Abstract
The study aimed to test if Briganti’s 2012 nomogram could be associated with the risk of prostate cancer (PCa) progression in European Association of Urology (EAU) intermediate-risk patients treated with robotic surgery. From January 2013 to December 2021, 527 consecutive patients belonging to the EAU intermediate-risk class were selected. Briganti’s 2012 nomogram, which predicts the risk of pelvic lymph node invasion (PLNI), was assessed as a continuous and dichotomous variable that categorized up to the median of 3.0%. Disease progression defined as biochemical recurrence and/or metastatic progression was evaluated by Cox proportional hazards (univariate and multivariate analysis). After a median follow-up of 95.0 months (95% confidence interval [CI]: 78.5–111.4), PCa progression occurred in 108 (20.5%) patients who were more likely to present with an unfavorable nomogram risk score, independently by the occurrence of unfavorable pathology including tumor upgrading and upstaging as well as PLNI. Accordingly, as Briganti’s 2012 risk score increased, patients were more likely to experience disease progression (hazard ratio [HR] = 1.060; 95% CI: 1.021–1.100; P = 0.002); moreover, it also remained significant when dichotomized above a risk score of 3.0% (HR = 2.052; 95% CI: 1.298–3.243; P < 0.0001) after adjustment for clinical factors. In the studied risk population, PCa progression was independently predicted by Briganti’s 2012 nomogram. Specifically, we found that patients were more likely to experience disease progression as their risk score increased. Because of the significant association between risk score and tumor behavior, the nomogram can further stratify intermediate-risk PCa patients, who represent a heterogeneous risk category for which different treatment paradigms exist.
Keywords: biochemical recurrence, Briganti’s 2012 nomogram, intermediate-risk prostate cancer, pelvic lymph node invasion, prostate cancer progression, robot-assisted radical prostatectomy
INTRODUCTION
Clinical prostate cancer (PCa) is a widespread problem of such magnitude to force both the European Association of Urology (EAU) and the National Comprehensive Cancer Network (NCCN) to continuously update their guidelines to give the urology community the best recommendations to treat the disease and avoid overtreatments, which has an important impact on patient’s quality of life.1,2,3,4 Clinical class risks are calculated using parameters including prostate-specific antigen (PSA), tumor stage, and grade according to the International Society of Urologic Pathology (ISUP).1,2 In both systems, the intermediate-risk class is the most heterogeneous and controversial one because it includes the largest group of patients that might present with PSA levels between 10 ng ml−1 and 20 ng ml−1 and/or ISUP tumor grade group 2–3 and/or clinical tumor stage cT2, which is up to cT-2b or cT-2c according to EAU and NCCN, respectively. Furthermore, this group is subdivided into favorable and unfavorable categories, the latter requiring active treatment if patients’ life expectancy exceeds 10 years. Active treatment includes surgery, which is more commonly performed by robot-assisted radical prostatectomy (RARP) eventually associated with extended pelvic lymph node dissection (ePLND), and radiation therapy (RT).1,2 However, more clinical factors are required to further stratify this large heterogeneous risk group. Although multiparametric magnetic resonance imaging (mpMRI) and molecular biology seem promising, the former is not reproducible in multicenter studies, while the latter is far from being used in routine practice.1,2,5 When surgery is the chosen option, there is a risk of pelvic lymph node invasion (PLNI), and the choice of performing a lymph node dissection might take into account validated nomograms such as Briganti’s 2012, which is one of the most used as it is simple to apply in clinical routine. Nevertheless, it has not yet been tested as a prognostic factor for this clinical risk category;1,2,5,6 therefore, we wanted to evaluate Briganti’s 2012 nomogram as a prognostic factor of PCa progression after robotic surgery in the EAU intermediate-risk category.
PATIENTS AND METHODS
EAU intermediate-risk cohort treated with robotic surgery
From January 2013 to December 2021, 527 patients meeting the EAU criteria for intermediate-risk PCa with available follow-up were selected at our institution (Integrated University Hospital, Verona, Italy). We previously excluded patients on androgen deprivation and/or with prior active treatments. RARP was performed by 5 experienced surgeons, eventually associated with ePLND including external iliac, obturator, Cloquet’s, and Marcille’s regions.7 All patients signed informed consent for the study, which was approved by the internal Institutional Review Board (Ethical Committee of Verona) in accordance with the 1964 Helsinki Declaration and its later amendments. Data were analyzed retrospectively. Patients were assessed for age (year), body mass index (BMI; kg m−2), prostate-specific antigen (PSA; ng ml−1), prostate volume (PV; ml), and biopsy-positive cores (BPC; %). Physical status was evaluated through the American Association of Anesthesiologists (ASA) system.7 Assessment of surgical specimens was conducted according to guidelines.1,2 Patients were followed up according to guidelines in a multidisciplinary setting where further treatments were eventually addressed.1,2
Design and endpoint evaluation of the study in the investigated patient population
We wanted to test the hypothesis that Briganti’s 2012 nomogram could be associated with PCa progression in EAU intermediate-risk patients treated with robotic surgery. The nomograph was examined as a continuous and categorical variable that was dichotomized up to the median, which was 3.0%. PCa progression was defined as the occurrence of biochemical recurrence/persistence and/or local recurrence and/or distant metastases. Biochemical recurrence was defined as any increase in PSA from undetectable to detectable on blood tests.
Statistical methods
Continuous variables were assessed for median with interquartile range (IQR). Categorical factors were assessed for frequency (percentage). Associations of the nomogram with clinical and pathological factors were calculated using the binomial logistic regression model (univariate and multivariate analysis). The time from surgery to disease progression or the last follow-up was measured as time to event occurrence. The association with the risk of PCa progression was assessed by Cox proportional hazards (univariate and multivariate analysis). Unadjusted Kaplan–Meier estimator curves for PCa progression were eventually generated. The software used for the analyses was IBM-SPSS version 26 (IBM SPSS Statistics, Armonk, NY, USA). All tests were two-sided with P < 0.05 considered to indicate statistical significance.
RESULTS
PCa progression in the EAU intermediate-risk cohort treated with robotic surgery
Table 1 shows the demographics of the cohort including 527 patients who were stratified by Briganti’s 2012 nomogram. The distribution of the nomogram score is shown in Figure 1. Patients with a risk score >3.0% were clinically more likely to have higher percentages of BPC, palpable tumors (cT-2b), and unfavorable tumor grades (ISUP 3); they were also more likely to harbor unfavorable cancers in the surgical specimen (tumor upgrading and upstaging). The ASA system resulted in score 1 in 47 (8.9%), score 2 in 427 (81.0%), and score 3 in 53 (10.1%) patients. A total of 372 patients underwent ePLND and 24 (6.5%) patients were found to have PLNI. The median number (IQR) of counted lymph nodes was 25 (19–31).
Table 1.
Distribution of factors stratified by Briganti’s 2012 nomogram in European Association of Urology intermediate-risk prostate cancer patients treated with robotic surgery
| Variable | Population (n=527) | Briganti’s score ≤3.0%, n=274 (52.0%) | Briganti’s score >3.0%, n=253 (48.0%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Clinical factor | |||||||
| Age (year), median (IQR) | 65 (60–70) | 65 (59–69) | 65 (61–70) | 1.020 (0.995–1.046) | 0.121 | 1.023 (0.986–1.062) | 0.226 |
| BMI (kg m−2), median (IQR) | 25.6 (23.7–27.8) | 25.7 (23.9–27.7) | 25.4 (23.6–28.1) | 1.003 (0.952–1.058) | 0.905 | 0.994 (0.918–1.076) | 0.877 |
| PV (ml), median (IQR) | 38 (30–49) | 38 (30–50) | 37 (28–47) | 0.995 (0.985–1.005) | 0.299 | 0.999 (0.985–1.104) | 0.899 |
| PSA <10 ng ml−1, n (%) | 421 (79.9) | 221 (80.7) | 200 (79.1) | Reference | Reference | ||
| PSA 10–20 ng ml−1, n (%) | 106 (20.1) | 53 (19.3) | 53 (20.9) | 1.105 (0.722–1.692) | 0.646 | 1.770 (0.920–3.405) | 0.087 |
| BPC (%), median (IQR) | 28.5 (18.7–47.1) | 21.4 (14.2–31.2) | 43.7 (28.5–63.6) | 1.074 (1.060–1.089) | <0.0001 | 1.105 (1.084–1.127) | <0.0001 |
| cT1c, n (%) | 304 (57.7) | 189 (69.0) | 115 (45.5) | Reference | Reference | ||
| cT2b, n (%) | 223 (42.3) | 85 (31.0) | 138 (54.5) | 2.688 (1.869–3.809) | <0.0001 | 3.879 (2.261–0.657) | <0.0001 |
| ISUP <3, n (%) | 348 (66.0) | 238 (86.9) | 110 (43.5) | Reference | Reference | ||
| ISUP =3, n (%) | 179 (34.0) | 36 (13.1) | 143 (56.5) | 8.594 (5.592–13.208) | <0.0001 | 27.362 (14.103–53.086) | <0.0001 |
| Pathology factor | |||||||
| ISUP 1–3, n (%) | 447 (84.8) | 248 (90.5) | 199 (78.7) | Reference | Reference | ||
| ISUP 4–5, n (%) | 80 (15.2) | 26 (9.5) | 54 (21.3) | 2.588 (1.564–4.283) | <0.0001 | 2.117 (1.243–3.606) | 0.006 |
| pT2, n (%) | 433 (82.2) | 241 (88.0) | 192 (75.9) | Reference | Reference | ||
| pT3, n (%) | 94 (17.8) | 33 (12.0) | 61 (24.1) | 2.320 (1.459–3.690) | <0.0001 | 1.947 (1.188–3.191) | 0.008 |
| R0, n (%) | 401 (76.1) | 211 (77.0) | 190 (75.1) | Reference | Reference | ||
| R1, n (%) | 126 (23.9) | 63 (23.0) | 63 (24.9) | 1.111 (0.744–1.657) | 0.608 | 0.839 (0.546–1.290) | 0.424 |
| pNx-0, n (%) | 503 (95.4) | 268 (97.8) | 235 (92.9) | Reference | Reference | ||
| pN1, n (%) | 24 (4.6) | 6 (2.2) | 18 (7.1) | 3.421 (1.336–8.762) | 0.010 | 2.154 (0.805–5.768) | 0.127 |
IQR: interquartile range; OR: odds ratio; CI: confidence interval; EAU: European Association of Urology; ISUP: International Society of Urologic Pathology; R0: negative surgical margins; R1: positive surgical margins; BMI: body mass index; PV: prostate volume; PSA: prostate-specific antigen; BPC: biopsy-positive cores; PNx-0: regional lymph nodes cannot be assessed, or no regional lymph node metastasis is present at pathological examination; PN1: regional lymph node metastasis is present at pathological examination
Figure 1.
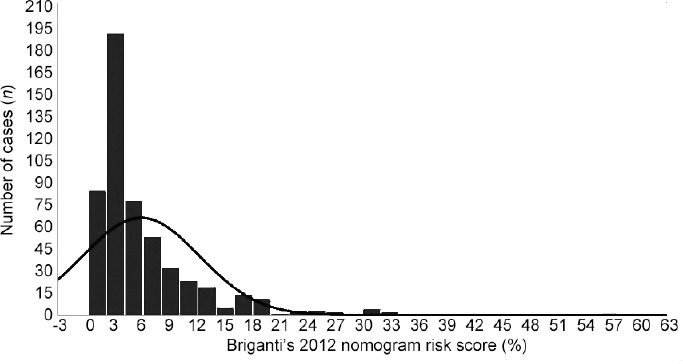
Distribution of Briganti’s 2012 nomogram predicting the risk of pelvic lymph node invasion. Data are from 527 EAU intermediate-risk patients treated with robot-assisted radical prostatectomy eventually associated with extended pelvic lymph node dissection. The median (interquartile range) and mean (standard deviation) were 3.0% (2.0%–7.0%) and 5.7% (6.4%), respectively. EAU: European Association of Urology; PCa: prostate cancer.
Prognostic impact of Briganti’s 2012 nomogram in the EAU intermediate-risk PCa population
After a median follow-up of 95.0 (95% confidence interval [CI]: 78.5–111.4) months, PCa progression occurred in 108 (20.5%) patients who were more likely to present with an unfavorable nomogram risk score, independently by the occurrence of unfavorable pathology including tumor upgrading and upstaging as well as PLNI, as shown in Table 2. Accordingly, as Briganti’s 2012 risk score increased, patients were more likely to experience disease progression (HR = 1.060; 95% CI: 1.021–1.100; P = 0.002). The independent prognostic impact of Briganti’s 2012 risk score on PCa progression after adjusting for clinical and pathological factors in EAU intermediate-risk patients treated with robotic surgery is shown in Table 3. Even after adjusting for clinical and pathological factors, Briganti’s 2012 risk score was effective as a continuous (HR = 1.052; 95% CI: 1.025–1.079; P < 0.0001) as well as a dichotomized variable (HR = 1.678; 95% CI: 1.506–2.675; P = 0.03). As the nomogram risk score increased, patients were more likely to experience disease progression, independently by the concomitance of other clinical and pathological adverse factors. Kaplan–Meier survival risk curves for PCa progression stratified by Briganti’s 2012 risk score are illustrated in Figure 2. Deaths occurred in 19 (3.6%) patients, of whom 2 (0.4%) were related to PCa. Radiation therapy was delivered in 87 (16.5%) patients, with salvage intent in 43 (8.2%) patients. Eighty-seven (16.5%) patients received androgen deprivation therapy.
Table 2.
Risk of prostate cancer progression after robotic surgery by clinical and pathological factors including Briganti’s 2012 nomogram in 527 European Association of Urology intermediate-risk prostate cancer patients
| Variable | No PCa progression, n=419 (79.5%) | PCa progression, n=108 (20.5%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age (year), median (IQR) | 65 (60–70) | 64 (60–68) | 0.993 (0.966–1.022) | 0.641 | 0.989 (0.960–1.020) | 0.498 |
| BMI (kg m−2), median (IQR) | 25.7 (23.8–27.8) | 25.1 (23.4–27.6) | 0.972 (0.912–1.037) | 0.391 | 0.958 (0.899–1.022) | 0.198 |
| PV (ml), median (IQR) | 38 (30–49) | 36 (28.2–47.7) | 1.001 (0.990–1.013) | 0.825 | 1.002 (0.990–1.015) | 0.711 |
| PSA 10–20 ng ml−1, n (%) | 76 (18.1) | 30 (27.8) | 1.348 (0.880–2.065) | 0.17 | 1.210 (0.782–1.873) | 0.392 |
| BPC (%), median (IQR) | 28.5 (18.1–44.4) | 39.2 (20–59.2) | 1.017 (1.010–1.024) | <0.0001 | 1.004 (0.993–1.015) | 0.484 |
| cT2b, n (%) | 183 (43.7) | 40 (37.0) | 1.430 (0.962–2.124) | 0.077 | 1.101 (0.723–1.676) | 0.655 |
| ISUP =3 (biopsy), n (%) | 136 (32.5) | 43 (39.8) | 1.590 (1.077–2.349) | 0.02 | 1.087 (0.677–1.746) | 0.731 |
| ISUP >3 (pathology), n (%) | 47 (11.2) | 33 (30.6) | 2.791 (1.843–4.227) | <0.0001 | 1.976 (1.234–3.163) | 0.005 |
| pT3, n (%) | 59 (14.1) | 35 (32.4) | 1.954 (1.298–2.940) | 0.001 | 1.082 (0.663–1.766) | 0.752 |
| R1, n (%) | 88 (21.0) | 38 (35.2) | 1.870 (1.251–2.794) | 0.002 | 1.491 (0.960–2.314) | 0.075 |
| pN1, n (%) | 5 (1.2) | 19 (17.6) | 4.832 (2.908–8.028) | <0.0001 | 3.217 (1.843–5.613) | <0.0001 |
| Briganti’s 2012 nomogram (%), median (IQR) | 3.0 (2.0–6.0) | 4.0 (2.0–10.7) | 1.070 (1.050–1.091) | <0.0001 | 1.060 (1.021–1.100) | 0.002 |
IQR: interquartile range; PCa: prostate cancer; BMI: body mass index; PV: prostate volume; PSA: prostate-specific antigen; BPC: biopsy-positive cores; R1: positive surgical margins; HR: hazard ratio; CI: confidence interval; ISUP: International Society of Urological Pathology; PN1: regional lymph node metastasis is present at pathological examination
Table 3.
Multivariate analysis of the prognostic impact of Briganti’s 2012 nomogram on prostate cancer progression in 527 European Association of Urology intermediate-risk prostate cancer patients treated with robotic surgery
| Briganti’s 2012 nomogram risk score | HR (95% CI) | P |
|---|---|---|
| After adjusting for clinical factors | ||
| Continuous variable | 1.071 (1.046–1.096) | <0.0001 |
| ≤3.0% | 1 | |
| >3.0% | 2.052 (1.298–3.243) | 0.002 |
| After adjusting for pathological factors | ||
| Continuous variable | 1.051 (1.027–1.074) | <0.0001 |
| ≤3.0% | 1 | |
| >3.0% | 1.791 (1.186–2.706) | 0.006 |
| After adjusting for all factors | ||
| Continuous variable | 1.052 (1.025–1.079) | <0.0001 |
| ≤3.0% | 1 | |
| >3.0% | 1.678 (1.506–2.675) | 0.03 |
HR: hazard ratio; CI: confidence interval
Figure 2.
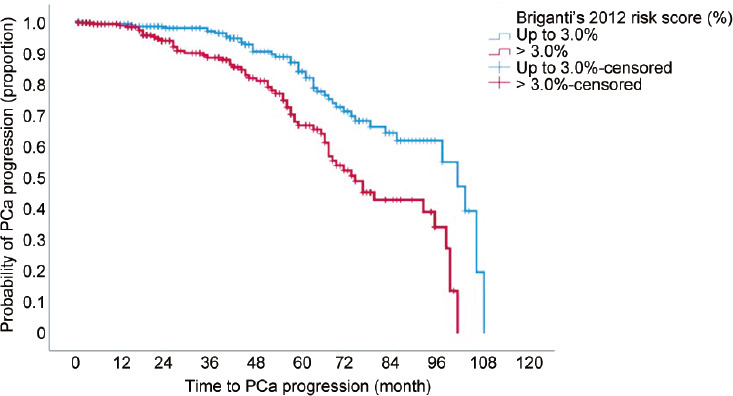
Kaplan–Meyer survival risk curves of prostate cancer (PCa) progression in 527 EAU intermediate-risk patients treated with robot-assisted radical prostatectomy eventually associated with extended pelvic lymph node dissection. The median survival time of PCa progression was longer for a risk score of up to 3.0% (87.5 months; 95% CI: 82.8–92.2 months) than that for a risk score >3.0% (72.6 months; 95% CI: 67.6–77.6 months) with significant difference (Mantel–Cox log-rank test: P < 0.0001). EAU: European Association of Urology; PCa: prostate cancer; PLNI: pelvic lymph node dissection; CI: confidence interval.
DISCUSSION
Clinical PCa has a variable natural history including recurrence rates of 30%–35% and cancer-specific deaths of 16.4% after primary active treatment; nevertheless, early biochemical recurrence and adverse pathology are unfavorable prognostic factors.1,2,8,9,10,11 In intermediate-risk PCa, it is important to stratify patients into favorable and unfavorable groups that will benefit from different treatment modalities.12 EAU guidelines recommend the use of nomograms to predict lymph node invasion and so to decide whether to perform ePLND in this class of patients.1 Many nomograms have been developed, based on systematic biopsies, such as the well-established Briganti’s 2012 nomogram.6 Remarkably, new nomograms have been recently developed following increasing usage of targeted biopsy of suspected lesions seen at mpMRI of the prostate. These new nomograms are intended to fit new clinical practices and to allow the prediction of node involvement in cases when a targeted biopsy is performed without concomitant systematic biopsies.13 Adverse pathology is still an issue in the subgroup presenting with favorable prognostic features, as shown by one study reporting a threefold rate of adverse pathology in favorable intermediate-risk patients compared to low-risk cases.14 Another study showed that nearly 25% of intermediate-risk patients presenting with favorable features harbored unfavorable pathology, indicating that clinical factors were not sufficient to identify a favorable subset even in this risk category.15 Favorable intermediate-risk patients treated with brachytherapy have low estimates of cancer-specific mortality during a follow-up of 10 years.16 While it is important to classify intermediate-risk patients into favorable and unfavorable risk categories, it is also critical to identify additional clinical factors that allow for further prognostic stratification. In our study, we showed that Briganti’s 2012 nomogram was able to predict PCa progression after robotic surgery, independently by the occurrence of unfavorable pathology. As the nomogram risk score increased, patients were more likely to progress; conversely, patients presenting with a risk score of up to 3.0% were less likely to experience disease progression. Moreover, patients having unfavorable pathology reports, but presenting with a favorable risk score, were also less likely to recur compared with those associating with a risk score >3.0%. To date, the EAU intermediate-risk PCa population can be classified into favorable and unfavorable risk groups, which can be further stratified into prognostic risk groups according to a favorable or unfavorable nomogram risk score at clinical presentation.
The ISUP system is closely associated with the natural history of PCa as it has a prognostic impact on disease recurrence after a primary active treatment including surgery or radiotherapy. The probability of biochemical recurrence-free progression decreases as the ISUP system increases from grade group 1 to 5.17,18 However, although tumor grade ranking is associated with unfavorable pathology and early biochemical recurrence, the natural history of tumor behavior in EAU intermediate-risk patients remains largely unknown. In our study, unfavorable pathology was an adverse prognostic factor for PCa progression in the EAU intermediate-risk cohort treated with robotic surgery; nevertheless, Briganti’s 2012 nomogram was independently associated with the risk of disease progression after predicting unfavorable cancers, which were harbored in the surgical specimens. Consequently, patients presenting with an unfavorable risk nomogram >3.0% were more likely not only to have unfavorable pathology, including upgrading and upstaging issues, but also to experience disease progression; conversely, patients presenting with a favorable risk score of up to 3.0% were less likely to have unfavorable pathology and PCa progression. Briganti’s 2012 nomogram was an effective tool for predicting disease progression after assessing the risk of unfavorable pathology, thus demonstrating a strong association with tumor behavior in the natural history of PCa. Our results have a clinical impact on the management of EAU intermediate-risk patients, who can be further stratified at clinical presentation according to Briganti’s 2012 risk score beyond the standard classification into favorable and unfavorable groups; accordingly, patients presenting with adverse and non-adverse nomogram risk score will benefit from different treatment paradigms.
Our results showed that Briganti’s 2012 risk score was associated with tumor behavior for predicting unfavorable adverse pathology and disease progression. As the risk score of the nomogram increased, patients were more likely to experience PCa progression, independently by the occurrence of unfavorable disease in the surgical specimen. Theoretically, these results might be explained by assuming that the nomogram associates with tumor behavior along multidimensional patterns determined by complex interactions and integrations between the set of factors composing the nomogram model. Increasing adverse risk scores are more likely to be associated with adverse cancer phenotypes, which are genetically unstable and thus more likely to progress along the cancer’s natural history. Still, controlled studies are needed to test this hypothesis.
Our study has limitations for being retrospective, RARP being performed by multiple surgeons, not evaluating cancer extension in each biopsy core, and not evaluating mpMRI findings, which were not available in all cases; however, it also has several strengths for specimens being evaluated by a single dedicated pathologist and for the primary endpoint, which was stronger than just biochemical recurrence.
Soon, with the growing usage of artificial intelligence in the field of urology, it is possible that machine learning will facilitate the prediction of outcomes of prostate cancer and replace existing tools. The use of artificial intelligence can accurately identify prostate cancer and aid in predicting patient outcomes. This can lead to a greater possibility of enhanced patient care. Anyway, several limitations, last but not least concerns about the ethical implications, still limit its usage today.19 Until the day that those powerful tools become available, practical tools such as Briganti’s 2012 nomogram remain irreplaceable allies for better management of patients with prostate cancer.
CONCLUSIONS
In the EAU intermediate-risk prostate cancer population treated with robotic surgery, PCa progression was predicted by Briganti’s 2012 nomogram risk score, independently by the occurrence of unfavorable pathology in the surgical specimen. Specifically, as the nomogram risk score increased, patients were more likely to experience disease progression. Accordingly, the intermediate-risk category of EAU, beyond favorable and unfavorable categories, can be further stratified according to Briganti’s 2012 nomogram, which is associated with tumor behavior.
AUTHOR CONTRIBUTIONS
ABP conducted the study design and conception, drafted the manuscript, and carried out the statistical analyses. F Montanaro, A Baielli, and SC performed the drafting of the manuscript and analyses and interpretation of data. ES drafted the manuscript and carried out data collection. FA, CB, AF, SG, and A Bianchi carried out data collection. AV, RR, MB, F Migliorini, SS, MAC, RGB, and AA provided supervision and critical revision of the manuscript for important intellectual contents. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Mottet N, Cornford P, van den Bergh RCN, Briers E. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Arnhem: EAU Guidelines Office; 2023. [[Last accessed on 2023 Dec 18]]. Expert Patient Advocate. Available from: https://www.uroweb.org/guidelines/prostate-cancer . [Google Scholar]
- 2.Schaeffer E, Srinivas S, Adra N, An Y, Barocas D, et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2023. [[Last accessed on 2023 Dec 18]]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [DOI] [PubMed]
- 3.Wallis CJ, Zhao Z, Huang LC, Penson DF, Koyama T, et al. Association of treatment modality, functional outcomes, and baseline characteristics with treatment-related regret among men with localized prostate cancer. JAMA Oncol. 2022;8:50. doi: 10.1001/jamaoncol.2021.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388:1547–58. doi: 10.1056/NEJMoa2214122. [DOI] [PubMed] [Google Scholar]
- 5.Oderda M, Diamand R, Albisinni S, Calleris G, Carbone A, et al. Indications for and complications of pelvic lymph node dissection in prostate cancer:accuracy of available nomograms for the prediction of lymph node invasion. BJU Int. 2021;127:318–25. doi: 10.1111/bju.15220. [DOI] [PubMed] [Google Scholar]
- 6.Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection:the essential importance of percentage of positive cores. Eur Urol. 2012;61:480–7. doi: 10.1016/j.eururo.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Porcaro AB, Rizzetto R, Bianchi A, Gallina S, Serafin E, et al. American Society of Anesthesiologists (ASA) physical status system predicts the risk of postoperative Clavien–Dindo complications greater than one at 90 days after robot-assisted radical prostatectomy:final results of a tertiary referral center. J Robot Surg. 2022;17:987–93. doi: 10.1007/s11701-022-01505-7. [DOI] [PubMed] [Google Scholar]
- 8.Van den Broeck T, van den Bergh RC, Arfi N, Gross T, Moris L, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer:a systematic review. Eur Urol. 2019;75:967–87. doi: 10.1016/j.eururo.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Tilki D, Preisser F, Graefen M, Huland H, Pompe RS. External validation of the European Association of Urology biochemical recurrence risk groups to predict metastasis and mortality after radical prostatectomy in a European cohort. Eur Urol. 2019;75:896–900. doi: 10.1016/j.eururo.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Gnanapragasam VJ, Bratt O, Muir K, Lee LS, Huang HH, et al. The cambridge prognostic groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer:a validation study. BMC Med. 2018;16:31. doi: 10.1186/s12916-018-1019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parry MG, Cowling TE, Sujenthiran A, Nossiter J, Berry B, et al. Risk stratification for prostate cancer management:value of the Cambridge Prognostic Group classification for assessing treatment allocation. BMC Med. 2020;18:114. doi: 10.1186/s12916-020-01588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zumsteg ZS, Spratt DE, Pei I, Zhang Z, Yamada Y, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Fiori C, Checcucci E, Stura I, Amparone D, De Cillis S, et al. Development of a novel nomogram to identify the candidate to extended pelvic lymph node dissection in patients who underwent mpMRI and target biopsy only. Prostate Cancer Prostatic Dis. 2022;26:388–94. doi: 10.1038/s41391-022-00565-y. [DOI] [PubMed] [Google Scholar]
- 14.Patel HD, Gupta M, Tosoian JJ, Carter HB, Partin AW, et al. Subtyping the risk of intermediate risk prostate cancer for active surveillance based on adverse pathology at radical prostatectomy. J Urol. 2018;200:1068–74. doi: 10.1016/j.juro.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Patel HD, Tosoian JJ, Carter HB, Epstein JI. Adverse pathologic findings for men electing immediate radical prostatectomy:defining a favorable intermediate-risk group. JAMA Oncol. 2018;4:89–92. doi: 10.1001/jamaoncol.2017.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raldow AC, Zhang D, Chen MH, Braccioforte MH, Moran BJ, et al. Risk group and death from prostate cancer:implications for active surveillance in men with favorable intermediate-risk prostate cancer. JAMA Oncol. 2015;1:334–40. doi: 10.1001/jamaoncol.2014.284. [DOI] [PubMed] [Google Scholar]
- 17.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping:data based on the modified Gleason scoring system. BJU Int. 2013;111:753–60. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, et al. A contemporary prostate cancer grading system:a validated alternative to the Gleason score. Eur Urol. 2016;69:428–35. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu TN, Wong EY, Ma R, Yang CH, Dalieh IS, et al. Exploring the use of artificial intelligence in the management of prostate cancer. Curr Urol Rep. 2023;24:231. doi: 10.1007/s11934-023-01149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


