Abstract
Cerebral blood flow at rest declines with age. However, age-related changes in functional measures of cerebrovascular health including cerebrovascular reactivity and neurovascular coupling are not well understood. Additionally, the effect of apolipoprotein E (APOE) ε4, a strong genetic risk factor for Alzheimer’s disease, on cerebral blood flow and cerebrovascular function remains unclear. APOEε4 positive (APOEε4+; n = 37, age = 63±4y) and APOEε4 negative (APOEε4-; n = 50, age = 63±4y) cognitively unimpaired adults participated in this study. Macrovascular cerebral blood flow and microvascular cerebral perfusion were measured using 4D flow MRI and pseudo-continuous arterial spin labeling MRI, respectively. Cerebrovascular reactivity and neurovascular coupling were assessed by measuring middle cerebral artery blood velocity in response to hypercapnia and the n-back test, respectively. Neurovascular coupling was lower in APOEε4+ compared with APOEε4- adults (P<0.05), despite higher cerebral blood flow and cerebrovascular reactivity to hypercapnia. Alterations in neurovascular coupling may occur early, prior to changes in cognition, in aging APOEε4 carriers.
Introduction
The apolipoprotein E (APOE) ε4 allele increases the risk of Alzheimer’s disease (AD) such that APOEε4 carriers have a 2 to 15 times greater risk of developing AD [1,2]. However, although there is a strong influence of APOEε4 on AD risk, APOEε4 positivity does not guarantee development of AD [3]. As such, additional risk factors may provide enhanced insight for APOEε4 carriers at genetic predisposition to develop mild cognitive impairment (MCI) and AD [4].
Alterations in cerebral blood flow (CBF) may occur prior to significant accumulation of AD neuropathology, changes in brain structure, and manifestation of AD symptoms [5,6]. Indeed, cross-sectional [7,8] and longitudinal [9,10] studies indicate a decline in CBF with advancing age, which is likely mediated by APOE genotype [11–13]. For example, CBF at rest is greater in APOEε4 positive (APOEε4+) compared with APOEε4 negative (APOEε4-) young adults yet lower in APOEε4+ compared with APOEε4- older adults [13]. These findings, as well as others [14–16], suggest a cerebrovascular compensation hypothesis which proposes a biphasic relationship between APOEε4 and CBF across the lifespan, complicating the use of CBF at rest as an AD risk factor in aging adults. Functional measures of cerebrovascular health, in addition to CBF at rest, may provide insight into AD risk in APOEε4 carriers across the lifespan.
Cerebrovascular function, or the cerebral blood flow or velocity response to chemical or cognitive stimuli, may be differentially affected by APOE genotype [17]. Cerebrovascular reactivity (CVR) to elevated carbon dioxide (hypercapnia) is a commonly used functional measure of cerebrovascular health. Previous research suggests a decline in CVR across the lifespan [8,18], with direct comparisons demonstrating lower CVR in older adults compared with young adults [19–21]. As a result, CVR has been hypothesized as a potential diagnostic tool to detect vascular dysfunction prior to declines in cognitive function in individuals at risk of MCI and AD [22]. Limited previous work suggests that CVR is lower in APOEε4+ compared with APOEε4- young [23] and older adults [24]; however, data in healthy, cognitive unimpaired adults in the 55–69 age range are lacking.
Another functional measure of cerebrovascular health is neurovascular coupling (NVC). NVC is the tight coupling between neuronal activity and CBF to maintain delivery of oxygenated blood and nutrients to the brain [25]. Impairments in NVC may be an early indicator of cerebrovascular dysfunction [26]. Consistent with the idea of cerebrovascular compensation in APOEε4 carriers, APOEε4+ young adults demonstrate augmented blood oxygen level dependent (BOLD) responses to memory tasks compared with APOEε4- young adults [14,27]; however, similar studies in aging adults report mixed findings [28]. Importantly, many studies that assess the impact of APOEε4 on functional measures of cerebrovascular health do not include tests that assess cerebrovascular responses to both chemical and cognitive stimuli. Furthermore, data in aging participants without multiple comorbidities such as poor vascular or cognitive health is lacking.
As vascular dysfunction may manifest prior to AD symptoms, it is possible that changes in cerebrovascular function occur earlier in APOEε4+ carriers. Therefore, the purpose of this study was to comprehensively evaluate the influence of the APOEε4 allele on both resting CBF and cerebrovascular function (measured by CVR and NVC) in cognitively unimpaired aging adults. We hypothesized that, compared with APOEε4- adults, APOEε4+ adults would demonstrate greater CBF at rest; however, they would demonstrate reduced responses to measures of cerebrovascular function including attenuated CVR to hypercapnia and impaired NVC in response to an acute cognitive challenge.
Materials and methods
Participants
Participants were recruited from cohorts within the Wisconsin Alzheimer’s Disease Research Center (ADRC) including the Investigating Memory in Preclinical Alzheimer’s Disease–Causes and Treatments (IMPACT) cohort and the Healthy Older Controls cohort. All participants included in this study were considered cognitively unimpaired at the time of enrollment and have been described in detail previously [29,30]. In addition, a portion of these results have been reported in the postmenopausal females who participated in this study [31]. The recruitment period started on April 6th, 2018, and ended on August 28th, 2019. All study procedures were approved by the University of Wisconsin–Madison Institutional Review Board and performed according to the Declaration of Helsinki by obtaining written informed consent with signed consent documentation from each participant.
Ninety-five cognitively unimpaired adults (32 males, 63 females) between 55–69 years of age participated in this study. Females were postmenopausal for greater than 1 year and were not currently taking oral menopausal hormone therapy. Exclusion criteria consisted of a confirmed diagnosis of MCI or dementia of any kind, body mass index greater than 34.9 kg/m2, uncontrolled hypertension, significant surgical history, history of clinically significant stroke, cerebrovascular disease, or other major neurological disorders. Participants with controlled hypertension, defined as taking prescribed blood pressure (BP) medication to manage their BP were included in the study. If a participant reported to have hypertension and was not taking any anti-hypertensive medication, they were excluded. Initial study inclusion and exclusion was determined by a phone screening.
Experimental procedures
Participants visited the Bruno Balke Biodynamics Laboratory at the University of Wisconsin-Madison on two separate occasions for a screen day and experimental study day. On a separate day, participants visited the Wisconsin Institutes for Medical Research in Madison, WI for an MRI scan. On the experimental study day, participants arrived at the laboratory after a 4-hour fast and refrained from performing strenuous exercise during the previous 24 hours. Participants were instructed to avoid consumption of caffeine or chocolate during the previous 24 hours, alcohol during the previous 24 hours, and aspirin or non-steroidal anti-inflammatory drugs during the previous 48 hours prior to the experimental study day visit. Participants were asked to withhold over-the-counter medications on the experimental study day. This purpose of withholding over-the-counter medications and refraining from exercise, caffeine or chocolate was to minimize the acute impact of these substances on cerebral blood flow regulation. All experimental study day procedures were performed while participants laid supine in a dimly lit, temperature-controlled room kept between 22–24°C and all study personnel were blinded to APOEε4 status. All experimental study day visits and MRI scans took place prior to the COVID-19 pandemic.
Screen day measurements
The screen day visit took approximately 1 hour. Upon arrival at the laboratory, height and weight were obtained using a standard scale and stadiometer. The screen day visit consisted of a brief familiarization with study procedures, a supine arterial BP measurement in triplicate using a brachial cuff (Datex Ohmeda, GE Healthcare, Fairfield, CT, United States) after resting quietly in a dimly lit room for 10 min, and a middle cerebral artery (MCA) screening using transcranial Doppler (TCD) ultrasound. The angle of the TCD ultrasound probe, anatomical location, depth of the signal, and mean velocity were recorded to be used as a reference on the experimental study day to ensure repeatability of measurements. Participants were familiarized with the CVR protocol and the n-back working memory test (n-back).
Experimental study day measurements
The experimental study day measurements took approximately 2 hours. Participants remained supine during instrumentation and throughout the entire protocol. An overview of the experimental study day protocol is as follows: instrumentation, baseline hemodynamic measurements, and middle cerebral artery velocity (MCAv) signal acquisition. This was followed by neurovascular coupling assessments, a 10 min washout period, and stepped hypercapnia assessments. Following 10 min of supine rest, arterial BP was measured in triplicate using a brachial BP cuff and the average value was recorded. Heart rate (HR) was measured using a 3-lead electrocardiogram (CardioCap, Datex Ohmeda, GE Healthcare, Fairfield, CT, United States). Mean arterial blood pressure (MAP) was continuously measured using a non-invasive finger cuff (NOVA, Finapres Medical Systems, Amsterdam, The Netherlands). End-tidal CO2 (ETCO2) was measured using a nasal cannula. MCAv of the left MCA was measured using a 2-MHz TCD ultrasound probe (Spencer Technologies, Seattle, WA, United States). Quality MCAv signals were obtained using established guidelines [32] and signal quality was confirmed prior to and throughout hypercapnia and the n-back test.
Experimental study day protocol
NVC was assessed by measuring the percent change in MCAv in response to the n-back test. Participants completed a 3 min n-back test, a test of working memory [33], as previously described [34]. Briefly, participants were asked to identify if the object on the screen was the same object as two slides ago. Participants were instructed to perform the n-back test to the best of their abilities.
A 10 min washout period was observed between the CVR protocol and NVC protocol. Participants were fitted with a mask covering their nose and mouth with a one-way valve to prevent re-breathing (7450-V2, Hans Rudolph, Inc. Shawnee, KS, United States). Participants completed a stepped hypercapnia protocol as previously described [19,31]. Briefly, participants breathed normocapnic room air for 5 min followed by stepwise elevations of hypercapnic air at 2%, 4%, and 6% inspired CO2 for 5 min each.
Apolipoprotein E genotyping
Apolipoprotein status was determined using competitive allele-specific polymerase chain reaction-based genotyping assays (LGC Genomics, Beverly, MA) [29]. Participants were considered APOEε4+ if they had one or more copies of the ε4 allele (i.e., APOE ε2/ε4, ε3/ε4, or, ε4/ε4). Participants were considered APOEε4- if they did not have one or more copies of the ε4 allele (i.e., APOE ε2/ε2, ε2/ε3, or ε3/ε3).
MRI measurements
On a separate visit from the cerebrovascular function testing, MRI brain scans were completed on a 3T clinical MRI scanner (GE Healthcare, Waukesha, WI, United States) at the Wisconsin Institutes for Medical Research. The MRI scan took approximately 1 hour. Participants remained supine in the MRI scanner throughout the entire protocol. A brief overview of the MRI measurement protocol is as follows: instrumentation, brain volume scan, microvascular cerebral perfusion scan, and macrovascular blood flow scan. Brain volumes were measured using a T1-weighted structural brain volume (BRAVO) scan with the following scan parameters: fast spoiled gradient echo sequence, inversion time = 450 ms, repetition time = 8.1 ms, echo time = 3.2 ms, flip angle = 12°, acquisition matrix = 256 × 256, field of view (FOV) = 256 mm, slice thickness = 1.0 mm, and scan time ∼8 min.
Microvascular cerebral prefusion was measured using background-suppressed pseudocontinuous arterial spin labeling (ASL) MRI utilizing a 3D fast spin-echo stack of spiral sequence described previously [12,35] with the following scan parameters: echo spacing = 4.9 ms, TE = 10.5 ms with centric phase encoding, spiral arms = 8, spiral readout duration = 4 ms, FOV = 240 × 240 × 176 mm, 4 mm isotropic spatial resolution, reconstructed matrix size = 128 × 128 × 44, number of averages (NEX) = 3, labeling RF amplitude = 0.24 mG, and scan time ~4.5 min. Post-labeling delay was 2025 ms for all scans. Immediately after each ASL scan, a proton density (PD) reference scan was performed with identical imaging acquisition parameters without ASL labeling but with a saturation pulse applied 2.0 seconds prior to imaging. This PD image was used for ASL flow quantification as well as for image registration.
Macrovascular blood flow of the large intracranial vessels was measured using 4D flow MRI using a 3D radially undersampled sequence (PC-VIPR) as previously described [36,37] with the following scan parameters: velocity encoding (Venc) = 80cm/s, imaging volume = 220 mm x 220 mm x 160 mm, acquired isotropic spatial resolution = 0.7mm×0.7mm×0.7mm, TR = 7.8ms, TE = 2.7ms, flip angle = 8°, bandwidth = 83.3kHz, 14,000 projection angles, and scan time ∼7min.
CVR and NVC data analysis
Cardiovascular and cerebrovascular variables were recorded using LabChart 8 at 250 Hz (AD Instruments, Dunedin, New Zealand) and stored offline for analysis. CVR was calculated as the linear relationship between the percent change in ETCO2 and the percent change in MCAv in response to hypercapnia [31]. NVC was calculated as the percent change in MCAv in response to the n-back test as previously described [34].
MRI data analysis
Individual structural MRI scans were segmented in Statistical Parametric Mapping version 12 (SPM12) into gray matter, white matter, and cerebrospinal fluid [38]. Total brain volume was calculated as the sum of gray and white matter volumes. Intracranial volume was calculated as the sum of gray matter, white matter, and cerebrospinal fluid volumes.
4D flow MRI scans were analyzed as previously described [21]. Time-resolved velocity and magnitude data were reconstructed offline by retrospectively gating into 20 cardiac phases using temporal interpolation [39]. Flow was calculated from the velocity and diameter measurements for each of the 20 cardiac phases. All scans underwent background phase offset correction, eddy current correction, and automatic phase unwrapping to minimize potential for velocity aliasing [40]. Individual vessel segmentation of left and right internal carotid arteries (ICA), left and right MCAs, and basilar artery were performed in MATLAB using an in-house tool for semi-automated cerebrovascular flow analysis [41]. Blood flow was averaged along the length of each vessel. The ICAs were measured below the carotid siphon along the cervical and petrous portions. The MCAs were measured at the M1 segment. The basilar artery was measured above the bifurcation of the vertebral arteries and below the superior cerebellar artery. Total CBF through the major arteries was calculated as the sum of the left and right ICAs and the basilar artery [42].
In addition to 4D flow CBF data, cerebral perfusion data were included to evaluate microvascular gray matter perfusion. Cerebral perfusion data were extracted from pseudocontinuous ASL cerebral perfusion images using SPM12 as previously described [12]. Each participant’s PD image was first registered to the T1 image and the derived transformation matrix was applied to the average quantitative cerebral perfusion map. With resampling to a 2 x 2 x 2 mm3 voxel size, the T1 volume and associated cerebral perfusion image were subsequently spatially normalized to the Montreal Neurological Institute (MNI) template. The normalized cerebral perfusion maps were then smoothed using an 8-mm full-width at half-maximum Gaussian kernel. Total gray matter cerebral perfusion was analyzed and reported.
Statistical analysis
Normality of all variables was assessed using Shapiro-Wilk tests and visually inspected using histograms and QQ plots. Equal variance of all variables was assessed using Levene’s test. Participant characteristics, cardiovascular variables at rest, brain volumes, blood flow through each artery, and cerebral perfusion were compared between APOEε4+ and APOEε4- adults using independent samples t-tests (two-tailed). Cardiovascular and cerebrovascular variables at baseline, in response to hypercapnia, and in response to the n-back test were compared between APOEε4+ and APOEε4- adults using independent samples t-tests (two-tailed). In the event of unequal variance between APOEε4+ and APOEε4- adults, Welch’s t-tests were used. Effect sizes for all comparisons between groups were calculated using Cohen’s d interpreted as a small (d = 0.2), medium (d = 0.5), or large (d = 0.8) effect [43]. The relationship between total CBF measured using 4D flow MRI and cerebral perfusion measured using ASL was assessed using a Pearson correlation. All statistical analyses were completed using R software. The mean ± standard deviation for all variables is presented. Statistical significance was set a priori at P < 0.05.
Results
Participants
Out of the initial 95 participants recruited for this study, eight participants were excluded from the final analysis due to inadequate data quality (MAP or MCAv signal loss; n = 7) or incomplete APOE genotype data (n = 1). Of the remaining 87 participants, 37 participants were APOEε4+ and 50 participants were APOEε4-. Participant characteristics and cardiovascular variables at rest are presented in Table 1. There were no differences in participant characteristics and cardiovascular variables at rest between APOEε4+ and APOEε4- adults (Table 1). Within the APOEε4+ group, four participants were ε2/ε4, 28 participants were ε3/ε4, and five participants were ε4/ε4. Within the APOEε4- group, 11 participants were ε2/ε3 and 39 participants were ε3/ε3.
Table 1. Participant characteristics and selected cardiovascular variables at rest.
| Variable | APOEε4+ | APOEε4- |
P-value (Effect Size) |
||||
|---|---|---|---|---|---|---|---|
| n | 37 | 50 | |||||
| Sex (M/F) | 13 / 24 | 14 / 36 | |||||
| Age (y) | 63 | ± | 4 | 63 | ± | 4 | 0.848 (0.042) |
| Ethnicity | |||||||
| Hispanic | 0 | 1 | |||||
| Non-Hispanic | 37 | 49 | |||||
| Race | |||||||
| African American | 1 | 0 | |||||
| Asian | 0 | 1 | |||||
| White | 36 | 49 | |||||
| Education (y) | 17 | ± | 2 | 17 | ± | 3 | 0.507 (0.145) |
| Height (cm) | 171 | ± | 9 | 168 | ± | 8 | 0.143 (0.321) |
| Weight (kg) | 78 | ± | 15 | 76 | ± | 16 | 0.553 (0.129) |
| Body Mass Index (kg/m2) | 27 | ± | 4 | 27 | ± | 4 | 0.854 (0.040) |
| HR (bpm) | 60 | ± | 9 | 60 | ± | 8 | 0.907 (0.025) |
| Systolic BP (mmHg) | 127 | ± | 17 | 125 | ± | 14 | 0.599 (0.115) |
| Diastolic BP (mmHg) | 76 | ± | 8 | 75 | ± | 8 | 0.385 (0.189) |
| Mean Arterial BP (mmHg) | 93 | ± | 10 | 92 | ± | 9 | 0.457 (0.162) |
| MoCA | 28 | ± | 2 | 28 | ± | 2 | 0.684 (0.089) |
| Family History of Dementia (n, %) | 26, 70 | 34, 68 | |||||
| Controlled Hypertension (n, %) | 6, 16 | 12, 24 | |||||
Values expressed as mean ± SD. APOE, apolipoprotein; BP, blood pressure; HR, heart rate; MoCA, Montreal Cognitive Assessment. P-value indicates result of independent samples t-test comparing APOEε4 positive (APOEε4+) and APOEε4 negative (APOEε4-) adults. Effect size calculated using Cohen’s d.
Brain volumes
There were no differences in gray matter volume, white matter volume, total brain volume, cerebrospinal fluid, or intracranial volume between APOEε4+ and APOEε4- adults (Table 2).
Table 2. Brain volumes between APOEε4+ and APOEε4- adults.
| Variable |
APOEε4+ n = 37 |
APOEε4- n = 50 |
P-value (Effect Size) |
||||
|---|---|---|---|---|---|---|---|
| Gray Matter (L) | 0.7 | ± | 0.1 | 0.7 | ± | 0.1 | 0.203 (0.278) |
| White Matter (L) | 0.4 | ± | 0.1 | 0.4 | ± | 0.1 | 0.165 (0.304) |
| Total Brain Volume (L) | 1.1 | ± | 0.1 | 1.1 | ± | 0.1 | 0.151 (0.315) |
| Cerebrospinal Fluid (L) | 0.3 | ± | 0.1 | 0.3 | ± | 0.1 | 0.868 (0.036) |
| Intracranial Volume (L) | 1.5 | ± | 0.1 | 1.4 | ± | 0.1 | 0.262 (0.245) |
Values expressed as mean ± SD. APOE, apolipoprotein. Total brain volume was calculated as the sum of gray and white matter volumes. Intracranial volume was calculated as the sum of gray matter, white matter, and cerebrospinal fluid. P-value indicates result of independent samples t-test comparing APOEε4 positive (APOEε4+, n = 37) and APOEε4 negative (APOEε4-, n = 50) adults. Effect size calculated using Cohen’s d.
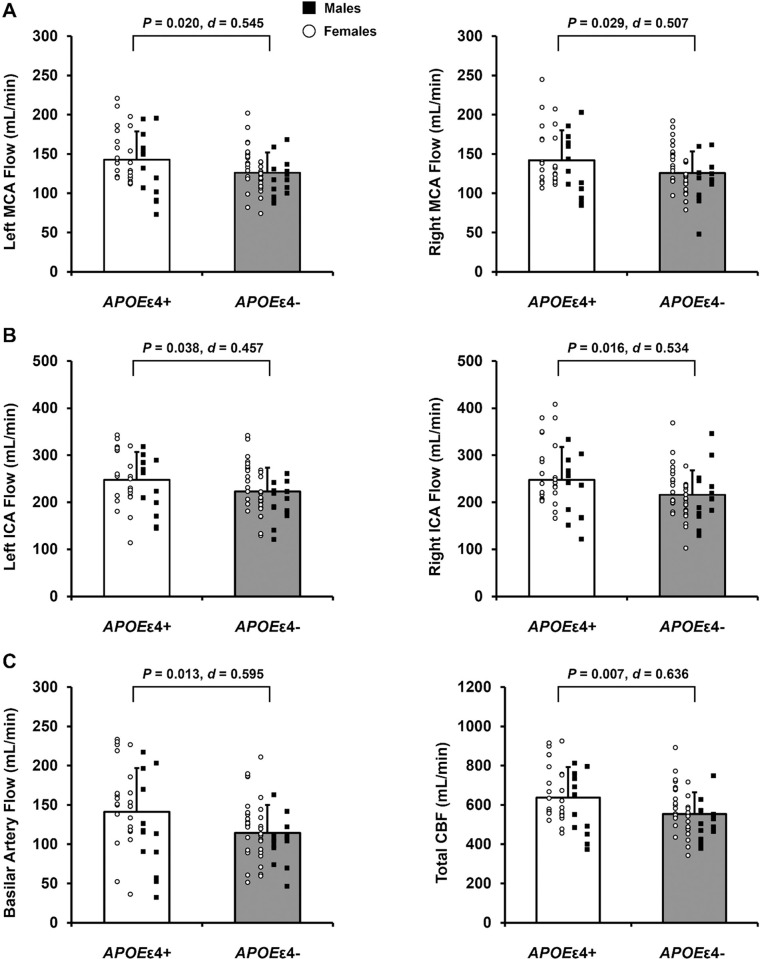
Blood flow through the intracranial arteries
Blood flow through the left and right MCA (Fig 1A), left and right ICA (Fig 1B), basilar artery (Fig 1C, left panel), and total CBF (Fig 1C, right panel) was greater in APOEε4+ compared with APOEε4- adults.
Fig 1. Blood flow through the intracranial arteries in apolipoprotein ε4 positive (APOEε4+) and apolipoprotein ε4 negative (APOEε4-) adults.
Data expressed as individual data and means ± standard deviation. APOEε4+ means represented by white bars and APOEε4- means represented by gray bars. Within each group, females are represented as white circles and males are represented as black squares. A: Middle cerebral artery (MCA) blood flow. B: Internal carotid artery (ICA) blood flow. C: Basilar artery blood flow and total cerebral blood flow (CBF). P-value indicates result of independent samples t-test comparing blood flow in each artery between APOEε4+ (n = 37) and APOEε4- (n = 50) adults*. Effect size calculated as Cohen’s d. *Note: For right MCA flow, n = 37 for APOEε4+ and n = 49 for APOEε4-.
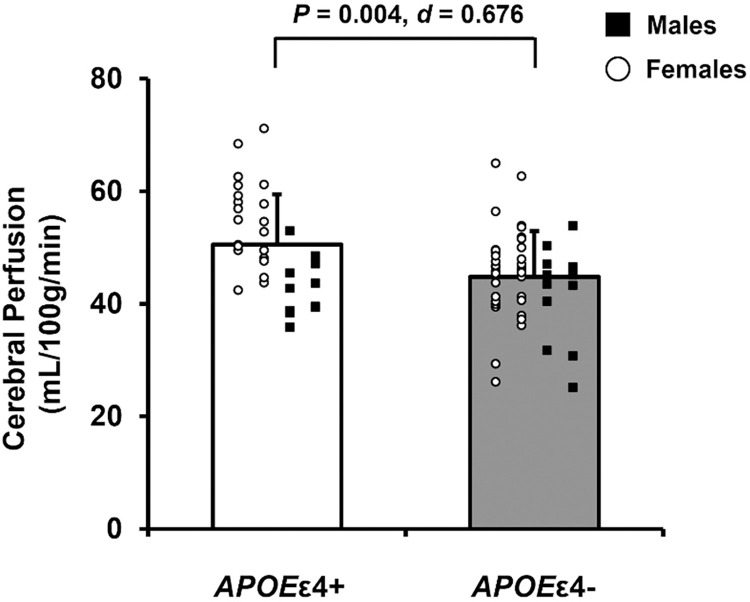
Cerebral perfusion
Five APOEε4+ and three APOEε4- adults were excluded from the cerebral perfusion analysis due to incomplete data (n = 6) or incompatible inversion times for participant specific flow (n = 2). In the remaining 79 participants, cerebral perfusion was greater in APOEε4+ compared with APOEε4- adults (Fig 2).
Fig 2. Microvascular cerebral perfusion in apolipoprotein ε4 positive (APOEε4+) and apolipoprotein ε4 negative (APOEε4-) adults.
Data expressed as individual data and means ± standard deviation. APOEε4+ means represented by white bars and APOEε4- means represented by gray bars. Within each group, females are represented as white circles and males are represented as black squares. P-value indicates result of independent samples t-test comparing cerebral perfusion between APOEε4+ (n = 32) and APOEε4- (n = 47) adults. Effect size calculated as Cohen’s d.
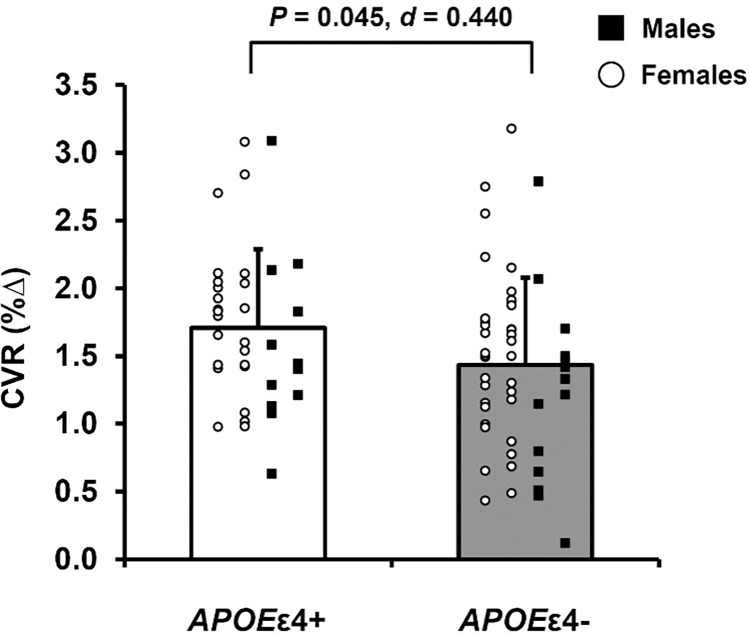
CVR
APOEε4+ adults had greater CVR compared with APOEε4- adults (Fig 3). Importantly, there were no differences in HR, MAP, or MCAv at baseline during room air or in response to hypercapnia between APOEε4+ and APOEε4- adults (S1 Table).
Fig 3. Cerebrovascular reactivity (CVR) to hypercapnia in apolipoprotein ε4 positive (APOEε4+) and apolipoprotein ε4 negative (APOEε4-) adults.
Data expressed as individual data and means ± standard deviation. APOEε4+ means represented by white bars and APOEε4- means represented by gray bars. Within each group, females are represented as white circles and males are represented as black squares. CVR expressed as the linear relationship between the percent change from baseline in middle cerebral artery blood velocity and the percent change in end-tidal CO2. P-value indicates result of independent samples t-test comparing CVR to hypercapnia between APOEε4+ (n = 37) and APOEε4- (n = 50) adults. Effect size calculated as Cohen’s d.
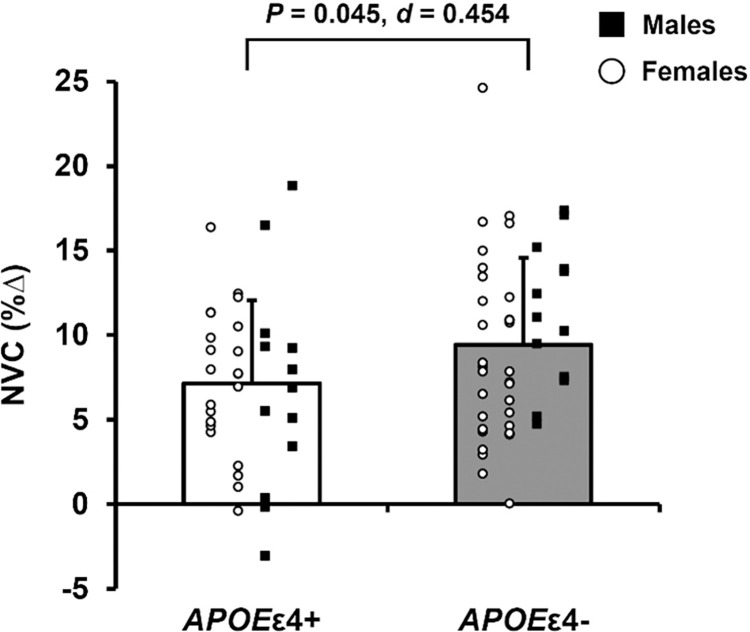
NVC
One APOEε4+ and four APOEε4- adults were excluded from the n-back analysis due to inadequate data quality (MAP or MCAv signal loss). In the remaining 82 participants NVC, expressed as the percent change in MCAv in response to the n-back test, was lower in APOEε4+ compared with APOEε4- adults (Fig 4). There were no differences in HR, MAP, or MCAv at baseline or in response to the n-back test between APOEε4+ and APOEε4- adults (S2 Table).
Fig 4. Neurovascular coupling (NVC) in response to the n-back working memory test in apolipoprotein ε4 positive (APOEε4+) and apolipoprotein ε4 negative (APOEε4-) adults.
Data expressed as individual data and means ± standard deviation. APOEε4+ means represented by white bars and APOEε4- means represented by gray bars. Within each group, females are represented as white circles and males are represented as black squares. NVC is expressed as the percent change from baseline in middle cerebral artery blood velocity. P-value indicates result of independent samples t-test comparing NVC in response to the n-back test between APOEε4+ (n = 36) and APOEε4- (n = 46) adults. Effect size calculated as Cohen’s d.
Discussion
The primary findings of this study were that APOEε4+ adults had greater CBF at rest and CVR, yet demonstrated lower NVC responses, compared with APOEε4- adults. Collectively, the novel findings of this study suggest that despite greater CBF at rest, impaired NVC, indicated by a blunted MCAv response to the n-back working memory test, may occur early in the time course of declines in cognitive function in cognitively unimpaired adult APOEε4 carriers.
Consistent with previous research, we report greater MCA blood flow at rest in APOEε4+ compared with APOEε4- adults [11]. Unique to the present study is the inclusion of blood flow through the ICAs, basilar artery, and total CBF using 4D flow MRI. These data provide insight into intracranial CBF supply and, with the addition of ASL data, microvascular cerebral perfusion. In support of previous research [11,14–16], we report greater total CBF and cerebral perfusion at rest in APOEε4+ compared with APOEε4- adults, despite no differences in brain volumes. These findings add to existing evidence supporting the cerebrovascular compensation hypothesis suggesting that APOEε4+ adults have greater CBF and gray matter perfusion to compensate for the detrimental effects of the APOEε4 allele across the lifespan until a hemodynamic breaking point at which rapid declines in CBF and cerebral perfusion occur. Indeed, cerebral perfusion is lower in older APOEε4+ adults (74 ± 7 years) compared with APOEε4- older adults [13] suggesting that a rapid decline in cerebral perfusion occurs in older age. For example, an ~8-year longitudinal study in cognitively unimpaired older adults (69 ± 7 years) found that declines in cerebral perfusion were greater in APOEε4+ compared with APOEε4- older adults [16]. As participants recruited for the present study were younger (62 ± 4 years), generally healthy, and cognitively unimpaired, the findings of this study provide insight into a critical period where clinically relevant declines in total CBF and microvascular cerebral perfusion likely begin or are occurring.
A novel aspect of the present study is the inclusion of cerebrovascular responses to both chemical and cognitive stimuli. Importantly, these tests of cerebrovascular function may provide insight into cerebrovascular health, especially in aging adults who do not demonstrate evidence of cognitive dysfunction. Declines in CVR to chemical stimuli such as hypercapnia occur across the lifespan [8,18]. Longitudinal data suggest that attenuated CVR is associated with greater declines in cognitive performance and increased risk of AD, with greater rates of decline in APOEε4+ adults [44]. To our knowledge, the present study is the first to evaluate the effect of the APOEε4 allele on CVR to hypercapnia in cognitively unimpaired, healthy adults 55–69 years of age. Our finding that APOEε4+ adults have greater CVR compared with APOEε4- adults is opposite of our original hypothesis and in conflict with previous work on APOEε4 and CVR [23,24]. However, the previous studies evaluated only young (24 ± 5 years) [23] or older adults (78 ± 5 years) [24]. Although CVR was lower in APOEε4+ young adults, CVR of the hippocampi was evaluated using BOLD MRI which reflects changes in deoxyhemoglobin of the left and right hippocampus. While Hajjar et al. 2015 utilized techniques similar to the present study, participants were older, had a history of stroke and cardiovascular disease, and the majority (74%) were hypertensive [24], which limits the applicability of these findings in the context of healthy aging. Therefore, it is possible that methodological and age group differences could explain the conflicting findings. Moreover, augmented CVR in APOEε4+ adults observed in the present study (in addition to greater total CBF) supports the proposed cerebrovascular compensation hypothesis such that responses to chemical stimuli may be amplified before declines start to occur.
CBF responses to acute cognitive activity are indicative of day-to-day tasks which elicit an increase in neuronal activity and metabolic demand of the brain. Impairments in NVC in the form of attenuated BOLD MRI and cerebral artery blood velocity responses to increased metabolic demand of the brain occur with advancing age [20,45] and impaired NVC may be an early indication of vascular dysfunction [26]. APOEε4 modulates cerebrovascular function directly at the neurovascular unit and indirectly through its effect on the peripheral and cerebral vasculature, ultimately contributing to cognitive decline [17]. In the present study, APOEε4+ adults demonstrated blunted MCAv responses to an increase in metabolic demand of the brain during the n-back test. These findings suggest that APOEε4+ adults have impaired NVC in response to a test of working memory. Previous work reported that the BOLD signal change in response to a memory task is greater in APOEε4+ relative to APOEε4- young adults [27]. In a follow-up study that included aging adults (64 ± 7 years), presence of the APOEε4 allele had the opposite effect such that the BOLD response to a memory task was lower in APOEε4+ relative to APOEε4- adults [14]. Collectively, the Filippini et al. 2011 and 2009 studies suggest a biphasic effect of APOEε4 on NVC responses to a memory task, similar to the biphasic patterns reported with CBF and cerebral perfusion. However, the current literature on the effect of APOEε4 on BOLD responses to acute cognitive activity in aging adults remains unclear [28]. In agreement with the findings of the present study, Fleisher et al. 2009 report attenuated BOLD responses to cognitive activity [46], while other studies suggest greater BOLD signal responses [47,48] or no difference between APOEε4 carriers and non-carriers [49]. Conflicts in the literature are likely due to the broad age range of participants, differences in the overall health and cognitive status of participants, as well as methodological differences between studies. In addition, the mechanisms by which cerebral blood velocity responses to chemical stimuli are augmented but responses to cognitive stimuli are impaired in cognitively unimpaired APOEε4 carriers are unknown.
Alternatively, it is possible that lower NVC and a blunted MCAv response to acute cognitive activity could be interpreted as a lesser need for blood flow to match an increase in metabolic demand of the brain. This is a plausible explanation given the observed greater total CBF and cerebral perfusion in APOEε4+ compared with APOEε4- adults in the present study; however, there were no differences in MCAv at rest between groups. Measurement of MCAv via TCD ultrasound is considered an estimate of flow because it does not include vessel diameter. Therefore, we cannot completely rule out that possibility that changes in MCA vessel diameter occurred in response to hypercapnia and acute cognitive activity. However, previous studies investigating MCA diameter changes in response to hypercapnia suggest that changes in the diameter of the MCA is minimal in older adults [21,50]. Therefore, this likely does not influence the results of the present study.
A better understanding of the underlying mechanisms that contribute to vascular dysfunction in AD and identification of novel, noninvasive biomarkers of vascular dysfunction in the context of cognitive and neurological impairments have been identified as two critical areas to move the field forward [51]. In support of the latter, impaired NVC may serve as a noninvasive, early indication of vascular dysfunction in individuals at genetic predisposition to develop AD [52]. In a healthy brain, the neurovascular unit works to direct blood flow to regions of the brain with higher metabolic activity (i.e. neurovascular coupling). However, if blood flow does not match neuronal activation and metabolic need, there could be cognitive consequences. Indeed, neurovascular coupling is disrupted in multiple neurological conditions including Alzheimer’s disease [4,5]. APOE genotype differentially modulates the cerebral and peripheral vasculature, and as a result, may affect cerebrovascular function [17]. For example, compared with cognitively unimpaired APOEε4- middle-aged adults, APOEε4+ adults have greater transcranial pulse wave velocity despite no difference in CBF which is indicative of vascular stiffening within the cerebrovasculature [53]. In mouse models, APOEε4+ mice have lower CBF, impaired NVC, disrupted white matter integrity, and exhibit cognitive dysfunction compared with APOEε4- mice [54]. Further, APOEε4 expression may impede amyloid beta clearance leading to amyloid beta deposition [55]. In humans, APOEε4 exacerbates the detrimental effects of cardiovascular disease risk factors such as hypertension and hypercholesterolemia [56] and disrupts the blood brain barrier [57]. Taken together, APOEε4 affects the cerebral and peripheral vasculature, potentially beginning at an early age and exerting its effect throughout the lifespan, resulting in compensatory mechanisms, such as augmented CBF and CVR to offset the detrimental effects of APOEε4.
There are several limitations of the present study. This study includes participants that are relatively healthy in attempt to isolate the effect of APOE genotype. The participants were also primarily white and well-educated; thus, this sample may not be representative of the general population. Some participants reported they were prescribed common medications such as blood pressure medication, statins, metformin, thyroid medication, or medication for depression or anxiety. The impact of chronic medication use on cerebral blood flow warrants further investigation. As mentioned above, measurement of MCAv via TCD ultrasound is considered an estimate of flow; however, use of TCD ultrasound enhances temporal resolution by evaluating beat-to-beat changes in MCAv which provides insight into rapid changes in CBF. Future studies may assess the influence of APOEε4+ on cerebrovascular function in other cerebral arteries besides the MCA. To quantify NVC, we used the n-back test, a cognitive test of working memory, but the test was not scored due to the experimental setup and instrumentation. Participants were instructed to complete the test to the best of their ability and were not informed about the purpose of completing the test until the conclusion of the experimental study visit. We cannot assess the “dose” of the NVC task based on perceived difficulty of the task. However, this is why NVC was calculated as a percent change in blood velocity from baseline. Future studies may also assess the interaction of sex, APOE genotype (ε2/ε4, ε3/ε3, etc.), and/or other genetic or environmental factors on cerebral blood flow regulation. We grouped all the APOEε4+ carriers together. However, within APOEε4+ carriers, different genotypes (for example ε2/ε4 vs. ε3/ε4) may have differential impacts cerebrovascular regulation and subsequent AD risk. Lastly, MRI data used for this study were collected as part of the ongoing longitudinal Wisconsin ADRC research study on early detection of AD [29]. The average time between MRI scan and the experimental study day visit was 1.8 ± 1.4 years, with MRI scans occurring either before or after the experimental study day visit depending on the participant.
In summary, despite no differences in brain volumes, cognitively unimpaired APOEε4+ adults had greater CBF through the large intracranial arteries, microvascular cerebral perfusion, and CVR to hypercapnia compared with APOEε4- adults 55–69 years of age. Importantly, APOEε4+ adults demonstrated lower cerebral blood velocity responses to a cognitive test suggesting impaired NVC. These findings suggest that impairments in NVC may occur early in the time course of declines in cognitive function in adult APOEε4+ carriers. Functional measures of cerebrovascular health may be a useful diagnostic tool in determining future risk of AD in individuals at genetic predisposition to develop AD.
Supporting information
Values expressed as mean ± SD. APOE, apolipoprotein; MAP, mean arterial blood pressure; MCAv, middle cerebral artery blood velocity. P-value indicates result of independent samples t-test comparing APOEε4 positive (APOEε4+, n = 37) and APOEε4 negative (APOEε4-, n = 50) adults during room air and in response to 6% CO2. Effect size calculated using Cohen’s d.
(DOCX)
Values expressed as mean ± SD. APOE, apolipoprotein; MAP, mean arterial blood pressure; MCAv, middle cerebral artery blood velocity. P-value and effect size in the column indicates result of independent samples t-test comparing APOEε4 positive (APOEε4+, n = 36) and APOEε4 negative (APOEε4-, n = 46) adults at baseline and in response to the n-back test. Effect size calculated using Cohen’s d.
(DOCX)
Acknowledgments
The authors would like to thank the research participants who volunteered to participate in this study and the Wisconsin Alzheimer’s Disease Research Center. The authors would also like to thank Howard A. Rowley for his assistance with this study.
Data Availability
Data Availability Statement: There are restrictions to data sharing for this study because participants are part of a long-term observational cohort study. Public availability of this data would compromise patient privacy. Data supporting the primary outcomes of this manuscript are available for qualified investigators upon approved request to the Wisconsin Alzheimer’s Disease Research Center by using the link provided: (https://wrap.wisc.edu/data-requests/). On a monthly basis requests are reviewed by an executive committee, independent of the study co-authors. Participants consent for information to be shared in a deidentified format for approved requests. For additional information or questions about a data request, please contact the Wisconsin Alzheimer’s Disease Research Center (address: 600 Highland Avenue, MC 2420, Madison, Wisconsin, USA 53792-2420, phone: +1 608-265-0407, email: adrc@medicine.wisc.edu).
Funding Statement
This research was supported by the Wisconsin Alzheimer's Disease Research Center (P30-AG062715). This research was also supported by the National Institutes of Health grants HL118154 (to JB) and HL007936 (to KM, T32 award to University of Wisconsin–Madison Cardiovascular Research Center), and an Alzheimer's Association Research Grant (#17-499398 to JB).
References
- 1.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261:921–3. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 2.Troutwine BR, Hamid L, Lysaker CR, Strope TA, Wilkins HM. Apolipoprotein E and Alzheimer’s disease. Acta Pharmaceutica Sinica B 2022;12:496–510. doi: 10.1016/j.apsb.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106–18. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hays CC, Zlatar ZZ, Wierenga CE. The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cell Mol Neurobiol 2016;36:167–79. doi: 10.1007/s10571-015-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis 2014;42 Suppl 4:S411–419. doi: 10.3233/JAD-141467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990;113 (Pt 1):27–47. doi: 10.1093/brain/113.1.27 [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011;21:1426–34. doi: 10.1093/cercor/bhq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leidhin CN, McMorrow J, Carey D, Newman L, Williamson W, Fagan AJ, et al. Age-related normative changes in cerebral perfusion: Data from The Irish Longitudinal Study on Ageing (TILDA). NeuroImage 2021;229:117741. doi: 10.1016/j.neuroimage.2021.117741 [DOI] [PubMed] [Google Scholar]
- 10.Staffaroni AM, Cobigo Y, Elahi FM, Casaletto KB, Walters SM, Wolf A, et al. A longitudinal characterization of perfusion in the aging brain and associations with cognition and neural structure. Hum Brain Mapp 2019;40:3522–33. doi: 10.1002/hbm.24613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dounavi M-E, Low A, McKiernan EF, Mak E, Muniz-Terrera G, Ritchie K, et al. Evidence of cerebral hemodynamic dysregulation in middle-aged APOE ε4 carriers: The PREVENT-Dementia study. J Cereb Blood Flow Metab 2021;41:2844–55. 10.1177/0271678X211020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Oh JM, Motovylyak A, Ma Y, Sager MA, Rowley HA, et al. Impact of sex and APOE ε4 on age-related cerebral perfusion trajectories in cognitively asymptomatic middle-aged and older adults: A longitudinal study. J Cereb Blood Flow Metab 2021;41:3016–27. 10.1177/0271678X211021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wierenga CE, Clark LR, Dev SI, Shin DD, Jurick SM, Rissman RA, et al. Interaction of Age and APOE Genotype on Cerebral Blood Flow at Rest. J Alzheimers Dis 2013;34:921–35. doi: 10.3233/JAD-121897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 2011;54:602–10. doi: 10.1016/j.neuroimage.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Hays CC, Zlatar ZZ, Meloy MJ, Bondi MW, Gilbert PE, Liu T, et al. Interaction of APOE, cerebral blood flow, and cortical thickness in the entorhinal cortex predicts memory decline. Brain Imaging Behav 2020;14:369–82. doi: 10.1007/s11682-019-00245-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010;67:93–8. doi: 10.1001/archneurol.2009.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, et al. The Role of APOE in Cerebrovascular Dysfunction. Acta Neuropathol 2016;131:709–23. doi: 10.1007/s00401-016-1547-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker SLM, de Leeuw F-E, den Heijer T, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral haemodynamics in the elderly: the rotterdam study. Neuroepidemiology 2004;23:178–84. doi: 10.1159/000078503 [DOI] [PubMed] [Google Scholar]
- 19.Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol (1985) 2012;112:1884–90. doi: 10.1152/japplphysiol.01270.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flück D, Beaudin AE, Steinback CD, Kumarpillai G, Shobha N, McCreary CR, et al. Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Front Physiol 2014;5:49. doi: 10.3389/fphys.2014.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, et al. Age-Related Reductions in Cerebrovascular Reactivity Using 4D Flow MRI. Front Aging Neurosci 2019;11:281. doi: 10.3389/fnagi.2019.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glodzik L, Randall C, Rusinek H, de Leon MJ. Cerebrovascular reactivity to carbon dioxide in Alzheimer’s disease. J Alzheimers Dis 2013;35:427–40. doi: 10.3233/JAD-122011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement 2015;11:648–657.e1. 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- 24.Hajjar I, Sorond F, Lipsitz LA. Apolipoprotein E, Carbon Dioxide Vasoreactivity, and Cognition in Older Adults: Effect of Hypertension. J Am Geriatr Soc 2015;63:276–81. 10.1111/jgs.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–35. doi: 10.1152/japplphysiol.00966.2005 [DOI] [PubMed] [Google Scholar]
- 26.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004;5:347–60. doi: 10.1038/nrn1387 [DOI] [PubMed] [Google Scholar]
- 27.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 2009;106:7209–14. doi: 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trachtenberg AJ, Filippini N, Mackay CE. The effects of APOE-ε4 on the BOLD response. Neurobiol Aging 2012;33:323–34. 10.1016/j.neurobiolaging.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, et al. The Wisconsin Registry for Alzheimer’s Prevention: A review of findings and current directions. Alzheimers Dement (Amst) 2017;10:130–42. doi: 10.1016/j.dadm.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson AG, Miller KB, Corkery AT, Eisenmann NA, Howery AJ, Cody KA, et al. Sympathoexcitatory Responses to Isometric Handgrip Exercise Are Associated With White Matter Hyperintensities in Middle-Aged and Older Adults. Frontiers in Aging Neuroscience 2022;14. doi: 10.3389/fnagi.2022.888470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moir ME, Corkery AT, Senese KA, Miller KB, Pearson AG, Loggie NA, et al. Age at natural menopause impacts cerebrovascular reactivity and brain structure. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2023;324:R207–15. doi: 10.1152/ajpregu.00228.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeWitt LD, Wechsler LR. Transcranial Doppler. Stroke 1988;19:915–21. doi: 10.1161/01.str.19.7.915 [DOI] [PubMed] [Google Scholar]
- 33.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59. doi: 10.1002/hbm.20131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson AG, Miller KB, Corkery AT, Eisenmann NA, Howery AJ, Carl AE, et al. Impact of age and cyclooxygenase inhibition on the hemodynamic response to acute cognitive challenges. Am J Physiol Regul Integr Comp Physiol 2021;321:R208–19. doi: 10.1152/ajpregu.00048.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, et al. Cerebral Blood Flow is Diminished in Asymptomatic Middle-Aged Adults with Maternal History of Alzheimer’s Disease. Cereb Cortex 2014;24:978–88. doi: 10.1093/cercor/bhs381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008;60:1329–36. doi: 10.1002/mrm.21763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivera-Rivera LA, Schubert T, Turski P, Johnson KM, Berman SE, Rowley HA, et al. Changes in intracranial venous blood flow and pulsatility in Alzheimer’s disease: A 4D flow MRI study. J Cereb Blood Flow Metab 2017;37:2149–58. doi: 10.1177/0271678X16661340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison SL, Koscik RL, Cary RP, Jonaitis EM, Rowley HA, Chin NA, et al. Comparison of different MRI-based morphometric estimates for defining neurodegeneration across the Alzheimer’s disease continuum. Neuroimage Clin 2019;23:101895. doi: 10.1016/j.nicl.2019.101895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Redmond MJ, Brodsky EK, Alexander AL, Lu A, Thornton FJ, et al. Generation and visualization of four-dimensional MR angiography data using an undersampled 3-D projection trajectory. IEEE Trans Med Imaging 2006;25:148–57. doi: 10.1109/TMI.2005.861706 [DOI] [PubMed] [Google Scholar]
- 40.Loecher M, Schrauben E, Johnson KM, Wieben O. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging 2016;43:833–42. doi: 10.1002/jmri.25045 [DOI] [PubMed] [Google Scholar]
- 41.Roberts GS, Hoffman CA, Rivera-Rivera LA, Berman SE, Eisenmenger LB, Wieben O. Automated hemodynamic assessment for cranial 4D flow MRI. Magn Reson Imaging 2023;97:46–55. doi: 10.1016/j.mri.2022.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera‐Rivera LA, Eisenmenger L, Cody KA, Reher T, Betthauser T, Cadman RV, et al. Cerebrovascular stiffness and flow dynamics in the presence of amyloid and tau biomarkers. Alzheimers Dement (Amst) 2021;13:e12253. doi: 10.1002/dad2.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:863. doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolters FJ, de Bruijn RFAG, Hofman A, Koudstaal PJ, Ikram MA. Cerebral Vasoreactivity, Apolipoprotein E, and the Risk of Dementia. Arteriosclerosis, Thrombosis, and Vascular Biology 2016;36:204–10. doi: 10.1161/ATVBAHA.115.306768 [DOI] [PubMed] [Google Scholar]
- 45.Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, et al. Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. Neuroimage 2014;85: doi: 10.1016/j.neuroimage.2013.04.113 10.1016/j.neuroimage.2013.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage 2009;47:1678–90. doi: 10.1016/j.neuroimage.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med 2000;343:450–6. doi: 10.1056/NEJM200008173430701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, et al. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry 2006;163:1603–10. doi: 10.1176/ajp.2006.163.9.1603 [DOI] [PubMed] [Google Scholar]
- 49.Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, et al. The Influence of Alzheimer Disease Family History and Apolipoprotein E ε4 on Mesial Temporal Lobe Activation. J Neurosci 2006;26:6069–76. doi: 10.1523/JNEUROSCI.0959-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 2017;37:344–55. doi: 10.1177/0271678X15626156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 2015;11:710–7. doi: 10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 2017;94:52–8. doi: 10.1016/j.exger.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivera-Rivera LA, Cody KA, Eisenmenger L, Cary P, Rowley HA, Carlsson CM, et al. Assessment of vascular stiffness in the internal carotid artery proximal to the carotid canal in Alzheimer’s disease using pulse wave velocity from low rank reconstructed 4D flow MRI. J Cereb Blood Flow Metab 2021;41:298–311. doi: 10.1177/0271678X20910302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koizumi K, Hattori Y, Ahn SJ, Buendia I, Ciacciarelli A, Uekawa K, et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun 2018;9:3816. 10.1038/s41467-018-06301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008;118:4002–13. doi: 10.1172/JCI36663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caselli RJ, Dueck AC, Locke DEC, Sabbagh MN, Ahern GL, Rapcsak SZ, et al. Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology 2011;76:1078–84. 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 2016;36:216–27. doi: 10.1038/jcbfm.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values expressed as mean ± SD. APOE, apolipoprotein; MAP, mean arterial blood pressure; MCAv, middle cerebral artery blood velocity. P-value indicates result of independent samples t-test comparing APOEε4 positive (APOEε4+, n = 37) and APOEε4 negative (APOEε4-, n = 50) adults during room air and in response to 6% CO2. Effect size calculated using Cohen’s d.
(DOCX)
Values expressed as mean ± SD. APOE, apolipoprotein; MAP, mean arterial blood pressure; MCAv, middle cerebral artery blood velocity. P-value and effect size in the column indicates result of independent samples t-test comparing APOEε4 positive (APOEε4+, n = 36) and APOEε4 negative (APOEε4-, n = 46) adults at baseline and in response to the n-back test. Effect size calculated using Cohen’s d.
(DOCX)
Data Availability Statement
Data Availability Statement: There are restrictions to data sharing for this study because participants are part of a long-term observational cohort study. Public availability of this data would compromise patient privacy. Data supporting the primary outcomes of this manuscript are available for qualified investigators upon approved request to the Wisconsin Alzheimer’s Disease Research Center by using the link provided: (https://wrap.wisc.edu/data-requests/). On a monthly basis requests are reviewed by an executive committee, independent of the study co-authors. Participants consent for information to be shared in a deidentified format for approved requests. For additional information or questions about a data request, please contact the Wisconsin Alzheimer’s Disease Research Center (address: 600 Highland Avenue, MC 2420, Madison, Wisconsin, USA 53792-2420, phone: +1 608-265-0407, email: adrc@medicine.wisc.edu).