Abstract
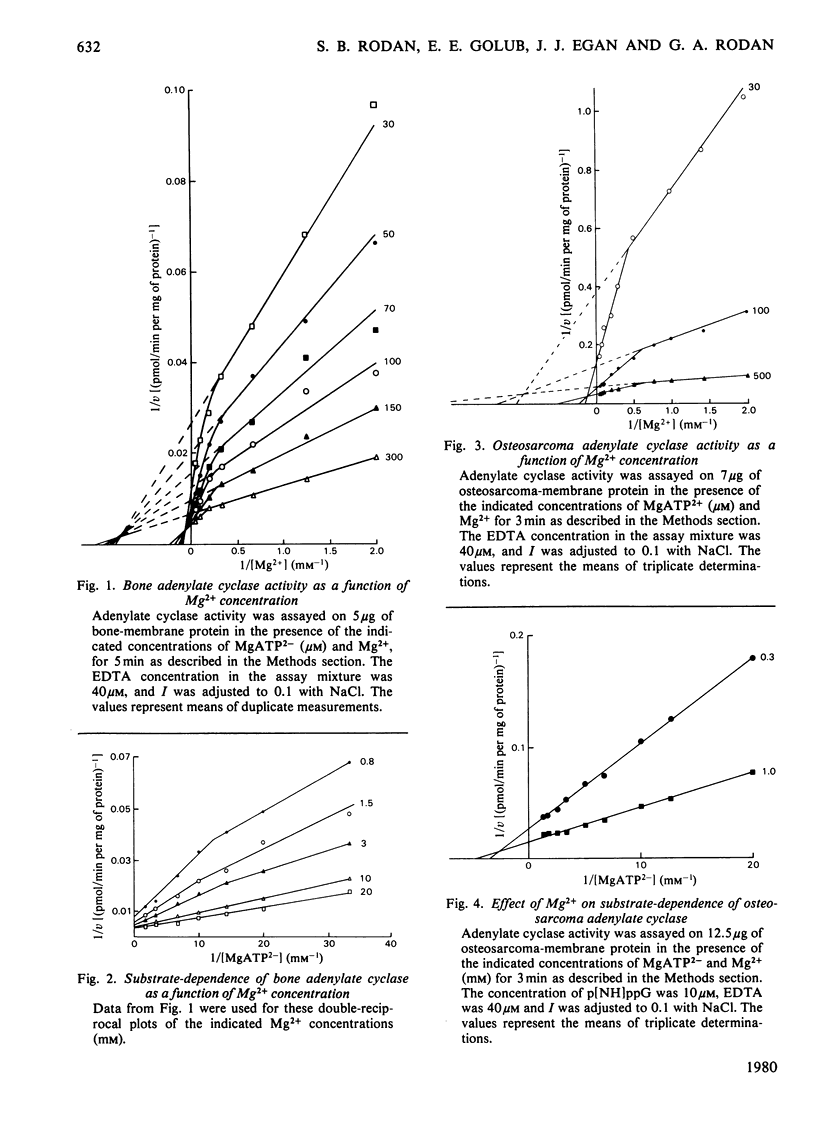
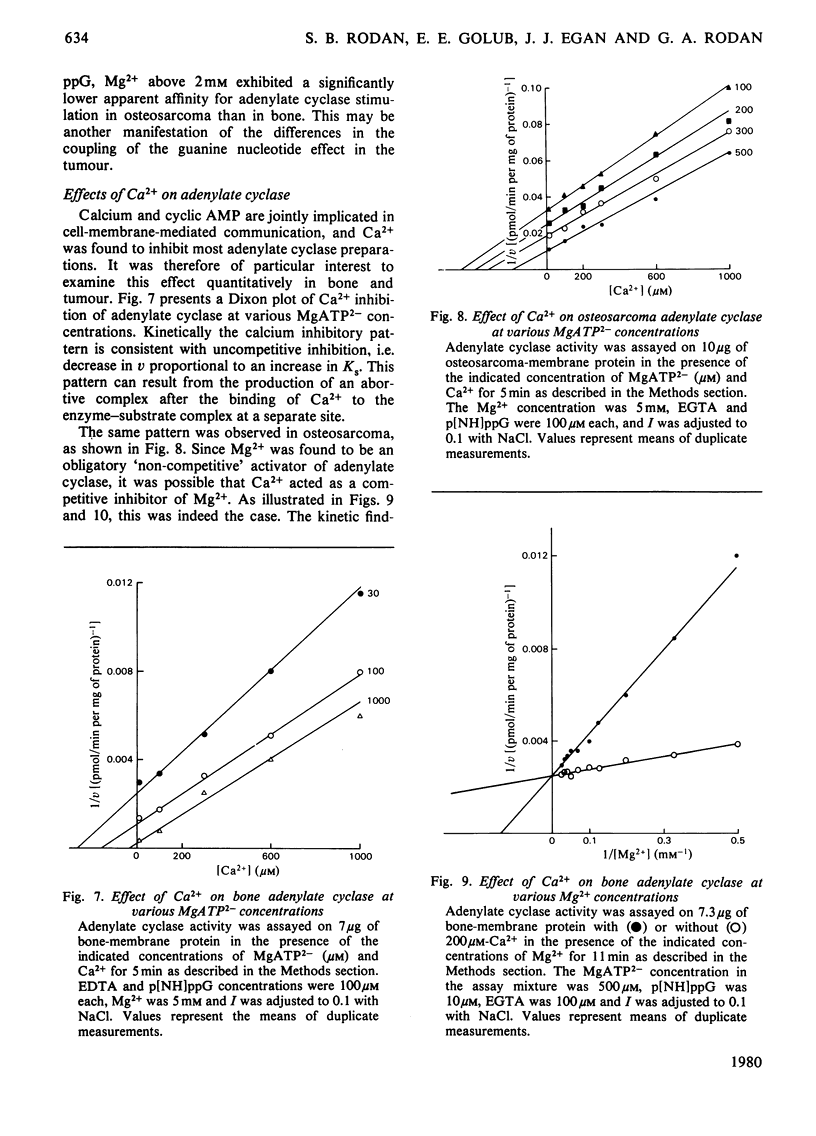
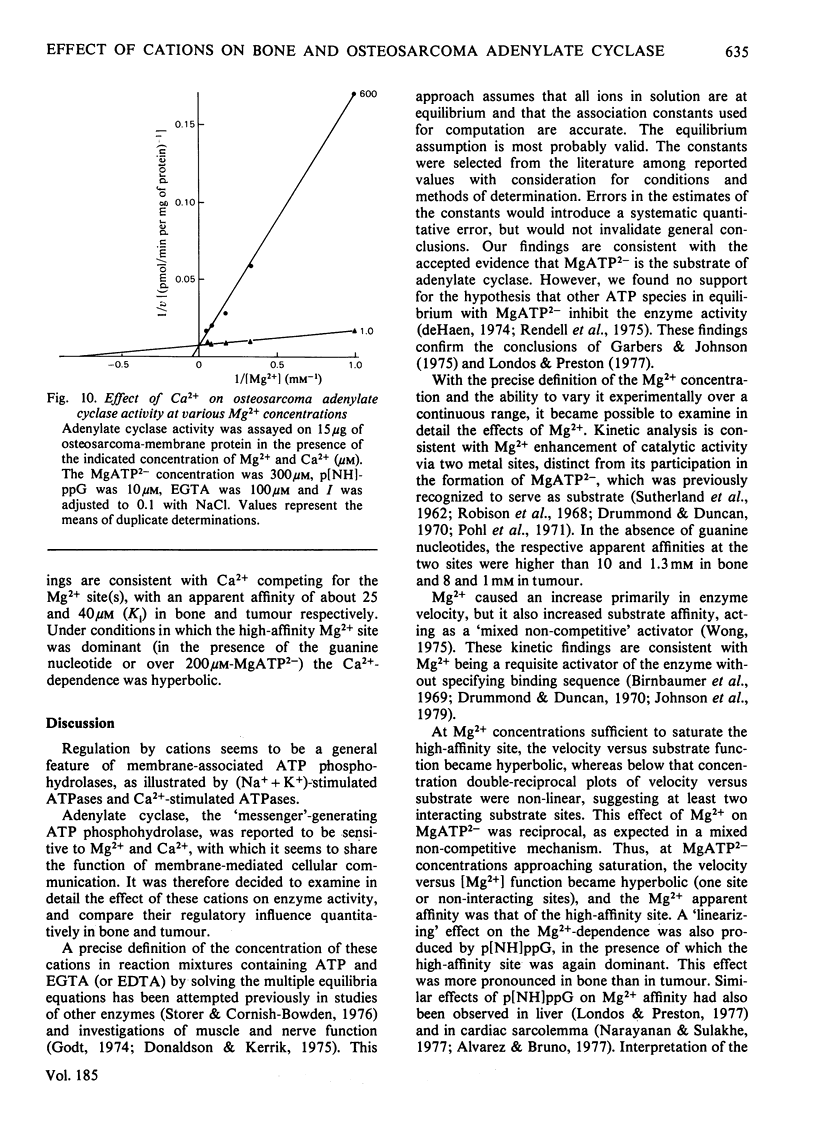
The effects of Mg2+ and Ca2+ on bone and osteosarcoma adenylate cyclase were investigated. The concentrations of the cations and other ionic species in the assay mixture were calculated by solving the simultaneous equations describing the relevant ionic interactions (multiple equilibria). We re-examined the effects of HATP3− and ATP4− on enzyme activity and found that (i) the concentration of the minor ATP species is less than 1% of that of MgATP2−, and their ratio to MgATP2− is constant if Mg2+ and H+ concentrations are unchanged; (ii) Mg2+ addition decreased the ratio of the minor species to MgATP2− and increased the enzyme activity, but no meaningful kinetic model could attribute this effect of HATP3− or ATP4−. On the other hand, kinetic analysis of Mg2+ effects showed: (i) stimulation via two metal sites, separate from the catalytic (MgATP2−) site, with apparent Km values of approximately 1 and 8mm; (ii) that the low affinity increased towards the higher one when the enzyme activity rose as a result of increased substrate or guanine nucleotide concentrations, this effect being less pronounced in tumour; (iii) conversely, that two apparent affinities for MgATP2− merged into one at high Mg2+ concentration; (iv) kinetically, that this relationship is of the mixed con-competitive type, which is consistent with a role for Mg2+ as a requisite activator, and binding occurring in non-ordered sequence. Analysis of the Ca2+ effects showed: (i) competition with Mg2+ at the metal site (Ki 20μm for bone and 40μm for tumour); (ii) that relative to the substrate the inhibition was uncompetitive, i.e. velocity decreased and affinity increased proportionally, which is consistent with Ca2+ binding after substrate binding. These findings support the existence of interacting enzyme complexes, losing co-operativity at increased enzyme activity. They also indicate a potential physiological role for Ca2+ in enzyme regulation and point to quantitative differences between bone and tumour with regard to these properties.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Alvarez R., Bruno J. J. Activation of cardiac adenylate cyclase: horminal modification of the magnesium ion requirement. Proc Natl Acad Sci U S A. 1977 Jan;74(1):92–95. doi: 10.1073/pnas.74.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Blume A. J., Foster C. J. Mouse neuroblastoma cell adenylate cyclase: regulation by 2-chloroadenosine, prostaglandin E1 and the cations Mg2+, Ca2+ and Mn2+. J Neurochem. 1976 Feb;26(2):305–311. doi: 10.1111/j.1471-4159.1976.tb04481.x. [DOI] [PubMed] [Google Scholar]
- Briggs F. N., Fleishman M. Calcium binding by particle-free supernatants of homogenates of skeletal muscle. J Gen Physiol. 1965 Sep;49(1):131–149. [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. I., Duncan L. Adenyl cyclase in cardiac tissue. J Biol Chem. 1970 Mar 10;245(5):976–983. [PubMed] [Google Scholar]
- Fogt E. J., Rechnitz G. A. Thermodynamics of ATP hydrolysis from membrane electrode measurements of metal-ion ATP and ADP complexation. Arch Biochem Biophys. 1974 Dec;165(2):604–614. doi: 10.1016/0003-9861(74)90288-4. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Johnson R. A. Metal and metal-ATP interactions with brain and cardiac adenylate cyclases. J Biol Chem. 1975 Nov 10;250(21):8449–8456. [PubMed] [Google Scholar]
- Glynn P., Cooper D. M., Schulster D. The regulation of adenylate cyclase of the adrenal cortex. Mol Cell Endocrinol. 1979 Feb;13(2):99–121. doi: 10.1016/0303-7207(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMES G. G., LEVISON S. A. A KINETIC INVESTIGATION OF THE INTERACTION OF ADENOSINE-5'-TRIPHOSPHATE WITH DIVALENT METAL IONS. Biochemistry. 1964 Oct;3:1504–1506. doi: 10.1021/bi00898a019. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Saur W., Jakobs K. H. Effects of prostaglandin E1 and adenosine on metal and metal-ATP kinetics of platelet adenylate cyclase. J Biol Chem. 1979 Feb 25;254(4):1094–1101. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Limbird L. E., Hickey A. R., Lefkowitz R. J. Unique uncoupling of the frog erythrocyte adenylate cyclase system by manganese. Loss of hormone and guanine nucleotide-sensitive enzyme activities without loss of nucleotide-sensitive, high affinity agonist binding. J Biol Chem. 1979 Apr 25;254(8):2677–2683. [PubMed] [Google Scholar]
- Londos C., Preston M. S. Activation of the hepatic adenylate cyclase system by divalent cations. J Biol Chem. 1977 Sep 10;252(17):5957–5961. [PubMed] [Google Scholar]
- Narayanan N., Sulakhe P. V. 5'-Guanylylimidodiphosphate-activated adenylate cyclase of cardiac sarcolemma displays higher affinity for magnesium ions. Mol Pharmacol. 1977 Nov;13(6):1033–1047. [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Perkins J. P. Adenyl cyclase. Adv Cyclic Nucleotide Res. 1973;3:1–64. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic data for the hydrolysis of adenosine triphosphate as a function of pH, Mg2+ ion concentration, and ionic strength. J Biol Chem. 1969 Jun 25;244(12):3330–3342. [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Jensen P., Lake W., Friedmann N., Goodman D. B. Cyclic nucleotides and cellular calcium metabolism. Adv Cyclic Nucleotide Res. 1975;5:375–394. [PubMed] [Google Scholar]
- Rechnitz G. A. Membrane electrode probes for biological systems. Science. 1975 Oct 17;190(4211):234–238. doi: 10.1126/science.1179205. [DOI] [PubMed] [Google Scholar]
- Rendell M., Salomon Y., Lin M. C., Rodbell M., Berman M. The hepatic adenylate cyclase system. III. A mathematical model for the steady state kinetics of catalysis and nucleotide regulation. J Biol Chem. 1975 Jun 10;250(11):4253–4260. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Rodan S. B., Egan J. J., Golub E. E., Rodan G. A. Comparison of bone and osteosarcoma adenylate cyclase. Partial purification of membranes and kinetic properties of enzyme. Biochem J. 1980 Mar 1;185(3):617–627. doi: 10.1042/bj1850617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Severson D. L., Drummond G. I., Sulakhe P. V. Adenylate cyclase in skeletal muscle. Kinetic properties and hormonal stimulation. J Biol Chem. 1972 May 10;247(9):2949–2958. [PubMed] [Google Scholar]
- Steer M. L., Levitzki A. The control of adenylate cyclase by calcium in turkey erythrocyte ghosts. J Biol Chem. 1975 Mar 25;250(6):2080–2084. [PubMed] [Google Scholar]
- Stolc V. Mechanism of regulation of adenylate cyclase activity in human polymorphonuclear leukocytes by calcium, guanosyl nucleotides, and positive effectors. J Biol Chem. 1977 Mar 25;252(6):1901–1907. [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Yamamoto T., Tonomura Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978 Jan;58(1):1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Lad P. M., Newby A. C., Yamamura H., Nicosia S., Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J Biol Chem. 1977 Sep 10;252(17):5947–5950. [PubMed] [Google Scholar]
- de Haën C. Adenylate cyclase. A new kinetic analysis of the effects of hormones and fluoride ion. J Biol Chem. 1974 May 10;249(9):2756–2762. [PubMed] [Google Scholar]


