Abstract
Background and Objectives
Apraxia is a frequently observed symptom in Alzheimer disease (AD), but the causal pathomechanism underlying this dysfunction is not well understood. Previous studies have demonstrated associations between various cognitive dysfunctions in AD and cortical tau deposition in specific brain areas, suggesting a causal relationship. Thus, we hypothesized that specific regional patterns of tau pathology in praxis-related brain regions may be associated with apraxic deficits in AD. For this purpose, we performed PET imaging with the second-generation tau-PET tracer [18F]PI-2620 in a well-defined group of patients with AD (N = 33) who had been systematically assessed for apraxia.
Methods
Patients with a biomarker-confirmed diagnosis of AD were recruited in addition to a sample of cognitively unimpaired (CU1) control participants. Both groups underwent apraxia assessments with the Dementia Apraxia Screening Test. In addition, PET imaging with [18F]PI-2620 was performed to assess tau pathology in the patients with AD. To specifically investigate the association of apraxia severity with regional tau pathology, we compared the PET data from this group with an independent sample of amyloid-negative cognitively intact participants (CU2) by generation of z-score deviation maps and submitted these maps to a voxel-based multiple regression analysis.
Results
A total of 120 participants (39% female) with a mean age of 67.9 (9.2) years were included in the study (AD = 33; CU1; N = 33; CU2; N = 54). We identified a significant correlation between circumscribed clusters of tau aggregation in praxis-related brain regions (including parietal (angular gyrus), temporal, and occipital regions) and severity of apraxia in patients with AD. By contrast, no significant correlations between tau tracer uptake in primary motor cortex or subcortical brain regions and apraxia were observed.
Discussion
These results suggest that tau deposition in specific cortical praxis-related brain regions may induce local neuronal dysfunction leading to a dose-dependent functional decline in praxis performance in AD. The awareness of this relationship could further refine a differentiated individual diagnostic characterization and classification of patients with AD.
Introduction
Clinically, Alzheimer disease (AD) is commonly strongly associated with typical cognitive deficits such as memory loss, language and orientation problems, and loss of executive functions, whereas motor deficits are usually not in the focus of clinical assessment. However, loss of higher order motor functions in particular is not uncommon in this disease. In particular, apraxia has been reported to be a core feature of AD.1 Generally, apraxia is defined as the “inability to perform specific and predefined actions or to execute learned and purposeful movements, independent of sensory, motor, and (other) cognitive deficits.”2 In mild stages of dementia, the pattern of apraxic deficits can even help to distinguish the different variants of frontotemporal lobar degeneration and AD.3,4 Apraxia can be considered to represent a highly relevant disability because it can lead to impairment in activities of daily living and, thus, contribute to increasing care needs and loss of patient independence. Despite the high prevalence and clinical relevance of apraxia in AD, its pathomechanisms remain largely unresolved.
Apraxia is typically not considered to be a disorder of the core motor system, which includes the primary motor cortex, basal ganglia, and cerebellum (i.e., not to be a consequence of basic motor deficits such as paresis, ataxia, rigidity, or tremor). Instead, it is believed to result from a higher order motor dysfunction of specific parietofrontal praxis networks that constitute the praxis system or from a deficient interaction between the praxis networks and (praxis-related) cognitive networks.5,6 Apraxia is therefore often considered to be a higher order motor-cognitive disorder.
Current disease concepts of apraxia rely heavily on evidence derived from studies in stroke patients. The underlying neuropathologies are not comparable (i.e., ischemic injury in stroke vs protein aggregation pathologies in AD, involving amyloid plaques and tau tangles leading to neurodegenerative damage). Apraxia in AD potentially results from an insidious and neurodegenerative process, leading to a gradual loss of function. In addition, AD pathology is typically bilateral and does not follow vascular territories, unlike stroke. Finally, it seems important to specifically analyze the molecular basis of apraxia in AD to improve our understanding of the underlying pathomechanisms and to possibly open a different perspective on apraxia in general.
Previous studies in AD suggest that tau pathology in particular impairs neuronal function in the brain in a region-specific manner7 Hyperphoshorylated tau proteins dissolve from the microtubuli, aggregate intraneuronally in the form of neurofibrils, and are believed to contribute to local neuronal dysfunction. Modern in vivo molecular imaging techniques using PET can noninvasively detect tau aggregations in the human brain.8 These methods may therefore be able to establish a specific link between regional AD-specific neuropathology and the resulting symptomatic deficits. In a recent study, we have already shown that increased tau deposition occurs also in higher motor regions of patients with biomarker-verified AD pathology.9 Analyzing tau-PET scans from the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort, we investigated how tau pathology in cytoarchitectonically mapped regions of the motor network varies across the clinical spectrum of AD. We were able to demonstrate significant tau pathology in predominantly higher motor regions in AD (e.g., supplementary motor area, superior parietal lobe, angular gyrus, and dorsal premotor cortex). Based on these findings, we hypothesized that tau pathology in praxis-related brain motor networks may contribute to apraxic deficits in AD.9 We also suggested that the spatial heterogeneity of AD pathology may contribute to the variability of apraxia severity in AD.
To test these hypotheses, we systematically assessed apraxia in a well-defined group of patients with AD and correlated the results with data derived from tau-PET imaging in these individuals.
We aimed to increase our knowledge of the mechanisms underlying apraxic deficits in AD at the molecular level and thus contribute to a better pathophysiologic understanding that will also allow more sound diagnostic classification of this severe dysfunction.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the ethics committee of the Medical Faculty at University of Cologne and by the Federal Office for Radiation Protection, Germany. Written informed consent was obtained from all participants before the study.
Participants
We recruited a group of biomarker-confirmed patients with AD, who had been thoroughly characterized by neuropsychological assessment (Consortium to Establish a Registry for Alzheimer's Disease; CERAD-PLUS) including an apraxia assessment with the Dementia Apraxia Screening Test (DATE).4 Patients with AD (N = 34) were referred from the Center for Memory Disorders of the University Hospital Cologne. Patients were recruited based on the following inclusion criteria: (1) older than 50 years, (2) symptoms of cognitive impairment as assessed by the Mini-Mental State Examination (MMSE), (3) biomarker-confirmed amyloid and tau pathology (i.e., positive amyloid and positive tau-PET scan [as defined by visual read] or pathologically decreased CSF β-amyloid [Aβ] 42 levels or pathologically increased CSF phosphorylated tau levels according to recently proposed guidelines),10-14 (4) ability to provide informed consent, (5) no medical radiation exposure of >60 mSv in the past 10 years, (6) no evidence of other forms of dementia than AD, and (7) no sign of neurologic or psychiatric disorder potentially responsible for cognitive decline or motor deficits (e.g., Parkinson disease). Patients who did not fulfill these criteria were excluded from potential study participation.
Two samples of cognitively unimpaired individuals were used in analyses described below (CU1 and CU2). Cognitive unimpaired status was determined based on the Clinical Dementia Rating (CDR), amyloid negativity, and/or detailed neuropsychological testing. The first CU sample was recruited at the University Hospital Cologne and underwent detailed neuropsychological testing to ensure that their cognitive status was within age-related norms. In addition, the DATE was administered to compute mean and SD z-scores for the DATE measuring praxis function (CU1). These individuals were included in the study if they (1) did not report subjective memory decline, (2) were free of any psychiatric or neurologic disease/symptoms, (3) had an MMSE ≥27, and (4) were not taking any medication affecting the CNS. Participants were excluded if they did not fulfill these criteria.
The second sample of CU participants (CU2) underwent static imaging with [18F]PI-2620 only and were selected from the Health & Aging Brain Study—Health Disparities (HABS-HD)15 study population. Participants were included if they (1) did not report subjective memory decline, (2) were amyloid negative on 18F-florbetaben based on visual read, and (3) had a CDR of 0. This group was used as a CU control sample to generate mean and SD images to compute z-score deviation maps for each patient with AD (for details, see imaging data before processing).
Determination of CSF/PET Biomarker Status
CSF concentration of Aβ42, Aβ40, p-tau, and total tau was quantified on the Elecsys assay (Roche) or Lumipulse G1200 platform (Fujirebio). The following thresholds were used to determine positivity on Aβ42, Aβ40, and p-tau levels, respectively: Fujirebio; Aβ1-42 (LQ): <629; Aβ1-42/1–40 (Quotient): >0.095; tau protein (LQ): >452; phospho-tau protein (LQ): >61. Roche; Aβ1-42 (Roche): <1,030 pg/mL; tau (Roche): ≥300 pg/mL; phospho-tau (Roche): ≥27 pg/mL; and pTau/Aβ1-42 (Roche): ≥0.0230. Determination of biomarker positivity indicative of AD pathophysiologic change in CSF was prioritized in the following order: (1) pathologic profile on Aβ42, Aβ40 and p-tau; (2) tau/Aβ1-42 ratio; and (3) pathologic profile on Aβ42 and Aβ40. CSF was obtained before the PET scan in all patients who received a lumbar puncture. The median difference between the time of CSF collection and PET scan was 250 days (SD = 347.8).
If only amyloid or tau-PET was available, positivity was determined based on visual read using recently published guidelines.16,17
Neuropsychological Assessment
The patients' overall cognitive status was assessed using the MMSE, a test that evaluates impairments in general cognitive functions including orientation, attention, working memory, language, and delayed recall.18 In addition, patients underwent neuropsychological testing using CERAD-PLUS, which includes verbal fluency, Boston naming test, MMSE, Word list learning, Rey figures, Word Free Recall, Word Recognition, Rey figure recall, Trail Making Test A/B, and phonetic fluency. Moreover, data from the DATE, a screening tool specifically designed to assess apraxia in individuals with dementia,4 were acquired. It assesses aspects of gesture imitation and recognition, pantomime and tool use, as well as aspects of buccofacial emblems and word imitation. The AD sample and CU1 sample performed the DATE.
PET
PET scans for the AD cohort were performed at the Department of Nuclear Medicine, University Hospital Cologne, Germany, with a Siemens Biograph mCT Flow 128 Edge scanner (Siemens, Knoxville, TN). All PET scans were iteratively reconstructed using a 3-D OSEM algorithm of 4 iterations and 12 subsets, postsmoothed by a Gaussian filter of 5 mm full width at half maximum (FWHM).
Patients were scanned for 30 minutes in listmode, 45 minutes after intravenous injection of approximately 185 MBq of [18F]PI-2620 (field of view: 500 mm; gap = 1.5 mm, slice thickness = 3 mm) following recommended procedures for tau-PET imaging with [18F]PI-2620.19 The PET images were reconstructed on a 128 × 128 matrix (3.18 × 3.18, slice thickness = 3 mm).
PET scans of the cohort of cognitively unimpaired individuals (CU2; N = 54) were acquired on a Siemens Biograph Vision 450 PET/CT scanner.15 All participants were injected with a 10.0 mCi (±10%) bolus of [18F]PI-2620 and scanned 45–75 minutes after injection. The PET images were reconstructed in a 440 reconstruction matrix with a zoom of 2, which results in 0.825 mm pixel (slice thickness = 1.64557 mm).
Imaging Data Before Processing
All PET data were preprocessed with Statistical Parametric Modeling 12 (SPM12, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College London). All PET images were aligned to the anterior-posterior commissure, coregistered to their corresponding MRI image, and spatially normalized to the tissue probability map implemented in SPM12. To create standardized uptake value ratios (SUVRs), individual images were normalized using the nonspecific binding of the vermis as the reference.9 Finally, all images were smoothed with an 8-mm FWHM Gaussian filter. Despite differences in scanner acquisition parameters and subsequent differences in image reconstruction, standardization of preprocessing steps similar to the ones used in the ADNI PET Core20 ensured comparability.
Imaging Data After Processing
All SUVR images from the CU2 sample were averaged and both SD and mean images were generated using Imcalc in SPM12. Mean and SD images were used to calculate z-score deviation maps for each individual AD SUVR scan to create patient-specific AD-related tau patterns.8 These images were then submitted for further analysis.
Data Analysis
Demographic variables were analyzed using analysis of variance and independent samples t tests for continuous data and χ2 tests for frequency variables. Significance was set at p < 0.05.
Behavioral Analysis
To dissociate age-related differences in praxis function from AD-specific apraxia, we calculated mean and SD values in the group of age-matched CU individuals (CU1) and calculated z-scores for each patient using the following equation:
| (1) |
Resulting z-scores were entered as an independent variable in the multiple regression analysis described below.
Imaging Analysis
Mean deviation maps to display the typical pattern of AD tau pathology were created in SPM12. Individual z-maps from the AD sample were averaged using the Imcalc function (mean(X)). The resulting average map, reflecting tauabnormalities in the entire patient population (see Results Figure 1A), was overlaid on the fs-average image provided in the computational anatomy toolbox (CAT12).21
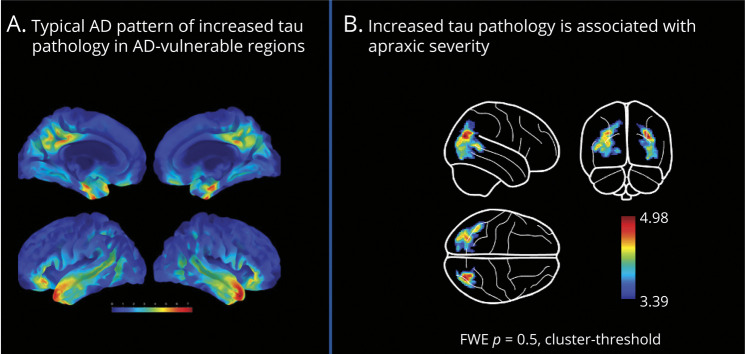
Figure 1. Regional Relationship of Tau-PET Clusters and Apraxia Severity.
(A) Mean increase in tau-PET in AD. (B) Multiple regression analysis between tau-PET and apraxia test scores (DATE: dementia apraxia screening test). Family-wise error (FWE) correction. Result maps are overlaid on a surface brain. Color scale indicates z-value ranges in the voxels.
Voxel-Based Regression Analysis
For voxel-based analysis, individual z-score deviation maps were subjected to multiple regression analysis in SPM12. A separate model was constructed to fit the following equation:
| (2) |
The statistical threshold was set at p = 0.05 with a family-wise error correction cluster threshold to account for multiple comparisons. The main contrast of interest was set to assess voxels of tau pathology associated with apraxia severity (DATE z-scores), controlling for potential age differences. The results were overlaid on the Hammersmith Brain Atlas22 to quantify the percentage overlap of each significant cluster with anatomically defined regions.
Sensitivity Analysis
To examine the potential contribution of disease severity toward the effect of tau pathology on apraxia, we extracted the z-values from the result map of the voxel-based regression analysis. These were submitted to a partial correlation controlling for the effect of MMSE. The significance threshold was set at p < 0.05.
Data Availability
Data used in preparation of this article are available upon request to the corresponding author.
Results
Demographic and Clinical Data
The total number of participants in this study was N = 120. Participant characteristics for the behavioral and imaging samples are summarized in Table 1. One outlier from the AD cohort was removed because of significant deviation (>3 SD) in the DATE resulting in a final sample of 33 patients with AD. In 27 patients (i.e., 81%) of the AD sample, biomarker positivity status was determined by CSF. Among these patients, 21 patients with AD had a pathologic Aβ and p-tau biomarker profile. In 5 patients, the ratio of amyloid and p-tau was abnormal, and 1 patient had a pathologic tau-PET scan, who displayed a significant tracer retention in bilateral medial temporal lobe regions and in the posterior cingulate. In the remainder of 6 patients, AD pathophysiologic change was determined based on both amyloid and tau-PET in 4 patients, whereas in 2 patients, only tau-PET was available. The spatial pattern of elevated tracer uptake in temporal and posterior cingulate regions in these 2 patients was indicative of underlying AD pathophysiologic change and in accordance with Braak stages III/IV.14,17
Table 1.
Demographics
| CU1 (N = 33) | CU2 (N = 54) | AD (n = 33) | |
| Behavioral controls (Cologne) | Imaging controls (HABS-HD) | Cologne | |
| Age | 67.7 (8.9) | 66.6 (7.9) | 69.5 (10.7) |
| Sex | 22/41 (53% male) | 30/54 (56% male) | 23/33 (69% male) |
| MMSE | 29 (IQR 2) | NA | 23.75 (4.03) |
| DATE | 57 (IQR 5) | NA | 46.09 (9.3) |
| CDR | NA | 0 | NA |
| PET-based AT classification | NA | 100% | 18.8% |
| Fluid-based AT classification | NA | 0% | 81.2% |
Abbreviations: A = amyloid status; CDR = Clinical Dementia Rating; DATE = Dementia Apraxia Screening Test; HABS-HD = Health & Aging Brain Study—Health Disparities; IQR = interquartile range; MMSE = Mini-Mental State Examination; T = tau status.
Demographic information for cognitively unimpaired individuals (CU1; behavioral sample; Cologne) and for the independent sample of cognitively unimpaired individuals (CU2; imaging controls; HABS-HD), from whom [18F]PI-2620 PET data were collected, as well as the sample of patients with Alzheimer disease. Median is displayed for non-normally distributed variables.
The 2 samples of cognitively unimpaired individuals consisted of 33 and 54 individuals in CU1 and CU2, respectively.
Patients with AD differed from the behavioral CU sample (CU1) in the MMSE and DATE scores (tDATE (36.17) = −5.83, p < 0.001; tMMSE(43.31) = 5.84, p < 0.001). The AD sample did not significantly differ from cognitively control samples (CU1 and CU2) in age (CU1 vs AD; tAGE (61) = −0.69, p = 0.491; CU2 vs AD; tAGE (72) = 1.61, p = 0.110) or in the distribution of male and female individuals within the group (χ2(1, N = 128) = 3.32, p = 0.19). Finally, age and sex also did not differ between the 2 control samples (i.e., CU1 and CU2; t(92) = −0.64, p = 0.52; χ2(1, N = 95) = 0.34, p = 0.85). Thus, both CU samples were sufficiently well matched to the AD sample.
Tau-PET Imaging
Figure 1A shows the mean deviation map across all patients with AD, which corresponds to the mean pattern of significant tau pathology in comparison with the group of cognitively unimpaired individuals (CU2). The spatial pattern of increased tau pathology included temporal regions, precuneus and cingulate cortex, as well as inferior and superior parietal and rostral middle frontal and lateral orbitofrontal regions, which is in correspondence to the spatial aggregation of tau in AD. No significant tau aggregation (i.e., z-score >2) was observed in basal ganglia in this analysis.
Voxel-Wise Analysis: Multiple Regression Analysis: DATE
Several clusters of increased tau pathology were associated with apraxic deficits as assessed by the DATE. A surface overlay of the result map is shown in Figure 1B. The associated regions and their percentage overlap with the Hammersmith Brain Atlas are listed in Table 2. In addition to lateral occipital regions, apraxic deficits were associated with increased tau pathology in posterior and superior temporal regions and angular gyrus. Of interest, these effects were observed in both hemispheres.
Table 2.
Regional Results From the Multiple Regression Analysis
| Hemisphere | Region label | Extent | Overlap with atlas region | t Value | MNI coordinates | ||
| x | y | z | |||||
| Main right hemispheric cluster | 1,311 | 5.0 | 34 | −76 | 28 | ||
| Lateral occipital lobe | 80% | ||||||
| Angular gyrus | 9% | ||||||
| Posterior temporal lobe | 6% | ||||||
| Superior parietal lobe | 4% | ||||||
| Main left hemispheric cluster | 2,389 | 4.6 | −26 | −70 | 34 | ||
| Lateral occipital lobe | 52% | ||||||
| Posterior temporal lobe | 19% | ||||||
| Superior parietal lobe | 12% | ||||||
| Angular gyrus | 10% | ||||||
| Lingual gyrus | 6% | ||||||
Abbreviations: DATE = Dementia Apraxia Screening Test; MNI = Montreal Neurological Institute.
Overlay of significant clusters with the Hammersmith Brain Atlas for the DATE voxel-wise multiple regression.
In both regression analyses, no correlation between tau tracer uptake and apraxia symptoms was observed in primary motor cortical regions, nor in the basal ganglia.
Sensitivity Analysis
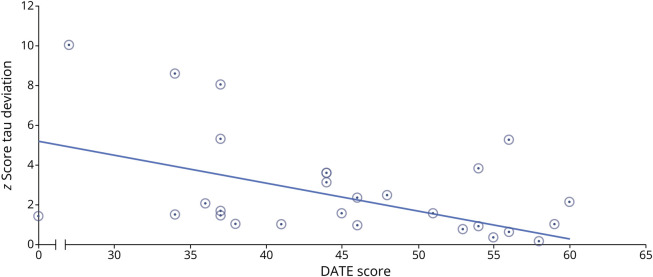
We extracted the individual z-values from the patient images using the result map displayed in Figure 1B. The partial correlation analysis revealed a significant correlation coefficient between DATE scores and the extracted z-values (r (MMSE) = −0.47, p = 0.007, corrected for variation in MMSE scores; Figure 2). The correlation of the extracted z-values and MMSE was not significant (r = −0.25, p = 0.145). These results suggest that our identified regions of tau deposition are distinctively associated with apraxic symptoms beyond the cognitive impairment that is attributable to the general disease severity.
Figure 2. Depiction of the Significant Z-Values in the Tau-PET Clusters and Apraxia Severity.
Results from partial correlation analysis. DATE = Dementia Apraxia Screening Test; R = Pearson correlation coefficient.
Discussion
In this study, we were able to demonstrate that apraxic deficits in AD are associated with specific cortical tau deposition. Some affected brain regions (i.e., angular gyrus) are well known for their involvement in praxis function.23 This suggests that tau deposition in these brain regions may induce local neuronal dysfunction, leading to a dose-dependent functional decline in praxis performance. However, we did not observe significant tau tracer retention in primary motor regions or the basal ganglia of patients with AD, supporting the notion that apraxia in AD represents a higher order motor dysfunction and is not caused by failure of the core primary motor system or subcortical involvement of the disease. In addition, our identified pattern of tau pathology was uniquely associated with apraxic deficits beyond variance caused by disease severity in the patient population. Although we have recently demonstrated the involvement of tau pathology in higher order motor regions in the context of AD severity,9 the current finding extend our previous work by showing a direct association with apraxia symptomatology.
The group comparison of tau tracer uptake between patients with AD and control subjects revealed a pattern of significant tau deposition in the brain, with maximum in diverse brain regions including precuneus, posterior cingulate temporal pole, entorhinal cortex, and inferior temporal regions, suggesting the group pattern of tau pathology extends beyond Braak III,24 which is consistent with a previously described typical tau pathology pattern in AD.8,25-27 These results indicate that the included AD population can be considered a representative sample.
Our findings are consistent with results from other studies, demonstrating high regional variance of tau deposition patterns in AD and symptom-dependent association with tau deposition in function-related brain regions.28-30 First, an association has been shown between the overall severity of cognitive impairment (as assessed by the MMSE) and tau burden, as measured in a large cortical meta-region of interest and in the lateral temporal cortex.28,29,31 Pronounced tau deposition in individual brain regions has been associated not only with overall cognitive impairment but also with specific decline of different cognitive functions associated with the respective brain areas.7,25 In particular, this refers to atypical variants of AD. Of interest, the clinical symptomatic variability of these atypical forms of AD (i.e., posterior cortical atrophy subtype, language problems in the logopenic aphasia subtype, or executive function problems in the frontal variant) has been shown to be reflected in corresponding variation in the distribution of tau aggregation as measured by tau-PET. Specifically, posterior/visual cortical regions were clearly affected in posterior cortical atrophy, asymmetric left hemispheric temporal accumulation was observed in logopenic aphasia, and pronounced frontal tau aggregation was observed in the frontal-executive variant, whereas bilateral tau tracer uptake in temporoparietal regions characterized typical AD suggesting that the regional distribution of tau-PET findings was strongly associated with the clinical and anatomical heterogeneity of AD.7,25,32
In this regard, it seems plausible that the expression of apraxia, representing a motor symptom of AD, was found to be associated with tau aggregation in the corresponding function-relevant cortical brain regions in this study. Tau aggregation in regions belonging to the praxis networks showed a consistent correlation with apraxia severity as measured by the DATE, including the posterior temporal lobes, angular gyrus, and higher order visual areas such as the lateral occipital cortex. Several of these regions have been previously demonstrated to be centrally involved in praxis functions. It was recently proposed that the lateral occipital cortex is crucial for action recognition, that is, an important praxis function.33 The angular gyrus itself is considered to be relevant even for more than 1 praxis processing stream.23 In addition, the posterior middle temporal gyrus/middle occipital gyrus is part of the praxis network23 and does partially overlap with the clusters identified in the temporal and occipital regions here. In summary, a potentially causal link between tau-induced neuronal dysfunction in the affected brain regions and observed apraxia symptoms appears in fact possible.
It is important to note that the detected clusters do not overlap with brain regions where tau deposition has been associated with overall severity of cognitive decline, such as the lateral temporal cortex.31
The severity of apraxia and specific regional tau pathology have not concomitantly been investigated in AD. However, in a previous study34 the tracer [18F]PI-2620 was used to distinguish subtypes of corticobasal syndrome (CBS). Higher tau levels, particularly in the dorsolateral prefrontal cortex, were observed in patients with amyloid-positive CBS compared with an amyloid-negative group. Patients were also assessed for apraxia using the DATE. Of interest, apraxia was found to be significantly more pronounced in amyloid-positive forms of CBS, supporting the notion that AD pathology is commonly associated with apraxic deficits. However, in CBS, no correlation between tau tracer retention and DATE apraxia scores was found. This may be due to the relatively small sample size and/or lower symptom variation in this group of CBS.
In the previous study, distinct tau deposition in the basal ganglia was observed in both patients with amyloid-positive and amyloid-negative CBS,34 which was not found in our AD patient sample. Our study included patients with typical AD symptoms,11 not CBS. The difference in tau deposition supports that AD with apraxia is a distinct subtype, not synonymous with amyloid-positive CBS. A strength of our study is the use of the [18F]PI-2620 tracer, which assesses tau aggregation in both the cortex and basal ganglia without nonspecific uptake, demonstrating motor deficits in AD can occur without involvement of the primary motor cortex or basal ganglia. In our group of patients, apraxia was a surprisingly common finding where severity of apraxia was not generally mild, but reached marked levels reflecting relevant disability. These findings underline that apraxia is a common symptom in AD that may require increased diagnostic attention and possibly therapeutic strategies.
This study has some limitations. First, the sample size of patients was still relatively small. However, all enrolled patients were deeply characterized and carefully matched to a set of controls. Nevertheless, replication of the results in larger samples of patients with AD may be warranted. Apraxia represents a multicomponential syndrome comprising different (impaired) cognitive processes.6 In addition to motor/cognitive deficits, these may include sensory processing such as impaired visual processing of spatial relations and/or structural features,35 as well as other cognitive dysfunctions, such as impaired auditory comprehension,36 which were not explicitly modeled here. In future studies, different components of the apraxia syndrome could be examined separately to see whether a differential tau-PET pattern might emerge from different components of the apraxia syndrome.
We sampled different CU control groups to evaluate behavioral age-related apraxia scores and age-related tau pathology in amyloid-negative individuals. Although these CU samples were matched by age and sex to the target population, the different samples of CU individuals may have introduced a bias, and our data may be less generalizable. However, the z-score deviation analysis approach provided us with the same scale for both tau pathology and apraxia score reducing the influence of the different samples.
Finally, the CU2 sample, which provided the basis for the z-score deviation analyses of the AD images, was scanned on a different scanner equipment compared with the AD cohort. Such differences can introduce a methodological bias, which we tried to reduce by applying a smoothing kernel and standard matrix normalization, following pre-preprocessing steps from ADNI to reduce differences in scanners.20 Again, a potential systematic scanner-dependent difference between the patient/control data sets would have applied to all studied patients with AD equally, rather not affecting z-score–based correlation analyses within the patient group. In addition, we collected a small sample of control subjects on the PET/CT scanner at the Cologne Hospital and compared the mean and SD maps with the CU2 sample and found no significant differences between the samples, suggesting that potential scanner-dependent differences are negligible.
In conclusion, we show a specific correlation of tau aggregation in praxis-related brain regions with the symptom of apraxia in a well-characterized group of patients with AD. The results suggest that tau in affected brain regions may contribute to local neuronal dysfunction and consequently to specific functional deficits. The here-observed relationship could be taken into account in the diagnostic workup and contribute to a better characterization and classification of patients with AD.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CBS
corticobasal syndrome
- CDR
Clinical Dementia Rating
- CERAD-PLUS
The Consortium to Establish a Registry for Alzheimer's Disease
- DATE
Dementia Apraxia Screening Test
- FWHM
full width at half maximum
- HABS-HD
Health & Aging Brain Study—Health Disparities
- MMSE
Mini-Mental State Examination
- SPM12
Statistical Parametric Modeling 12
- SUVR
standardized-uptake value ratio
Appendix. Authors
| Name | Location | Contribution |
| Gérard N. Bischof, PhD | Multimodal Neuroimaging Group, Department of Nuclear Medicine, Medical Faculty and University Hospital of Cologne, University of Cologne; Molecular Organization of the Brain, Institute for Neuroscience and Medicine II, Research Center Juelich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Elena Jaeger, MD | Multimodal Neuroimaging Group, Department of Nuclear Medicine, Medical Faculty and University Hospital of Cologne, University of Cologne, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Kathrin Giehl, PhD | Multimodal Neuroimaging Group, Department of Nuclear Medicine, Medical Faculty and University Hospital of Cologne, University of Cologne, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Frank Jessen, MD | Department of Psychiatry, Medical Faculty and University Hospital of Cologne, University of Cologne; German Center for Neurodegenerative Diseases, Bonn/Cologne, Germany | Major role in the acquisition of data |
| Oezguer A. Onur, MD | Department of Neurology, Medical Faculty and University Hospital of Cologne, University of Cologne, Germany | Major role in the acquisition of data |
| Sid O'Bryant, PhD | Institute for Translational Research, and Department of Family Medicine, Texas College of Osteopathic Medicine, University of North Texas Health Science Center, Fort Worth | Major role in the acquisition of data; study concept or design |
| Esra Kara, MD | Department of Neurology, Medical Faculty and University Hospital of Cologne, University of Cologne, Germany | Major role in the acquisition of data |
| Peter H. Weiss, MD | Department of Neurology, Medical Faculty and University Hospital of Cologne, University of Cologne, Cologne; Cognitive Neuroscience, Institute for Neuroscience and Medicine (INM-3), Research Center Juelich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Alexander Drzezga, MD | Multimodal Neuroimaging Group, Department of Nuclear Medicine, Medical Faculty and University Hospital of Cologne, University of Cologne; Molecular Organization of the Brain, Institute for Neuroscience and Medicine II, Research Center Juelich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
A. Drzezga, G.N. Bischof, E. Jaeger, and P.H. Weiss are funded by the Deutsche Forschungsgemeinschaft - Project-ID 431549029 - SFB 1451. G.N. Bischof received funding from Alzheimer Forschung Initiative e.V., Germany (AFI K1707). In addition, this study was supported by the German Research Foundation (DFG, DR 445/9-1). Precursor for the synthesis of the tracer [18F]PI-2620 was kindly provided by Life Molecular imaging. The [18F]PI-2620 control sample was kindly provided by Sid O'Bryant and were taken from the HABS-HD database (apps.unthsc.edu/itr/studies/habs). Research reported on this publication was supported by the National Institute on Aging of the NIH under Award Numbers R01AG054073, R01AG058533, P41EB015922, and U19AG078109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
A. Drzezga reports Research support: Siemens Healthineers, Life Molecular Imaging, GE Healthcare, AVID Radiopharmaceuticals, Sofie, Eisai, Novartis/AAA, Ariceum Therapeutics; Speaker Honoraria/Advisory Boards: Siemens Healthineers, Sanofi, GE Healthcare, Biogen, Novo Nordisk, Invicro, Novartis/AAA, Bayer Vital, Lilly; Stock: Siemens Healthineers, Lantheus Holding, Structured Therapeutics, Lilly; Patents: Patent for 18F-JK-PSMA-7 (Patent No.: EP3765097A1; Date of patent: January 20, 2021). The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Lesourd M, Le Gall D, Baumard J, Croisile B, Jarry C, Osiurak F. Apraxia and Alzheimer's disease: review and perspectives. Neuropsychol Rev. 2013;23(3):234-256. doi: 10.1007/s11065-013-9235-4 [DOI] [PubMed] [Google Scholar]
- 2.Osiurak F, Rossetti Y. Definition: limb apraxia. Cortex. 2017;93:228. doi: 10.1016/j.cortex.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 3.Johnen A, Tokaj A, Kirschner A, et al. Apraxia profile differentiates behavioural variant frontotemporal from Alzheimer's dementia in mild disease stages. J Neurol Neurosurg Psychiatry. 2015;86(7):809-815. doi: 10.1136/jnnp-2014-308773 [DOI] [PubMed] [Google Scholar]
- 4.Johnen A, Reul S, Wiendl H, Meuth SG, Duning T. Apraxia profiles: a single cognitive marker to discriminate all variants of frontotemporal lobar degeneration and Alzheimer's disease. Alzheimers Dement (Amst). 2018;10:363-371. doi: 10.1016/j.dadm.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt CC, Weiss PH. The cognitive neuroscience of apraxia. In: Della Sala S, editor. Encyclopedia of Behavioral Neuroscience, 2nd ed. Elsevier; 2022:668-677. [Google Scholar]
- 6.Schmidt CC, Achilles EIS, Fink GR, Weiss PH. Distinct cognitive components and their neural substrates underlying praxis and language deficits following left hemisphere stroke. Cortex. 2022;146:200-215. doi: 10.1016/j.cortex.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Dronse J, Fliessbach K, Bischof GN, et al. In vivo patterns of tau pathology, amyloid-β burden, and neuronal dysfunction in clinical variants of Alzheimer's disease. J Alzheimers Dis. 2017;55(2):465-471. doi: 10.3233/JAD-160316 [DOI] [PubMed] [Google Scholar]
- 8.Bischof GN, Jessen F, Fliessbach K, et al. Impact of tau and amyloid burden on glucose metabolism in Alzheimer's disease. Ann Clin Transl Neurol. 2016;3(12):934-939. doi: 10.1002/acn3.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischof GN, Jaeger E, Giehl K, Hönig MC, Weiss PH, Drzezga A. Affection of motor network regions by tau pathology across the Alzheimer's disease spectrum. eNeuro. 2024;11(1):ENEURO.0242-23.2023. doi: 10.1523/ENEURO.0242-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischof GN. Tau-PET bildgebung der demenzerkrankungen. Angewandte Nuklearmedizin. 2022;45(4):266-272. doi: 10.1055/a-1712-6020 [DOI] [Google Scholar]
- 11.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leuzy A, Smith R, Ossenkoppele R, et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 2020;77(8):955-965. doi: 10.1001/jamaneurol.2020.0989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer's Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109. doi: 10.1016/j.jalz.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 14.Hammes J, Bischof GN, Bohn KP, et al. One-stop shop: 18F-flortaucipir PET differentiates amyloid-positive and -negative forms of neurodegenerative diseases. J Nucl Med. 2021;62(2):240-246. doi: 10.2967/jnumed.120.244061 [DOI] [PubMed] [Google Scholar]
- 15.O'Bryant SE, Johnson LA, Barber RC, et al. The Health & Aging Brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimers Dement (Amst). 2021;13(1):e12202. doi: 10.1002/dad2.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischof GN, Bartenstein P, Barthel H, et al. Toward a universal readout for 18F-labeled amyloid tracers: the CAPTAINs study. J Nucl Med. 2021;62(7):999-1005. doi: 10.2967/jnumed.120.250290 [DOI] [PubMed] [Google Scholar]
- 17.Fleisher AS, Pontecorvo MJ, Devous MD, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77(7):829-839. doi: 10.1001/jamaneurol.2020.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 19.Mueller A, Bullich S, Barret O, et al. Tau PET imaging with 18F-PI-2620 in patients with Alzheimer disease and healthy controls: a first-in-humans study. J Nucl Med. 2020;61(6):911-919. doi: 10.2967/jnumed.119.236224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagust WJ, Landau SM, Koeppe RA, et al. The Alzheimer's disease neuroimaging initiative 2 PET core: 2015. Alzheimers Dement. 2015;11(7):757-771. doi: 10.1016/j.jalz.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaser C. Dahnke R. CAT: A Computational Anatomy Toolbox for SPM. Accessed January 20, 2024. https://neuro-jena.github.io/cat/
- 22.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19(4):224-247. doi: 10.1002/hbm.10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin M, Hermsdörfer J, Bohlhalter S, Weiss PH. [Networks involved in motor cognition: physiology and pathophysiology of apraxia]. Nervenarzt. 2017;88(8):858-865. doi: 10.1007/s00115-017-0370-7 [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960-969. doi: 10.1097/NEN.0b013e318232a379 [DOI] [PubMed] [Google Scholar]
- 25.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971-982. doi: 10.1016/j.neuron.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Benzinger TL, Su Y, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. JAMA Neurol. 2016;73(9):1070-1077. doi: 10.1001/jamaneurol.2016.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejanin A, Schonhaut DR, La Joie R, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain. 2017;140(12):3286-3300. doi: 10.1093/brain/awx243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8(338):338ra66. doi: 10.1126/scitranslmed.aaf2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel JW, Young AL, Oxtoby NP, et al. Four distinct trajectories of tau deposition identified in Alzheimer's disease. Nat Med. 2021;27(5):871-881. doi: 10.1038/s41591-021-01309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boccalini C, Ribaldi F, Hristovska I, et al. The impact of tau deposition and hypometabolism on cognitive impairment and longitudinal cognitive decline. Alzheimers Dement. 2024;20(1):221-233. doi: 10.1002/alz.13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips JS, Das SR, McMillan CT, et al. Tau PET imaging predicts cognition in atypical variants of Alzheimer's disease. Hum Brain Mapp. 2018;39(2):691-708. doi: 10.1002/hbm.23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Q, Lin D, Huang S, et al. Brain functional topology alteration in right lateral occipital cortex is associated with upper extremity motor recovery. Front Neurol. 2022;13:780966. doi: 10.3389/fneur.2022.780966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palleis C, Brendel M, Finze A, et al. Cortical [18 F]PI-2620 binding differentiates corticobasal syndrome subtypes. Mov Disord. 2021;36(9):2104-2115. doi: 10.1002/mds.28624 [DOI] [PubMed] [Google Scholar]
- 35.Milner D, Goodale M. The Visual Brain in Action. Oxford University Press; 2006. [Google Scholar]
- 36.Weiss PH, Ubben SD, Kaesberg S, et al. Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Struct Funct. 2016;221(1):563-576. doi: 10.1007/s00429-014-0925-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in preparation of this article are available upon request to the corresponding author.