Abstract
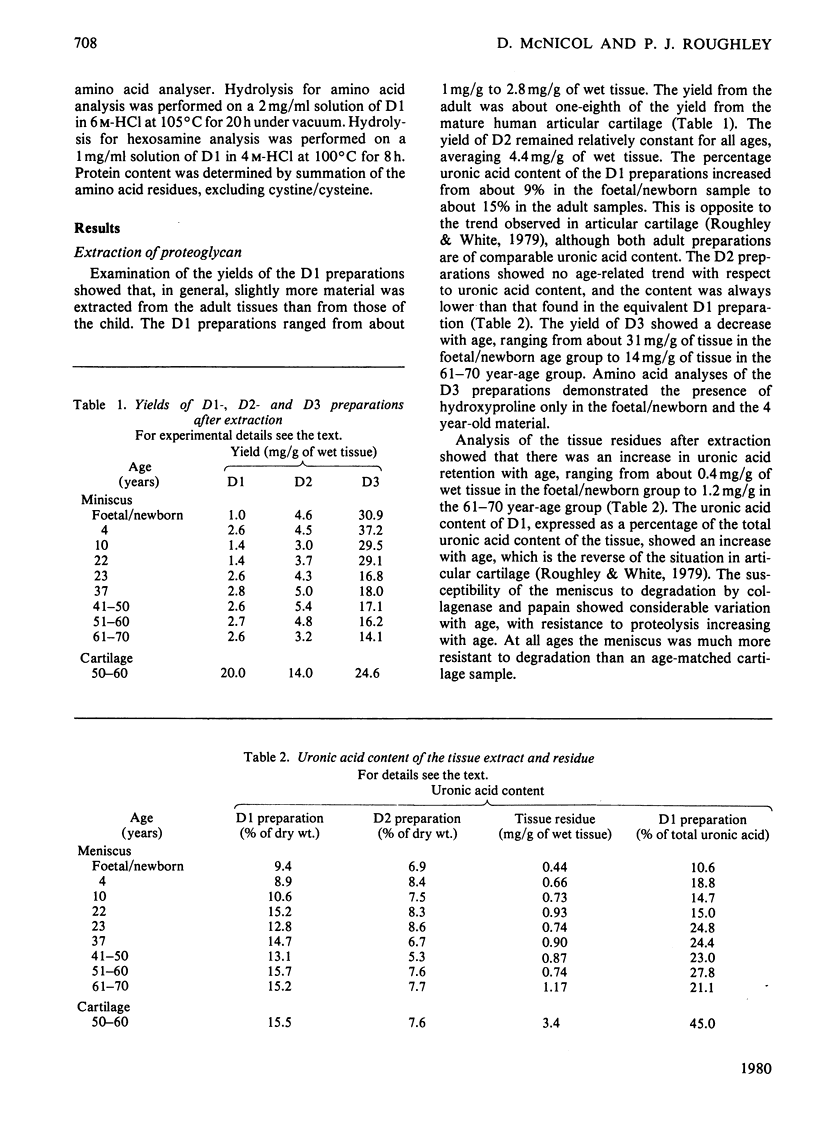
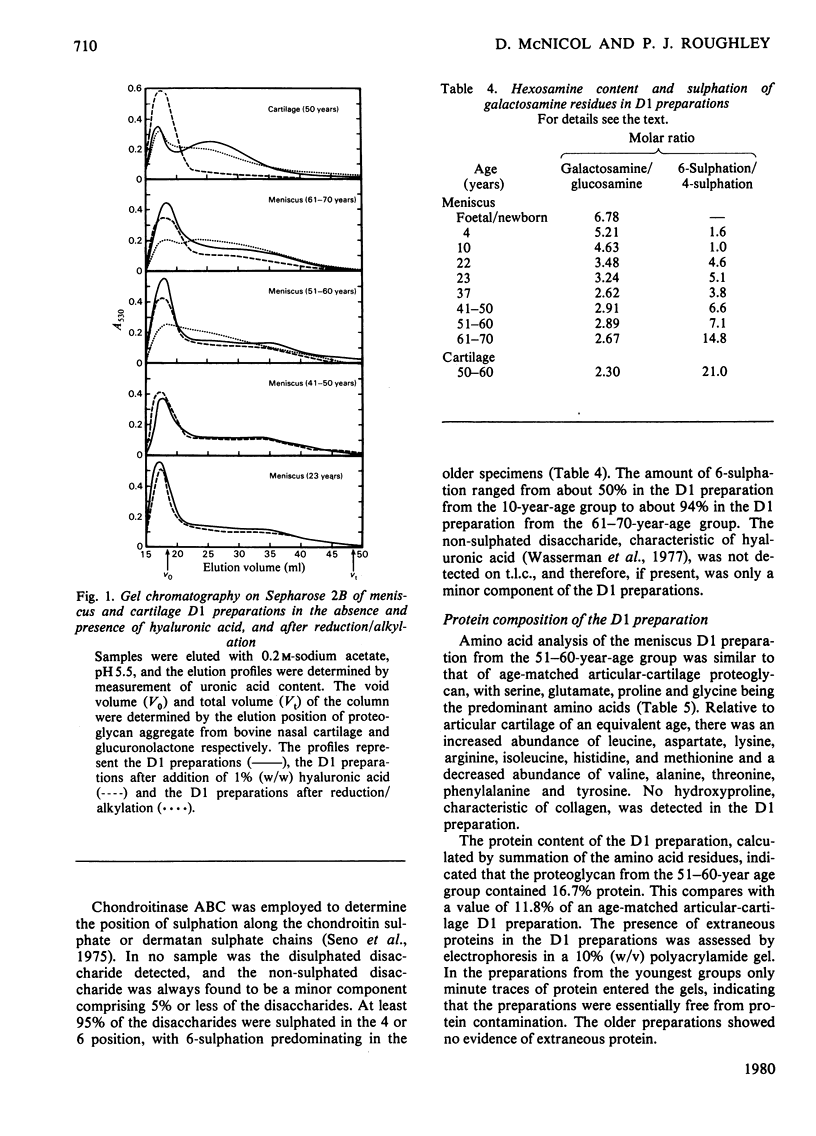
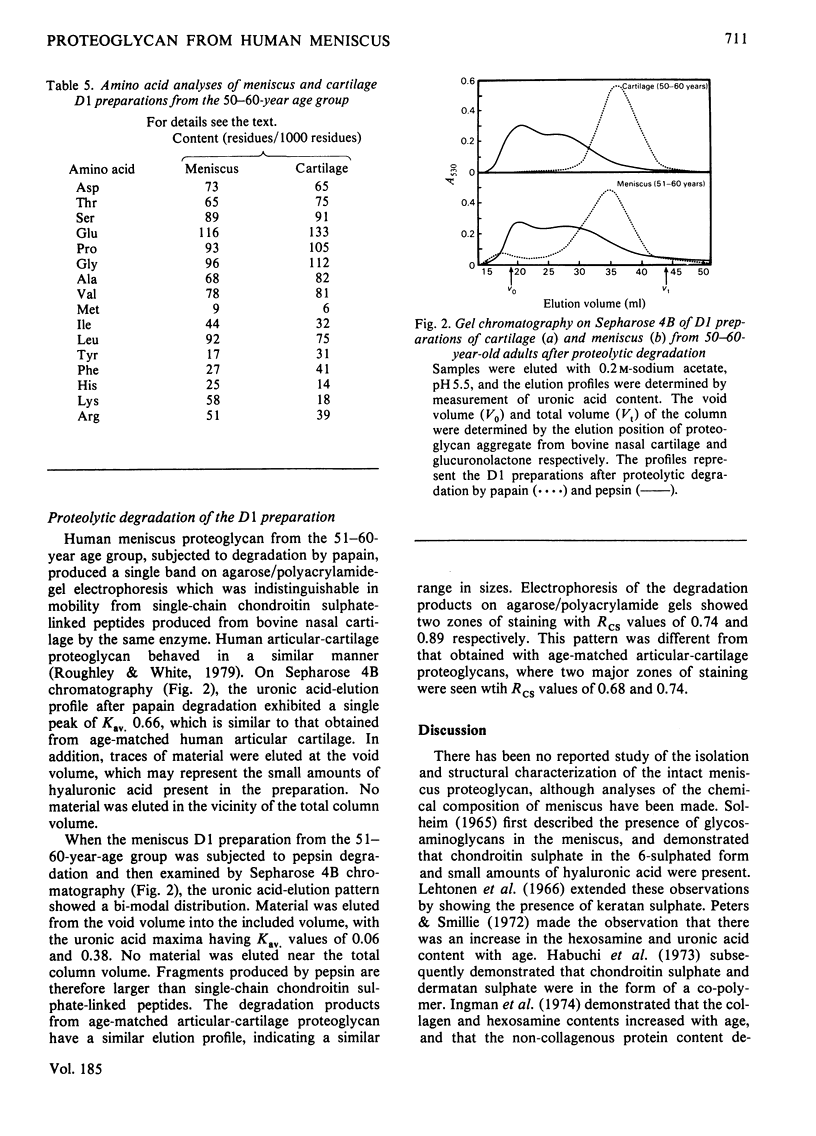
This study consists of (1) the extraction of proteoglycan from the human meniscus under dissociative conditions, (2) an investigation of the changes that occur in the abundance and structure of this proteoglycan with age and (3) a comparison of these findings with those for human articular-cartilage proteoglycan. Adult meniscus was found to possess proteoglycan molecules of similar size and glycosaminoglycan content to those present in cartilage, although tissue concentrations were considerably lower. In addition, age-related changes, with respect to the occurrence of keratan sulphate and the sulphation of chondroitin sulphate chains, were common to both tissues. The presence of aggregated proteoglycan was demonstrated, although specific interaction with hyaluronic acid was not conclusively shown biochemically. Differences were, however, noted in the structure of the proteoglycan between the two tissues: dermatan sulphate was found in the meniscus proteoglycan preparation and the core proteins exhibited some dissimilarities. A proteoglycan structure of this type would be compatible with its participation in meniscus elasticity, especially as the material is localized in a specific area.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Disorders of connective tissue function and the aging process: a synthesis and review of current concepts and findings. Mech Ageing Dev. 1976 Jul-Aug;5(4):305–314. doi: 10.1016/0047-6374(76)90030-0. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Sherblom A. P. Isolation and physical characterization of hyaluronic acid prepared from bovine nasal septum by cetylpyridinium chloride precipitation. J Biol Chem. 1977 Jan 25;252(2):420–426. [PubMed] [Google Scholar]
- Habuchi H., Yamagata T., Iwata H., Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J Biol Chem. 1973 Sep 10;248(17):6019–6028. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingman A. M., Ghosh P., Taylor T. K. Variation of collagenous and non-collagenous proteins of human knee joint menisci with age and degeneration. Gerontologia. 1974;20(4):212–223. doi: 10.1159/000212017. [DOI] [PubMed] [Google Scholar]
- Isemura M., Ikenaka T. Beta-Elimination and sulfite addition reaction of chondroitin sulfate peptidoglycan and the peptide structure of the linkage region. Biochim Biophys Acta. 1975 Nov 10;411(1):11–21. doi: 10.1016/0304-4165(75)90280-9. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Kettelkamp D. B., Jacobs A. W. Tibiofemoral contact area--determination and implications. J Bone Joint Surg Am. 1972 Mar;54(2):349–356. [PubMed] [Google Scholar]
- Koseki M., Kimura A., Tsurumi K. Micro determination of unsaturated disaccharide formed by the action of acidic glycosaminoglycan-endoeliminases. An application of the thiobarbituric acid method to the assay of D-gluco-4-enepyranosyluronic acid-containing disaccharides. J Biochem. 1978 Feb;83(2):553–558. doi: 10.1093/oxfordjournals.jbchem.a131943. [DOI] [PubMed] [Google Scholar]
- Krause W. R., Pope M. H., Johnson R. J., Wilder D. G. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976 Jul;58(5):599–604. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lehtonen A., Viljanto J., Kärkkäinen J. The mucopolysaccharides of herniated human intervertebral discs and semilunar cartilages. Acta Chir Scand. 1967;133(4):303–306. [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Muir H. The structure and metabolism of mucopolysaccharides (glycosaminoglycans) and the problem of the mucopolysaccharidoses. Am J Med. 1969 Nov;47(5):673–690. doi: 10.1016/0002-9343(69)90163-6. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Smillie I. S. Studies on the chemical composition of the menisci of the knee joint with special reference to the horizontal cleavage lesion. Clin Orthop Relat Res. 1972 Jul-Aug;86:245–252. doi: 10.1097/00003086-197207000-00037. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- Roughley P. J. A comparative study of the glycosaminoglycan-peptides obtained after degradation of cartilage proteoglycan by different proteinases, and their use in the characterization of different proteoglycans. Connect Tissue Res. 1978;6(3):145–153. doi: 10.3109/03008207809152624. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLHEIM K. THE GLYCOSAMINOGLYCANS OF HUMAN SEMILUNAR CARTILAGE. J Oslo City Hosp. 1965 Jun;15:127–132. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Seno N., Anno K., Yaegashi Y., Okuyama T. Microheterogeneity of chondroitin sulfates from various cartilages. Connect Tissue Res. 1975;3(1):87–96. doi: 10.3109/03008207509152345. [DOI] [PubMed] [Google Scholar]
- Wasserman L., Ber A., Allalouf D. Use of thin-layer chromatography in the separation of disaccharides resulting from digestion of chondroitin sulphates with chondroitinases. J Chromatogr. 1977 Jun 11;136(2):342–347. doi: 10.1016/s0021-9673(00)86291-3. [DOI] [PubMed] [Google Scholar]


