Abstract
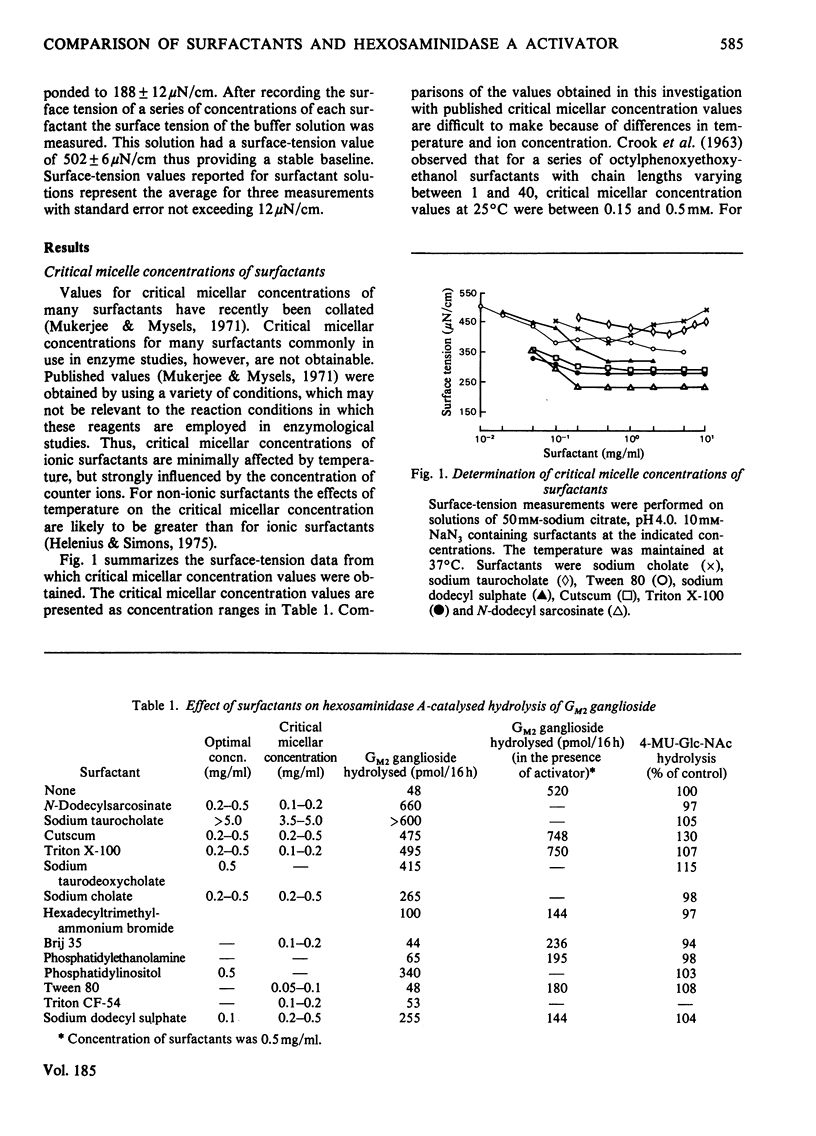
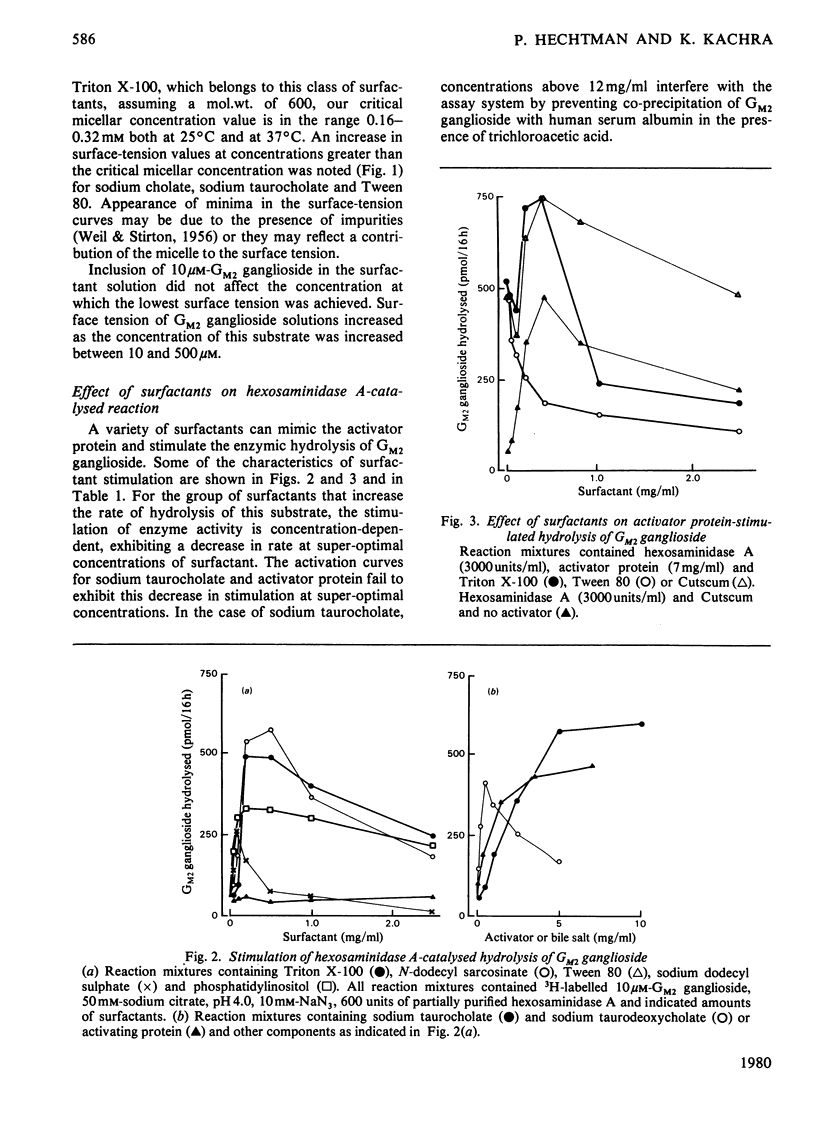
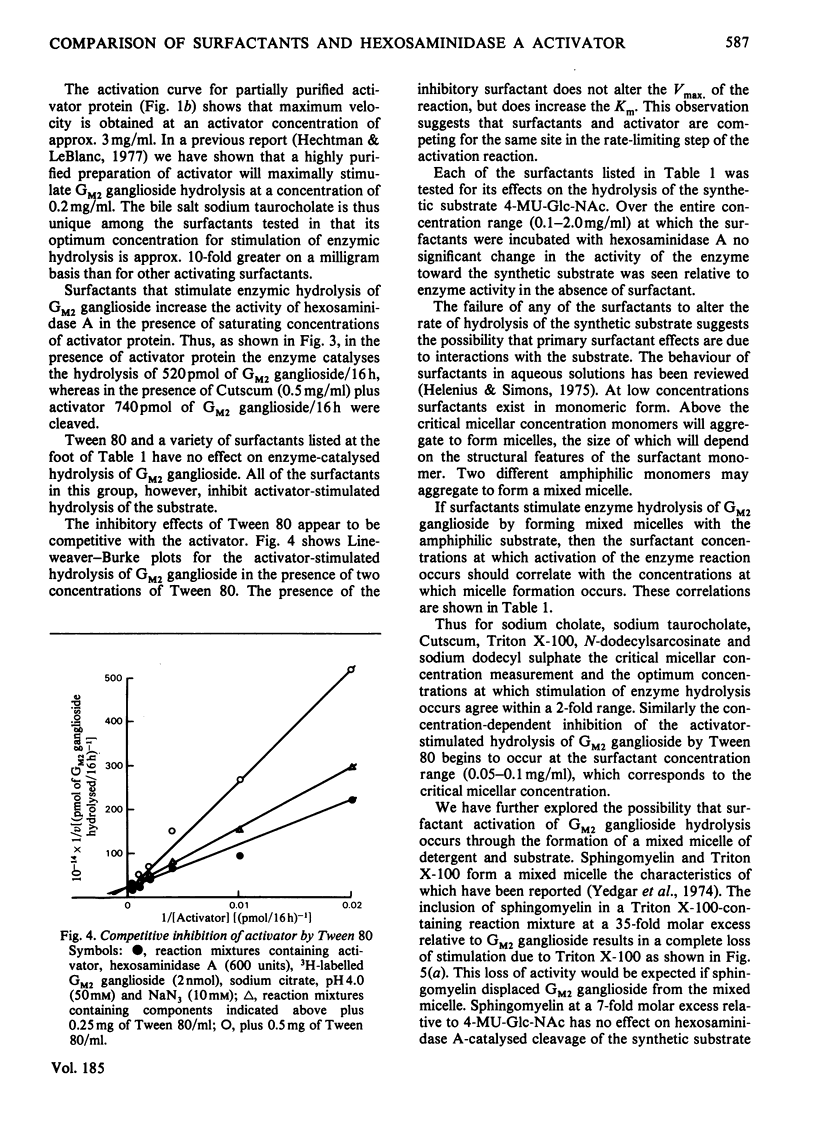
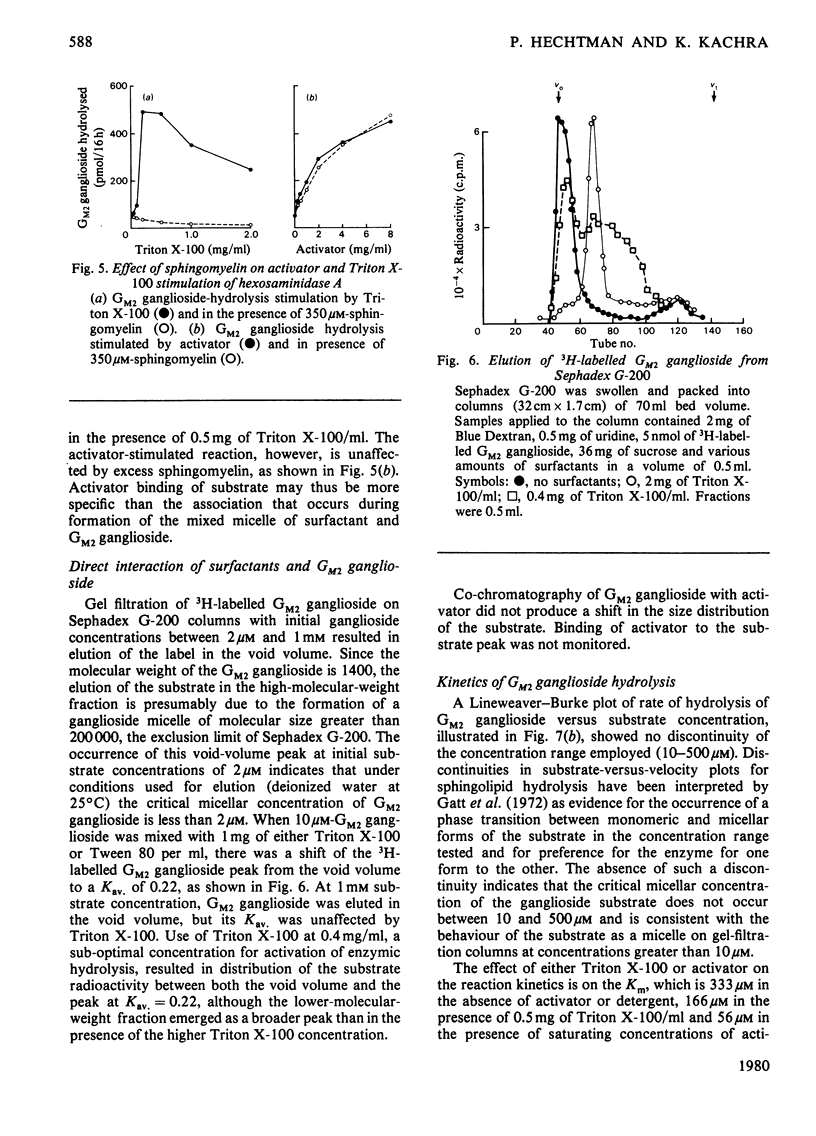
The effects of surfactants on the human liver hexosaminidase A-catalysed hydrolysis of Gm2 ganglioside were assessed. Some non-ionic surfactants, including Triton X-100 and Cutscum, and some anionic surfactants, including sodium taurocholate, sodium dodecyl sulphate, phosphatidylinositol and N-dodecylsarcosinate, were able to replace the hexosaminidase A-activator protein [Hechtman (1977) Can. J. Biochem. 55, 315–324; Hechtman & Leblanc (1977) Biochem. J. 167, 693–701) and also stimulated the enzymic hydrolysis of substrate in the presence of saturating concentrations of activator. Other non-ionic surfactants, such as Tween 80, Brij 35 and Nonidet P40, and anionic surfactants, such as phosphatidylethanolamine, did not enhance enzymic hydrolysis of Gm2 ganglioside and inhibited hydrolysis in the presence of activator. The concentration of surfactants at which micelles form was determined by measurements of the minimum surface-tension values of reaction mixtures containing a series of concentrations of surfactant. In the case of Triton X-100, Cutscum, sodium taurocholate, N-dodecylsarcosinate and other surfactants the concentration range at which stimulation of enzymic activity occurs correlates well with the critical micellar concentration. None of the surfactants tested affected the rate of hexosaminidase A-catalysed hydrolysis of 4-methylumbelliferyl N-acetyl-β-d-glucopyranoside. Both activator and surfactants that stimulate hydrolysis of Gm2 ganglioside decrease the Km for Gm2 ganglioside. Inhibitory surfactants are competitive with the activator protein. Evidence for a direct interaction between surfactants and Gm2 ganglioside was obtained by comparing gel-filtration profiles of 3H-labelled GM2 ganglioside in the presence and absence of surfactants. The results are discussed in terms of a model wherein a mixed micelle of surfactant or activator and GM2 ganglioside is the preferred substrate for enzymic hydrolysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED K., SCHOLEFIELD P. G. Biochemical studies on 1-aminocyclopentane carboxylic acid. Can J Biochem Physiol. 1962 Aug;40:1101–1110. [PubMed] [Google Scholar]
- BRADY R. O., KANFER J., SHAPIRO D. THE METABOLISM OF GLUCOCEREBROSIDES. I. PURIFICATION AND PROPERTIES OF A GLUCOCEREBROSIDE-CLEAVING ENZYME FROM SPLEEN TISSUE. J Biol Chem. 1965 Jan;240:39–43. [PubMed] [Google Scholar]
- Bach G., Suzuki K. Heterogeneity of human hepatic H-acetyl-beta-D-hexosaminidose. A activity toward natural glycosphingolipid substrates. J Biol Chem. 1975 Feb 25;250(4):1328–1332. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fischer G., Jatzkewitz H. The activator of cerebroside sulphatase. Binding studies with enzyme and substrate demonstrating the detergent function of the activator protein. Biochim Biophys Acta. 1977 Apr 12;481(2):561–572. doi: 10.1016/0005-2744(77)90288-1. [DOI] [PubMed] [Google Scholar]
- Fischer G., Jatzkewitz H. The activator of cerebroside sulphatase. Purification from human liver and identification as a protein. Hoppe Seylers Z Physiol Chem. 1975 May;356(5):605–613. doi: 10.1515/bchm2.1975.356.1.605. [DOI] [PubMed] [Google Scholar]
- Formisano S., Johnson M. L., Lee G., Aloj S. M., Edelhoch H. Critical micelle concentrations of gangliosides. Biochemistry. 1979 Mar 20;18(6):1119–1124. doi: 10.1021/bi00573a028. [DOI] [PubMed] [Google Scholar]
- GAMMACK D. B. PHYSICOCHEMICAL PROPERTIES OF OX-BRAIN GANGLIOSIDES. Biochem J. 1963 Aug;88:373–383. doi: 10.1042/bj0880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GATT S. ENZYMIC HYDROLYSIS AND SYNTHESIS OF CERAMIDES. J Biol Chem. 1963 Sep;238:3131–3133. [PubMed] [Google Scholar]
- Hechtman P. Characterization of an activating factor required for hydrolysis of Gm2 ganglioside catalyzed by hexosaminidase A. Can J Biochem. 1977 Apr;55(4):315–324. doi: 10.1139/o77-044. [DOI] [PubMed] [Google Scholar]
- Hechtman P., LeBlanc D. Purification and properties of the hexosaminidase A-activating protein from human liver. Biochem J. 1977 Dec 1;167(3):693–701. doi: 10.1042/bj1670693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Gaucher's disease: deficiency of 'acid' -glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. W. Specificity of low molecular weight glycoprotein effector of lipid glycosidase. FEBS Lett. 1975 May 1;53(2):243–247. doi: 10.1016/0014-5793(75)80029-9. [DOI] [PubMed] [Google Scholar]
- Kanfer J. N., Young O. M., Shapiro D., Brady R. O. The metabolism of sphingomyelin. I. Purification and properties of a sphingomyelin-cleaving enzyme from rat liver tissue. J Biol Chem. 1966 Mar 10;241(5):1081–1084. [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., Wan C. C., Mazzotta M. Y., Li Y. T. Requirement of an activator for the hydrolysis of sphingoglycolipids by glycosidases of human liver. Carbohydr Res. 1974 May;34(1):189–193. doi: 10.1016/s0008-6215(00)80383-3. [DOI] [PubMed] [Google Scholar]
- Norden A. G., O'Brien J. S. Ganglioside GM1 beta-galactosidase: studies in human liver and brain. Arch Biochem Biophys. 1973 Nov;159(1):383–392. doi: 10.1016/0003-9861(73)90465-7. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Norden G. W., Miller A. L., Frost R. G., Kelly T. E. Ganglioside GM2 N-acetyl-beta-D-galactosaminidase and asialo GM2 (GA2) N-acetyl-beta-D-galactosaminidase; studies in human skin fibroblasts. Clin Genet. 1977 Mar;11(3):171–183. doi: 10.1111/j.1399-0004.1977.tb01296.x. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Tennant L., Veath M. L., Scott C. R., Bucknall W. E. Characterization of unusual hexosaminidase A (HEX A) deficient human mutants. Am J Hum Genet. 1978 Nov;30(6):602–608. [PMC free article] [PubMed] [Google Scholar]
- Peters S. P., Coyle P., Coffee C. J., Glew R. H. Purification and properties of a heat-stable glucocerebrosidase activating factor from control and Gaucher spleen. J Biol Chem. 1977 Jan 25;252(2):563–573. [PubMed] [Google Scholar]
- Peters S. P., Coyle P., Glew R. H. Differentiation of beta-glucocerebrosidase from beta-glucosidase in human tissues using sodium taurocholate. Arch Biochem Biophys. 1976 Aug;175(2):569–582. doi: 10.1016/0003-9861(76)90547-6. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O. The catabolism of Tay-Sachs ganglioside in rat brain lysosomes. J Biol Chem. 1972 Dec 10;247(23):7570–7575. [PubMed] [Google Scholar]
- Yedgar S., Barenholz Y., Cooper V. G. Molecular weight, shape and structure of mixed micelles of Triton X-100 and sphingomyelin. Biochim Biophys Acta. 1974 Aug 21;363(1):98–111. doi: 10.1016/0005-2736(74)90009-1. [DOI] [PubMed] [Google Scholar]


