factors in platelet-rich plasma
| Growth factor | Concentration (ng ml−1), mean±s.d. |
|---|---|
| PDGF | 29.4±1.6 |
| VEGF | 2.0±0.7 |
| bFGF | 12.6±0.8 |
| IGF-1 | 40.7±3.5 |
PDGF: platelet-derived growth factor; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; IGF-1: insulin-like growth factor 1; s.d.: standard deviation
Metabolic parameters
Plasma Hcy levels (mean ± s.d.) in rats fed the Met-enriched diet were significantly higher than those in the age-matched controls during the 16th week (10.0 ± 1.7 μmol l−1 vs 4.1 ± 1.4 μmol l−1, P < 0.01), confirming the successful establishment of the HHcy rat model. Additionally, local PRP injections did not affect Hcy levels (data not shown).
PRP protects against HHcy-related ED
The mean arterial pressure (MAP) was calculated, and the maximum ICP/MAP (maxICP/MAP) ratio was used to assess erectile function. The maxICP/MAP ratio was significantly lower in rats fed the Met-enriched diet than that in the age-matched control group (P < 0.01). PRP treatment substantially improved the maxICP/MAP ratio compared to that in the saline-injected control group (P < 0.05), indicating that PRP injection partially restored erectile function in rats with HHcy-related ED (Figure 2).
Figure 2.

Platelet-rich plasma protects against hyperhomocysteinemia-related ED. (a) Representative ICP and real-time arterial pressure tracing showing neurogenic-mediated erectile responses at 2.5 V and 5.0 V for 1 min in age-matched controls and in rats fed a 4% Met-enriched diet and treated with saline or PRP. (b) Bar graph showing the ratios of the voltage-dependent maxICP/MAP. The data were expressed as the mean ± standard deviation (n = 6 rats per group). *P < 0.05, the value of the indicated group compared with that of the control group. #P < 0.05, the value of the indicated group compared with that of the Met + saline group. ICP: intracavernosal pressure; Met: methionine; PRP: platelet-rich plasma; ES: electrical stimulation; maxICP/MAP: maximal ICP to the mean arterial pressure; ED: erectile dysfunction; con: control.
PRP inhibits HHcy-induced oxidative stress in the penis
The ROS levels in rats fed the Met-enriched diet increased significantly, and these levels were subsequently reduced by PRP administration (P < 0.05). MDA levels were higher in the saline-injected control group than those in the age-matched control and PRP treatment groups (P < 0.05). SOD activity in rats fed a Met-enriched diet was lower than that in the age-matched control group; however, PRP treatment slightly increased SOD activity compared to that in the saline-injected control group (P < 0.05; Figure 3).
Figure 3.

Platelet-rich plasma inhibits hyperhomocysteinemia-induced oxidative stress in the penis. (a) Representative images of ROS production in the corpus cavernosum. Scale bars = 50 µm. (b) Semiquantitative analysis of the ROS-positive area in the corpus cavernosum was performed using Image-Pro Plus software. (c) MDA levels in the corpus cavernous was determined by ELISA. (d) SOD activity in the corpus cavernous was determined by ELISA. The data were expressed as the mean ± standard deviation (n = 6 rats per group). *P < 0.05, the value of the indicated group compared with that of the control group. #P < 0.05, the value of the indicated group compared with that of the Met + saline group. ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase; ELISA: enzyme-linked immunosorbent assay; Met: methionine; PRP: platelet-rich plasma; Con: control.
PRP attenuates HHcy-related histological changes in the penis
Immunofluorescence analysis was used to evaluate the expression of vWF and RECA-1 to determine whether the restoration of erectile function was associated with an increase in endothelial cell numbers. The results indicated a significant reduction in the cavernous endothelial area in rats fed a Met-enriched diet compared to that in age-matched controls. This decrease was partially mitigated by PRP treatment (P < 0.05; Figure 4). These findings were further confirmed by western blot analysis.
Figure 4.
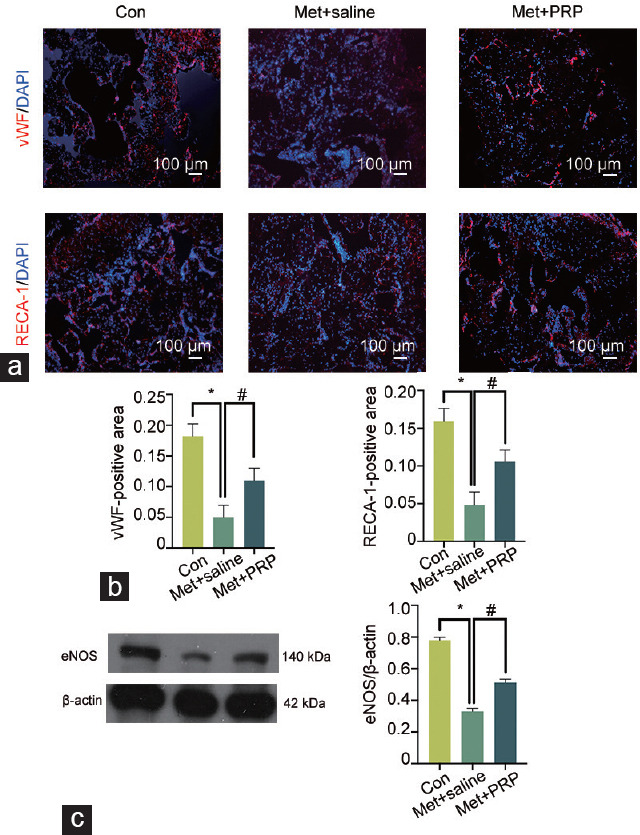
Platelet-rich plasma accelerates cavernosal endothelial cell repair in rats with hyperhomocysteinemia. (a) Representative images of the endothelium in the corpus cavernosum, as examined by immunofluorescence staining with antibodies against vWF and RECA-1 (red). Nuclei were stained with DAPI (blue). Scale bars = 100 μm. (b) Semiquantitative analysis of endothelial cells in the corpus cavernosum was performed. (c) Representative western blot results showing eNOS expression in the corpus cavernous. The data were expressed as the mean ± standard deviation (n = 6 rats per group). *P < 0.05, the value of the indicated group compared with that of the control group. #P < 0.05, the value of the indicated group compared with that of the Met + saline group. vWF: von-Willebrand factor; RECA-1: endothelial cell antigen; DAPI: 4’,6-diamidino-2-phenylindole; eNOS: endothelial nitric oxide synthase; Met: methionine; PRP: platelet-rich plasma; Con: control.
The expression of smooth muscle markers was significantly lower in rats fed a Met-enriched diet than that in the age-matched control group. PRP treatment partially restored the HHcy-related histological changes after 12 weeks of treatment (P < 0.05; Figure 5).
Figure 5.

Platelet-rich plasma improves cavernosal smooth cell levels in rats with hyperhomocysteinemia. (a) Representative images showing smooth muscle cells in the corpus cavernosum, as detected by immunofluorescence staining with antibodies against α-SMA and Desmin (red). Nuclei were stained with DAPI (blue). Smooth muscle and connective tissue were detected with Masson’s trichrome method. Scale bars = 100 μm. (b) Semiquantitative analysis of smooth muscle cells in the corpus cavernosum was performed. The data were expressed as the mean ± standard deviation (n = 6 rats per group). *P < 0.05, the value of the indicated group compared with that of the control group. #P < 0.05, the value of the indicated group compared with that of the Met + saline group. α-SMA: α-smooth muscle actin; DAPI: 4’,6-diamidino-2-phenylindole; Met: methionine; PRP: platelet-rich plasma; Con: control.
DISCUSSION
The current study demonstrated the restorative effects of repeated PRP injections on erectile function in a severe HHcy-related ED rat model. Our results suggested that HHcy-related ED was characterized by increased oxidative stress, impaired endothelial function, and decreased smooth muscle levels in erectile tissue. However, PRP treatment could partially reverse these changes.
HHcy has been reported to strongly correlate with the occurrence of ED and may be linked to nonresponsiveness to phosphodiesterase type 5 inhibitors.29 Although animal models for aging-induced, diabetes-induced, and bilateral cavernous nerve injury (BCNI)-induced ED have been developed,16,17,18,19 few authenticated studies have reported the efficacy of PRP treatment for reversing a severe HHcy-related ED rat model.
PRP contains high concentrations of signaling proteins, adhesion proteins, and proteases that may enhance processes involved in tissue repair.13,14 Notably, the autologous nature of PRP reduces inflammatory reactions and bypasses ethical issues.30 Following the adaptation of low-intensity shockwave therapy for ED management according to the European Association of Urology (EAU) and the American Urological Association (AUA) guidelines,31,32 intracavernous injection of PRP has emerged as a novel option for ED treatment. According to our results, rats fed 4% Met chow exhibited numerous abnormalities in oxidative stress, EDys, and cavernosal fibrosis, which affected erectile function, as previously reported.26 Therefore, this versatile restorative strategy may have beneficial effects on HHcy-induced CC impairment.
PRP relies on high concentrations of platelets to promote tissue regeneration. Earlier studies demonstrated that a two-step centrifugation procedure followed by chitosan activation and freeze-thaw cycle was an optimized PRP preparation protocol.25 According to this protocol, we quantified the concentrations of representative growth factors, and the results were consistent with those of a previous study.18
In related preclinical studies, there were two major limitations: (1) the APO test was not performed before treatment and (2) only single-dose intracavernous PRP injections were assessed with short-term follow-up. The long-term restorative effects of PRP on erectile function were not examined. Our data demonstrated the long-term effect of PRP on the restoration of impaired histologic structures and erectile function in an HHcy rat model and indicated that PRP could reverse severe ED. Importantly, a strength of the present study lies in the use of a repeated intracavernous injection protocol, which mimics protocols used in published RCTs and offers more reliable results for translating the findings to humans.
A major limitation of this study is the absence of a treatment group involving rats fed a Met-enriched diet that received a single injection of PRP. The effects of single versus repeat injection and systemic versus local administration of PRP need to be explored. Additionally, the mechanism of the therapeutic effect has not been adequately analyzed, although there are indications that PRP augments growth factor resources. Future studies need to address these limitations through further validation.
CONCLUSIONS
This study reported the use of repeated intracavernous PRP injections to restore erectile function in a rat model of severe HHcy-related ED. These findings showed a reduction in oxidative stress and the regeneration of endothelial and smooth muscle cells in vivo after PRP treatment. Given the limitations of the study, additional investigations are needed to determine the precise mechanisms underlying these therapeutic effects. These studies should also focus on treatment protocols to optimize the therapeutic potential of these factors for severe ED.
AUTHOR CONTRIBUTIONS
ZY and YZX participated in the design of the trial, conducted the data acquisition, interpreted and analyzed the data, and drafted and revised the manuscript. XLH designed the study and contributed to the study materials. SZH and XML pointed out deficiencies and ameliorated the manuscript. JXW guided the experiment directions and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENT
This work was supported by grants from the Quanzhou Science and Technology Project Funding (No. 2021N017S) and the Doctoral Nursery Fund of the Second Affiliated Hospital of Fujian Medical University (BS202102).
REFERENCES
- 1.Pellegrino F, Sjoberg DD, Tin AL, Benfante NE, Briganti A, et al. Relationship between age, comorbidity, and the prevalence of erectile dysfunction. Eur Urol Focus. 2023;9:162–7. doi: 10.1016/j.euf.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demir T, Comlekçi A, Demir O, Gülcü A, Calýpkan S, et al. Hyperhomocysteinemia:a novel risk factor for erectile dysfunction. Metabolism. 2006;55:1564–8. doi: 10.1016/j.metabol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hunayan A, Thalib L, Kehinde EO, Asfar S. Hyperhomocysteinemia is a risk factor for erectile dysfunction in men with adult-onset diabetes mellitus. Urology. 2008;71:897–900. doi: 10.1016/j.urology.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Xie X, Zhang Z, Wang X, Luo Z, Lai B, et al. Stachydrine protects eNOS uncoupling and ameliorates endothelial dysfunction induced by homocysteine. Mol Med. 2018;24:10. doi: 10.1186/s10020-018-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Z, Jiang X, Pansuria M, Fang P, Mai J, et al. Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via µ-calpain activation. Diabetes. 2015;64:947–59. doi: 10.2337/db14-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borkowska A, Ziolkowski W, Kaczor K, Herman-Antosiewicz A, Knap N, et al. Homocysteine-induced decrease in HUVEC cells'resistance to oxidative stress is mediated by Akt-dependent changes in iron metabolism. Eur J Nutr. 2021;60:1619–31. doi: 10.1007/s00394-020-02360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Zhang L, Miao Y, Yang J, Wang X, et al. Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol. 2019;20:46–59. doi: 10.1016/j.redox.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, et al. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J Biol Chem. 2001;276:35867–74. doi: 10.1074/jbc.M100747200. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VS, Trinath J, Reddy GB. Implication of homocysteine in protein quality control processes. Biochimie. 2019;165:19–31. doi: 10.1016/j.biochi.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CK, Luo JY, Lau CW, Cho WC, Ng CF, et al. A GLP-1 analog lowers ER stress and enhances protein folding to ameliorate homocysteine-induced endothelial dysfunction. Acta Pharmacol Sin. 2021;42:1598–609. doi: 10.1038/s41401-020-00589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S, Xu W, Chen Z, Cui C, Fan X, et al. Hydrogen sulphide reduces hyperhomocysteinaemia-induced endothelial ER stress by sulfhydrating protein disulphide isomerase to attenuate atherosclerosis. J Cell Mol Med. 2021;25:3437–48. doi: 10.1111/jcmm.16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins T, Alexander D, Barkatali B. Platelet-rich plasma:a narrative review. EFORT Open Rev. 2021;6:225–35. doi: 10.1302/2058-5241.6.200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9:721–30. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 15.Poulios E, Mykoniatis I, Pyrgidis N, Kalyvianakis D, Hatzichristou D. Platelet-rich plasma for the treatment of erectile dysfunction:a systematic review of preclinical and clinical studies. Sex Med Rev. 2023;11:359–68. doi: 10.1093/sxmrev/qead027. [DOI] [PubMed] [Google Scholar]
- 16.Tai HC, Tsai WK, Chang ML, Praveen Rajneesh C, Tseng XW, et al. Intracavernous injection of platelet-rich plasma reverses erectile dysfunction of chronic cavernous nerve degeneration through reduction of prostate hyperplasia evidence from an aging-induced erectile dysfunction rat model. FASEB J. 2023;37:e22826. doi: 10.1096/fj.202201443R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao CH, Lee KH, Chung SD, Chen KC, Praveen Rajneesh C, et al. Intracavernous injection of platelet-rich plasma therapy enhances erectile function and decreases the mortality rate in streptozotocin-induced diabetic rats. Int J Mol Sci. 2022;23:3017. doi: 10.3390/ijms23063017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YC, Wu CT, Chen MF, Kuo YH, Li JM. Intracavernous injection of autologous platelet-rich plasma ameliorates hyperlipidemia-associated erectile dysfunction in a rat model. Sex Med. 2021;9:100317. doi: 10.1016/j.esxm.2020.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu YN, Liao CH, Chen KC, Chiang HS. CXCL5 cytokine is a major factor in platelet-rich plasma's preservation of erectile function in rats after bilateral cavernous nerve injury. J Sex Med. 2021;18:698–710. doi: 10.1016/j.jsxm.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, Zhang Y, Tang Z, Song J, Gao X, et al. Intracavernosal adeno-associated virus-mediated S100A1 gene transfer enhances erectile function in diabetic rats by promoting cavernous angiogenesis via VEGF-A/VEGFR2 signaling. J Sex Med. 2019;16:1344–54. doi: 10.1016/j.jsxm.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Masterson TA, Molina M, Ledesma B, Zucker I, Saltzman R, et al. Platelet-rich plasma for the treatment of erectile dysfunction:a prospective, randomized, double-blind, placebo-controlled clinical trial. J Urol. 2023;210:154–61. doi: 10.1097/JU.0000000000003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulios E, Mykoniatis I, Pyrgidis N, Zilotis F, Kapoteli P, et al. Platelet-rich plasma (PRP) improves erectile function:a double-blind, randomized, placebo-controlled clinical trial. J Sex Med. 2021;18:926–35. doi: 10.1016/j.jsxm.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Ince B, Yildirim ME, Kilinc I, Oltulu P, Dadaci M. Investigation of the development of hypersensitivity and hyperalgesia after repeated application of platelet-rich plasma in rats:an experimental study. Aesthet Surg J. 2019;39:1139–45. doi: 10.1093/asj/sjz113. [DOI] [PubMed] [Google Scholar]
- 24.Glanzmann MC, Audigé L. Platelet-rich plasma for chronic lateral epicondylitis:is one injection sufficient? Arch Orthop Trauma Surg. 2015;135:1637–45. doi: 10.1007/s00402-015-2322-7. [DOI] [PubMed] [Google Scholar]
- 25.Wu YN, Wu CC, Sheu MT, Chen KC, Ho HO, et al. Optimization of platelet-rich plasma and its effects on the recovery of erectile function after bilateral cavernous nerve injury in a rat model. J Tissue Eng Regen Med. 2016;10:E294–304. doi: 10.1002/term.1806. [DOI] [PubMed] [Google Scholar]
- 26.Cui K, Luan Y, Tang Z, Li CC, Wang T, et al. Human tissue kallikrein-1 protects against the development of erectile dysfunction in a rat model of hyperhomocysteinemia. Asian J Androl. 2019;21:508–15. doi: 10.4103/aja.aja_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W, Xiong L, Yang B, Li W, Zhang J, et al. Hyperhomocysteinaemia in rats is associated with erectile dysfunction by impairing endothelial nitric oxide synthase activity. Sci Rep. 2016;6:26647. doi: 10.1038/srep26647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui M, Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater. 2012;8:1792–801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Lombardo F, Tsamatropoulos P, Piroli E, Culasso F, Jannini EA, et al. Treatment of erectile dysfunction due to C677T mutation of the MTHFR gene with vitamin B6 and folic acid in patients non responders to PDE5i. J Sex Med. 2010;7:216–23. doi: 10.1111/j.1743-6109.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- 30.Liao CH, Chang CJ, Chen KC, Rajneesh CP, Tseng XW, et al. Effects of platelet-rich plasma glue placement at the prostatectomy site on erectile function restoration and cavernous nerve preservation in a nerve-sparing prostatectomy rat model. Biomed Pharmacother. 2023;161:114499. doi: 10.1016/j.biopha.2023.114499. [DOI] [PubMed] [Google Scholar]
- 31.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, et al. European Association of Urology guidelines on sexual and reproductive health-2021 update:male sexual dysfunction. Eur Urol. 2021;80:333–57. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–41. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]


