Abstract
Objective
Although quetiapine has been approved for use in schizophrenic patients, its individualized dosage regimen remains unclear, especially with respect to drug–drug interactions (DDIs). Thus, we investigated the potential DDIs and optimal initial dosage of quetiapine in schizophrenic patients based on population pharmacokinetics (PPK).
Methods
Ninety-six schizophrenic patients treated with quetiapine were included to establish the PPK model, which also includes coadministration of multiple drugs.
Results
It was found that the patient weights and fluvoxamine or duloxetine coadministration affected quetiapine clearance in schizophrenic patients. Without fluvoxamine or duloxetine coadministration, 16 and 12 mg/kg/day of quetiapine were recommended to schizophrenic patients whose weights were in the ranges of 40–50 and 50–120 kg, respectively. With fluvoxamine coadministration, 8 mg/kg/day of quetiapine was recommended to patients with weights in the range of 40–120 kg. With duloxetine coadministration, 8 mg/kg/day of quetiapine was recommended to patients with weights in the 40–120 kg range. With simultaneous coadministration of fluvoxamine and duloxetine, 4 mg/kg/day of quetiapine was recommended to patients with weights in the 40–120 kg range.
Conclusion
The present study was a pilot effort at investigating the potential DDIs and optimal initial dosage of quetiapine in schizophrenic patients based on PPK. The initial dosages of quetiapine administered to the patients were optimized according to the coadministration of fluvoxamine or duloxetine.
Keywords: optimal initial dosage, quetiapine, schizophrenic patient, fluvoxamine, duloxetine, drug–drug interactions
1 Introduction
Schizophrenia is a mental disease occurring in late adolescence and young adulthood; it is often accompanied by sensory, thinking, emotional, will-based, and behavioral disorders in combination with social or occupational defects and is considered to be one of the most serious mental diseases (Charlson et al., 2018; Jauhar et al., 2022). The clinical treatment of schizophrenia involves severe challenges because of its complex etiology, interlaced symptoms, and high recurrence rate, for which drug therapy remains the main mode of treatment at present (Li et al., 2023; Wang et al., 2024; Zhang et al., 2024a; Zhang et al., 2024b).
Quetiapine is a dibenzothiazepine derivative containing low-affinity dopamine D2 and serotonin 5-HT2A antagonist belonging to atypical antipsychotics (Cheer and Wagstaff, 2004; Hao et al., 2023); it has been approved for use in schizophrenia and is presently the most commonly prescribed antipsychotic medication among adults aged 20–64 years in almost 71% of the countries globally (Kasper and Muller-Spahn, 2000; Cheer and Wagstaff, 2004; Hojlund et al., 2021; Hao et al., 2023).
In terms of pharmacokinetics, quetiapine is mainly metabolized by CYP3A4, CYP2C19, and CYP2D6 (Bakken et al., 2012; Cabaleiro et al., 2015; Xu et al., 2016; Liu et al., 2021; Stauble et al., 2021; Rohail et al., 2023; Yau et al., 2023). When drug combinations are used clinically, especially when there is inhibition or induction of CYP3A4, CYP2C19, or CYP2D6, quetiapine could have significant variations in terms of clearance and drug concentration. Quetiapine has been reported to have many interactions, especially with drugs used against cardiovascular diseases (Siwek et al., 2020) and other drugs such as erythromycin (Li et al., 2005), clarithromycin (Schulz-Du Bois et al., 2008), aprepitant (Patel et al., 2017), lovastatin (Furst et al., 2002), as well as medicinal products and diet supplements containing herbal extracts or grapefruit (Cinderella et al., 2021). From a clinical perspective, low concentrations of quetiapine have been associated with reduced drug effects and poor psychiatric control, whereas high quetiapine concentrations may cause adverse reactions (Hao et al., 2023). Thus, the present study was aimed at investigating the potential drug–drug interactions (DDIs) and optimal initial dosage of quetiapine in schizophrenic patients based on population pharmacokinetics (PPK).
2 Methods
2.1 Information collection
Schizophrenic patients treated with quetiapine at the Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University between July 2020 and November 2023 were enrolled in this investigation, which was a single-center study. We assessed quetiapine concentrations for therapeutic drug monitoring (TDM) while also collecting the physiological and biochemical indexes of the patients as well as information regarding drug combinations. The present study was approved by the Research Ethics Committee of Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University.
2.2 Modeling
We constructed a PPK model using the non-linear mixed-effect modeling (NONMEM) approach using the apparent oral clearance (CL/F), apparent volume of distribution (V/F), and absorption rate constant (Ka) fixed at 1.46/h (Zhou et al., 2015) as the assessment parameters.
Equation 1 is the expression for the interindividual variability:
| (1) |
where Bi is the individual parameter, TV(B) is the typical individual parameter, and ηi indicates symmetrical distribution.
Equation 2 gives the expression for the random residual variability:
| (2) |
where Di is the observed concentration, Fi is the individual predicted concentration, and εn indicates symmetrical distribution.
Equation 3 shows the relationship of the pharmacokinetic parameters with weight:
| (3) |
where Hi is the ith individual parameter, Li is the ith individual weight, Lstd is the standard weight of 70 kg, and Hstd is the typical individual parameter. The variable N is the allometric coefficient, which is 0.75 for CL/F and 1 for V/F (Anderson and Holford, 2008).
Equations (4, 5) show the pharmacokinetic parameters for the continuous and categorical covariates, respectively:
| (4) |
| (5) |
where Oi is the individual parameter, TV(O) is the typical individual parameter, p is the parameter to be estimated, Zi is the covariate of the ith individual, and Zm is the population median for the covariate.
A stepwise method was used to analyze the covariates in the PPK model of quetiapine in schizophrenic patients. In this process, a decrease in the objective function value (OFV) by more than 3.84 (P< 0.05) was accepted as the inclusion standard and an increase in OFV by more than 6.63 (P< 0.01) was considered as the exclusion standard.
2.3 Model evaluation
The final model was evaluated through visualization, and the bootstrap method was used to compare the final model parameters.
2.4 Simulation
Monte Carlo simulations were conducted regarding the optimal quetiapine concentrations for schizophrenic patients given that the recommended therapeutic window for quetiapine was 100–500 ng/mL (Lin et al., 2024). It was found that the patient weight as well as fluvoxamine or duloxetine coadministration significantly impacted quetiapine clearance in the patients. Hence, based on the coadministration of fluvoxamine or duloxetine, four different conditions were simulated in the present study: schizophrenic patients without fluvoxamine or duloxetine coadministration, schizophrenia patients with fluvoxamine coadministration, schizophrenic patients with duloxetine coadministration, and schizophrenic patients administered both fluvoxamine and duloxetine. Each condition was simulated with 1,000 virtual schizophrenic patients under five weight groups (40, 60, 80, 100, and 120 kg) and eight dosage groups (1, 4, 8, 12, 16, 20, 24, and 28 mg/kg/day) each. The probability of achieving the target concentration was selected as the evaluation criterion, and the probability of exceeding the upper limit of the treatment window (500 ng/mL) over 1,000 simulated concentrations was deemed the safety evaluation measure.
3 Results
3.1 Patient information
Ninety-six schizophrenic patients treated with quetiapine (immediate-release tablets) and 154 quetiapine concentrations were included in this study to establish the PPK model; the patients included 52 men and 44 women of age 43.53 ± 14.17 years weighing 70.88 ± 16.84 kg who were coadministered multiple drugs. The demographic data and drug combinations of the patients given quetiapine are summarized in Tables 1, 2, respectively.
TABLE 1.
Demographic data on the schizophrenic patients treated with quetiapine (n = 96).
| Characteristic | Mean ± SD |
|---|---|
| Gender (men/women) | 52/44 |
| Age (years) | 43.53 ± 14.17 |
| Weight (kg) | 70.88 ± 16.84 |
| Albumin (g/L) | 41.39 ± 3.27 |
| Globulin (g/L) | 27.14 ± 3.44 |
| Alanine transaminase (IU/L) | 29.57 ± 24.97 |
| Aspartate transaminase (IU/L) | 22.02 ± 11.78 |
| Creatinine (μmol/L) | 63.64 ± 15.19 |
| Urea (mmol/L) | 4.52 ± 1.30 |
| Total protein (g/L) | 68.53 ± 4.86 |
| Total cholesterol (mmol/L) | 4.55 ± 1.08 |
| Triglyceride (mmol/L) | 2.08 ± 1.30 |
| Direct bilirubin (μmol/L) | 2.62 ± 1.44 |
| Total bilirubin (μmol/L) | 8.07 ± 3.36 |
| Hematocrit (%) | 39.22 ± 4.81 |
| Hemoglobin (g/L) | 129.01 ± 17.10 |
| Mean corpuscular hemoglobin (pg) | 29.56 ± 2.37 |
| Mean corpuscular hemoglobin concentration (g/L) | 328.64 ± 10.87 |
TABLE 2.
Drug combinations administered to the schizophrenic patients (n = 96).
| Drug | Category | N | Drug | Category | N |
|---|---|---|---|---|---|
| Acarbose capsules | 0 | 91 | Lorazepam tablets | 0 | 80 |
| 1 | 5 | 1 | 16 | ||
| Agomelatine tables | 0 | 94 | Metformin hydrochloride tablets | 0 | 80 |
| 1 | 2 | 1 | 16 | ||
| Alprazolam tablets | 0 | 87 | Nifedipine sustained-release tablets | 0 | 93 |
| 1 | 9 | 1 | 3 | ||
| Amlodipine besylate tablets | 0 | 94 | Oxazepam tablets | 0 | 90 |
| 1 | 2 | 1 | 6 | ||
| Aripiprazole tablets | 0 | 80 | Perphenazine tablets | 0 | 92 |
| 1 | 16 | 1 | 4 | ||
| Aspirin enteric-coated tablets | 0 | 92 | Propranolol hydrochloride tablets | 0 | 79 |
| 1 | 4 | 1 | 17 | ||
| Atorvastatin calcium tablets | 0 | 91 | Risperidone tablets | 0 | 78 |
| 1 | 5 | 1 | 18 | ||
| Clonazepam tablets | 0 | 89 | Silymarin capsules | 0 | 94 |
| 1 | 7 | 1 | 2 | ||
| Clozapine tablets | 0 | 77 | Sodium valproate sustained-release tablets | 0 | 74 |
| 1 | 19 | 1 | 22 | ||
| Duloxetine hydrochloride enteric-coated capsules | 0 | 94 | Spironolactone tablets | 0 | 93 |
| 1 | 2 | 1 | 3 | ||
| Fluvoxamine maleate tablets | 0 | 94 | Trihexyphenidyl hydrochloride tablets | 0 | 73 |
| 1 | 2 | 1 | 23 | ||
| Glimepiride tablets | 0 | 91 | Valsartan capsules | 0 | 93 |
| 1 | 5 | 1 | 3 | ||
| Lithium carbonate sustained-release tablets | 0 | 80 | Zopiclone tablets | 0 | 87 |
| 1 | 16 | 1 | 9 |
Category, 0: without drug, 1: with drug; N, number of patients.
3.2 Modeling
The patient weight as well as coadministration of fluvoxamine or duloxetine affected quetiapine clearance in the schizophrenic patients. At the same weight, the quetiapine clearance rates were 1, 0.464, 0.463, and 0.214832 in the patients without fluvoxamine or duloxetine coadministration, with fluvoxamine coadministration, with duloxetine coadministration, and with both fluvoxamine and duloxetine coadministration, respectively. Thus, the PPK model of quetiapine in the schizophrenic patients is as follows (Equations 6, 7)
| (6) |
| (7) |
where CL/F is the apparent oral clearance, and V/F is the apparent volume of distribution; FLU and DUL refer to fluvoxamine and duloxetine, respectively. When the schizophrenic patients were administered fluvoxamine or duloxetine, the values of FLU and DUL were 1; otherwise, FLU and DUL were set to 0.
3.3 Evaluation
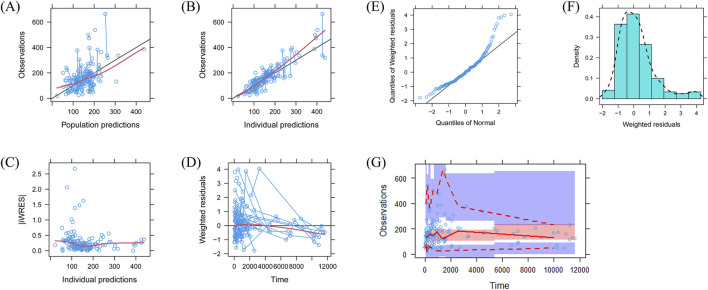
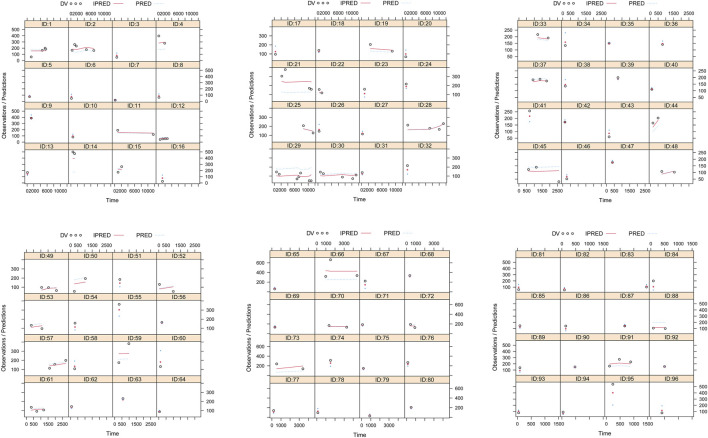
The quetiapine PPK model observations are shown in Figure 1A–G, which indicate that the quetiapine concentrations are well predicted. Figure 2 shows the plots of the individuals and shows that the quetiapine PPK model accurately predicts the quetiapine concentrations at the individual level. The bootstrap validation results are shown in Table 3, which indicates that the final model is accurate and reliable.
FIGURE 1.
Model evaluations: (A) observations vs. population predictions; (B) observations vs. individual predictions; (C) absolute value of the weighted residuals of the individuals (│iWRES│) vs. individual predictions; (D) weighted residuals vs. time; (E) quantiles of weighted residuals vs. normal quantiles; (F) density vs. weighted residuals; (G) visual predictive check of the model.
FIGURE 2.
Plots of the individual subjects. ID, patient ID number; DV, measured concentration; IPRED, individual predicted value; PRED, population predicted value.
TABLE 3.
Parameter estimates of the quetiapine final model and bootstrap validations in schizophrenic patients.
| Bootstrap | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | SE (%) | Median | 90% Confidence interval | Bias (%) |
| CL/F (L/h) | 118 | 6.9 | 117 | [100, 130] | −0.85 |
| V/F (L) | 2,460 | 33.5 | 2,505 | [1,222, 5,163] | 1.83 |
| Ka (h-1) | 1.46 (fixed) | — | — | — | — |
| θFLU | −0.536 | 4.7 | −0.535 | [–0.579, −0.487] | −0.19 |
| θDUL | −0.537 | 12.0 | −0.533 | [–0.642, −0.417] | −0.74 |
| ωCL/F | 0.333 | 12.9 | 0.325 | [0.230, 0.410] | −2.40 |
| σ1 | 0.267 | 15.3 | 0.258 | [0.168, 0.327] | −3.37 |
| σ2 | 29.917 | 34.7 | 31.780 | [2.380, 49.785] | 6.23 |
The 90% confidential interval is displayed as the 5th to 95th percentile of the bootstrap estimates. CL/F, apparent oral clearance (L/h); V/F, apparent volume of distribution (L); Ka, absorption rate constant (h-1); θFLU and θDUL are the coefficients of fluvoxamine and duloxetine, respectively; ωCL/F, inter-individual variability of CL/F; σ1, residual variability with proportional error; σ2, residual variability with additive error; Bias, prediction error given as [(median–estimate) × 100% / estimate].
3.4 Recommended dosage
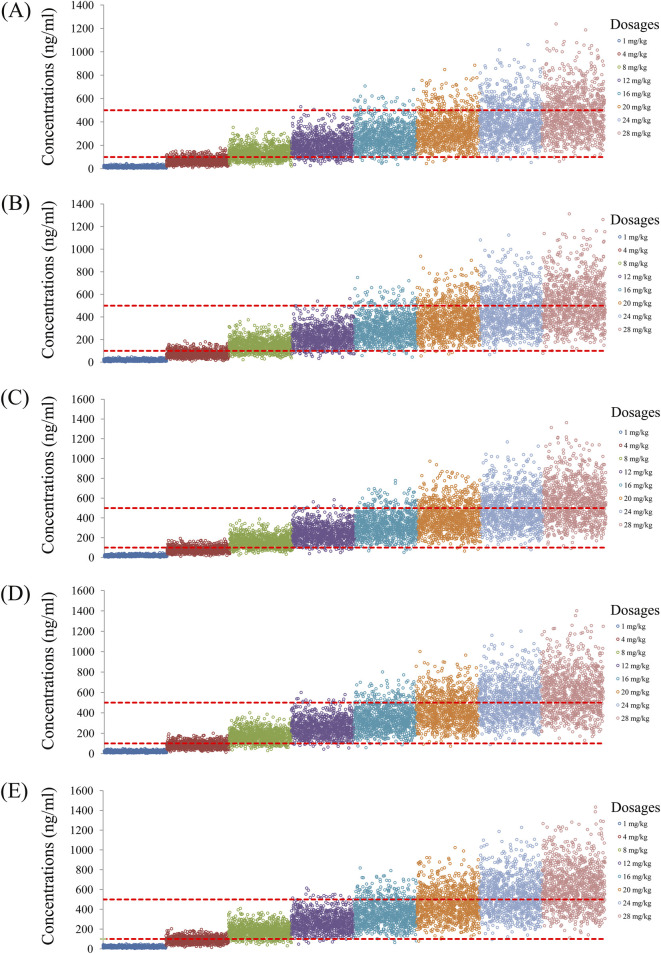
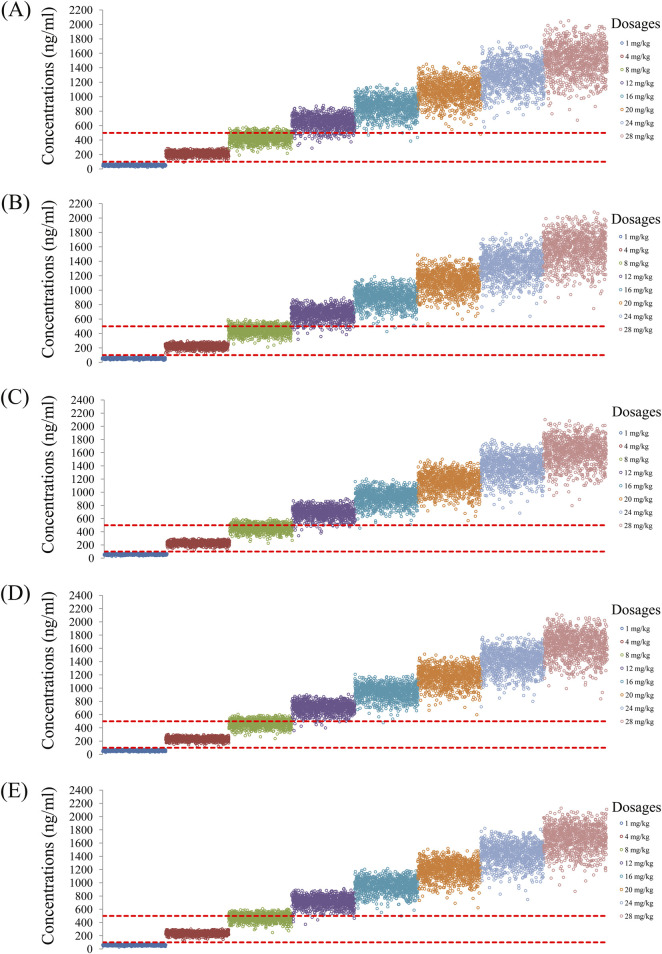
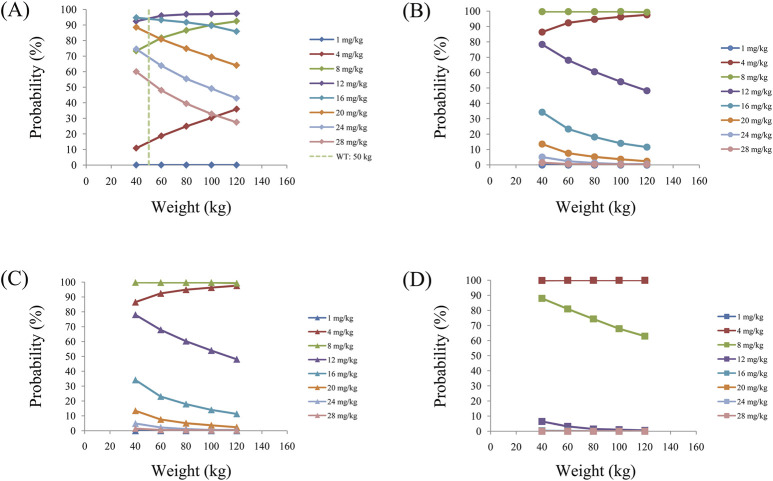
As noted earlier, four different conditions were simulated in this study, namely schizophrenia patients without fluvoxamine or duloxetine coadministration, with fluvoxamine coadministration, with duloxetine coadministration, and with both fluvoxamine and duloxetine coadministration, whose results are shown in Figures 3–6, respectively. The probabilities of achieving the target concentrations of quetiapine in the schizophrenic patients under the four conditions are demonstrated in Figure 7; here Figures 7A–D are the results for the schizophrenic patients without fluvoxamine or duloxetine coadministration, with fluvoxamine coadministration, with duloxetine coadministration, and with both fluvoxamine and duloxetine coadministration, respectively. The optimal initial dosages of quetiapine in the schizophrenic patients are summarized in Table 4. Accordingly, without fluvoxamine or duloxetine coadministration, 16 and 12 mg/kg/day of quetiapine are recommended to patients whose weights are in the 40–50 and 50–120 kg ranges, for which the probabilities of achieving the target concentrations are 94.0%–94.7% and 94.0%–97.3%, respectively. For fluvoxamine coadministration, 8 mg/kg/day of quetiapine is recommended to patients in the weight range of 40–120 kg, for which the probability of achieving the target concentration is 99.3%–99.8%. For duloxetine coadministration, 8 mg/kg/day of quetiapine is recommended to patients with weights in the range of 40–120 kg, for which the probability of achieving the target concentration is 99.3%–99.8%. For both fluvoxamine and duloxetine coadministration, 4 mg/kg/day of quetiapine is recommended to patients with weights in the range of 40–120 kg, for which the probability of achieving the target concentration is 99.9%–100.0%.
FIGURE 3.
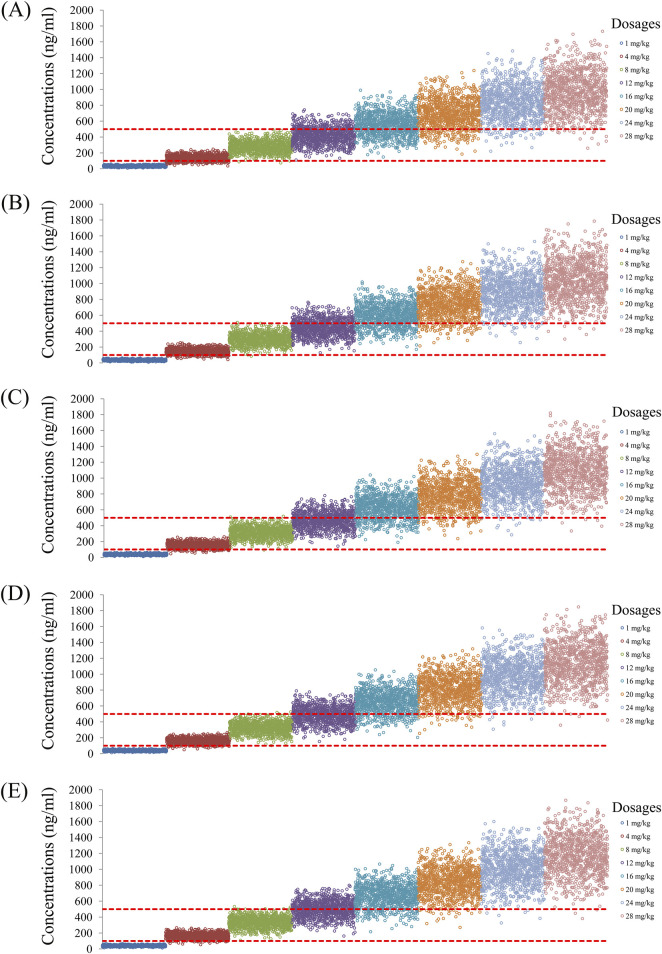
Simulated quetiapine concentrations without fluvoxamine or duloxetine coadministration for schizophrenic patients of weights (A) 40 kg, (B) 60 kg, (C) 80 kg, (D) 100 kg, and (E) 120 kg.
FIGURE 6.
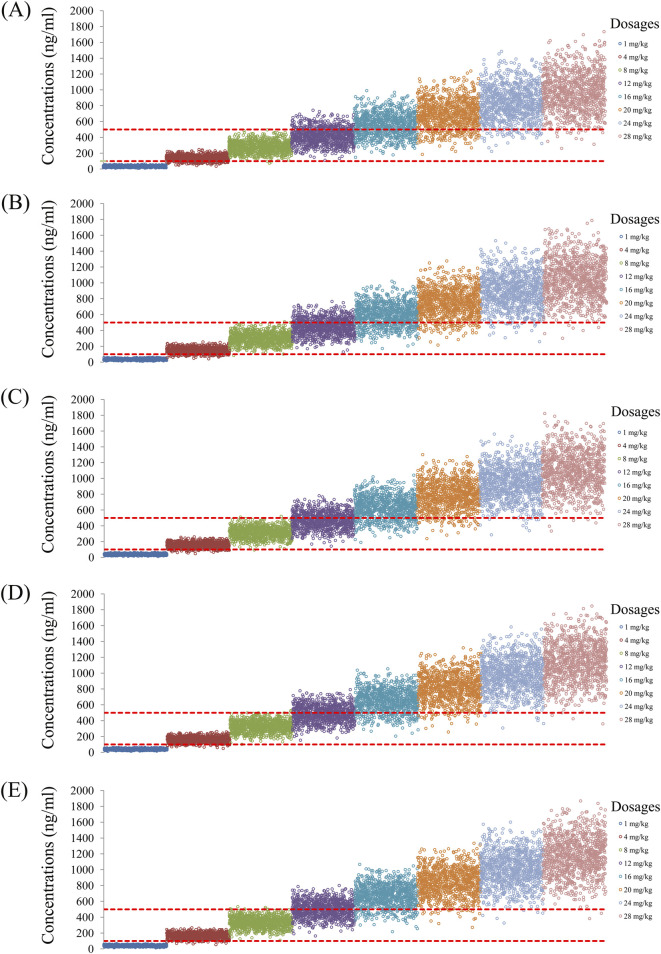
Simulated quetiapine concentrations with coadministration of both fluvoxamine and duloxetine for schizophrenic patients of weights (A) 40 kg, (B) 60 kg, (C) 80 kg, (D) 100 kg, and (E) 120 kg.
FIGURE 7.
Probabilities of achieving the target concentrations of quetiapine in schizophrenic patients (A) without fluvoxamine or duloxetine coadministration, (B) with fluvoxamine coadministration, (C) with duloxetine coadministration, and (D) with coadministration of both fluvoxamine and duloxetine.
TABLE 4.
Initial dosage recommendations of quetiapine for schizophrenic patients.
| Without fluvoxamine | With fluvoxamine | ||||||
|---|---|---|---|---|---|---|---|
| Without duloxetine | Without duloxetine | ||||||
| Body weight (kg) | Dosage (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) | Body weight (kg) | Dosage (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) |
| [40–50) | 16 | 94.0–94.7 | 3.1–4.6 | [40–120] | 8 | 99.3–99.8 | 0–0.7 |
| [50–120] | 12 | 94.0–97.3 | 0.2–1.8 | ||||
| With duloxetine | With duloxetine | ||||||
|---|---|---|---|---|---|---|---|
| Body weight (kg) | Dosage (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) | Body weight (kg) | Dosage (mg/kg/day) | Probability to achieve the target concentrations (%) | Probability to exceed the upper limit of the target concentrations (%) |
| [40–120] | 8 | 99.3–99.8 | 0–0.7 | [40–120] | 4 | 99.9–100.0 | 0 |
FIGURE 4.
Simulated quetiapine concentrations with fluvoxamine coadministration for schizophrenic patients of weights (A) 40 kg, (B) 60 kg, (C) 80 kg, (D) 100 kg, and (E) 120 kg.
FIGURE 5.
Simulated quetiapine concentrations with duloxetine coadministration for schizophrenic patients of weights (A) 40 kg, (B) 60 kg, (C) 80 kg, (D) 100 kg, and (E) 120 kg.
3.5 Safety evaluation
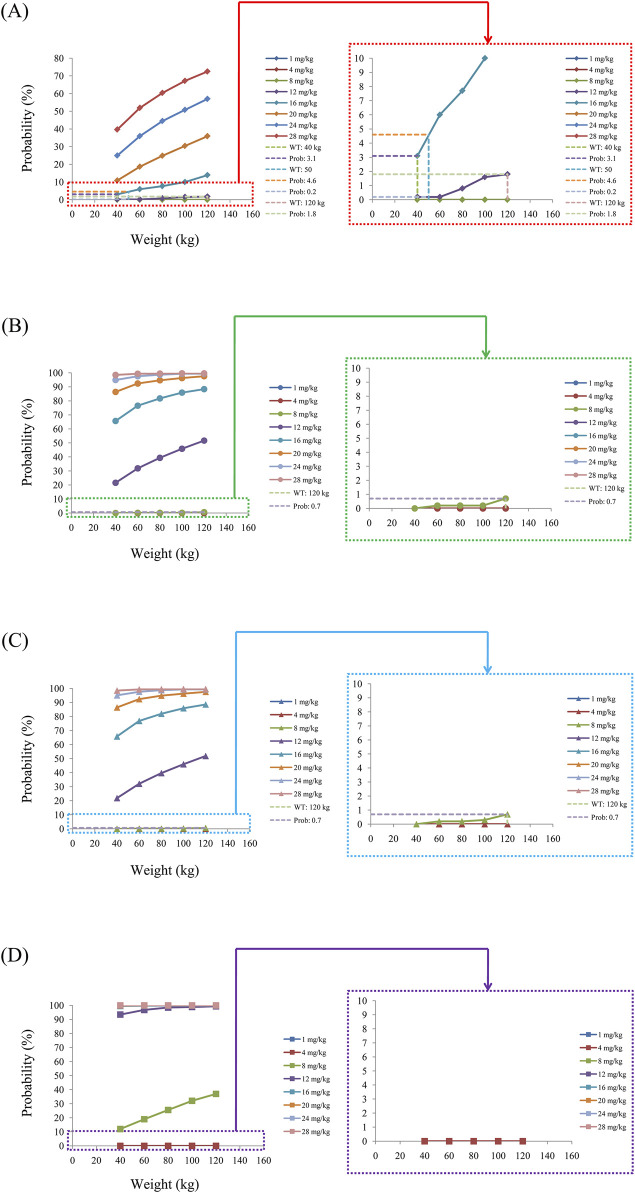
The probabilities of exceeding the upper limit of the treatment window (500 ng/mL) as a measure of safety under the four conditions are shown in Figure 8; here, Figures 8A–D are the schizophrenic patients without fluvoxamine or duloxetine coadministration, with fluvoxamine coadministration, with duloxetine coadministration, and with both fluvoxamine and duloxetine coadministration, respectively. For schizophrenic patients without fluvoxamine or duloxetine coadministration, the probabilities of exceeding the upper limit of the quetiapine target concentration are 3.1%–4.6% and 0.2%–1.8% when the recommended dosages are 16 and 12 mg/kg/day, respectively. For fluvoxamine coadministration, the probability of exceeding the upper limit of the quetiapine target concentration is 0%–0.7% when the recommended dosage is 8 mg/kg/day. For duloxetine coadministration, the probability of exceeding the upper limit of the quetiapine target concentration is 0%–0.7% when the recommended dosage is 8 mg/kg/day. For coadministration of both fluvoxamine and duloxetine, the probability of exceeding the upper limit of the quetiapine target concentration is 0 when the recommended dosage is 4 mg/kg/day. These data are also summarized in Table 4.
FIGURE 8.
Probabilities of exceeding the upper limit of the target concentration of quetiapine in schizophrenic patients (A) without fluvoxamine or duloxetine coadministration, (B) with fluvoxamine coadministration, (C) with duloxetine coadministration, and (D) with coadministration of both fluvoxamine and duloxetine.
4 Discussion
In clinical practice, TDM is one of the important methods of guaranteeing accurate dosage of antipsychotics with low risk of adverse drug reactions and high treatment efficacy (Guo et al., 2021; Hao et al., 2023). However, the premise of this personalized drug delivery approach is that there are reference drug concentrations available for the patients from TDM; based on these known drug concentrations, the subsequent dosages of medication can be accurately adjusted to achieve the clinically needed treatment concentrations. Therefore, if there are no references from TDM for the drug concentrations administered to patients, it is not possible to recommend appropriate initial dosages for patients who are given these drugs for the first time.
PPK was used as a means to discover DDIs and achieve precise drug delivery. Here, the PPK model helped realize clinical precision of drug delivery through quantitative pharmacology, and its core intent was to promote the formulation of drug delivery protocols for clinical patients through modeling and simulation. In practical applications, Monte Carlo simulations can be combined to screen the factors influencing the course of clinical treatment, especially DDIs, and further predicting the optimal dosage based on different DDIs. The combination of PPK and Monte Carlo simulations has been widely utilized and reported for dosage recommendations (Bai et al., 2024; Deng et al., 2024; Leegwater et al., 2024; Li et al., 2024; Shen et al., 2024; Sitaruno et al., 2024; Yang et al., 2024). Therefore, we used PPK and Monte Carlo simulations in this study to analyze the clinical TDM data and patient-related information, construct a precise administration model for quetiapine in schizophrenic patients, screen the influences of DDIs, and predict the optimal initial dosage of quetiapine in schizophrenic patients based on the filtered DDI results.
In this study, we collected information from ninety-six schizophrenic patients treated with quetiapine; simultaneously, we collected the physiological and biochemical indexes of these patients along with information regarding drug combinations. By constructing the PPK model of quetiapine in schizophrenic patients, we found that the patient weight as well as fluvoxamine or duloxetine coadministration affected quetiapine clearance. The main reason for the DDIs was that quetiapine was primarily metabolized by CYP3A4 and CYP2D6 (Liu et al., 2021; Stauble et al., 2021; Rohail et al., 2023; Yau et al., 2023); however, fluvoxamine inhibited CYP3A4 (Sugahara et al., 2009; Britz et al., 2019; Huth et al., 2022) while duloxetine inhibited CYP2D6 (Ma et al., 2017; Seggio et al., 2019; Margraff et al., 2023). From the findings, we concluded that for the same weight, the quetiapine clearance rates were 1, 0.464, 0.463, and 0.214832 in schizophrenic patients without fluvoxamine or duloxetine coadministration, with fluvoxamine coadministration, with duloxetine coadministration, and with both fluvoxamine and duloxetine coadministration, respectively. Furthermore, we recommended appropriate dosages for different DDI situations. In the absence of fluvoxamine or duloxetine coadministration, 16 and 12 mg/kg/day of quetiapine are recommended to schizophrenic patients with weights in the 40–50 and 50–120 kg ranges, respectively. For fluvoxamine coadministration, 8 mg/kg/day of quetiapine is recommended to patients with weights in the 40–120 kg range. For duloxetine coadministration, 8 mg/kg/day of quetiapine is recommended to patients with weights in the 40–120 kg range. For coadministration of both fluvoxamine and duloxetine, 4 mg/kg/day of quetiapine is recommended to patients with weights in the 40–120 kg range.
Regardless of the findings, there were some limitations to this study. First, this study was a retrospective, single-center study. Second, the quetiapine concentrations were sparse sampling data from TDM. Therefore, we intend to conduct a prospective multicenter intensive sampling study in the future to further validate the recommended dosages.
5 Conclusion
The present study constitutes a pilot effort at investigating the potential DDIs and optimal initial dosages of quetiapine in schizophrenic patients based on PPK. Furthermore, the initial dosages of quetiapine administered to the schizophrenic patients were optimized on the basis of coadministration of fluvoxamine or duloxetine.
Funding Statement
The authors declare that financial support was received for the research, authorship, and/or publication of this article. The present study was supported by the Xuzhou Special Fund for Promoting Scientific and Technological Innovation (Nos KC23217 and KC23254), The Medical Research Project of Jiangsu Provincial Health Commission (No. Z2023010), Jiangsu Province Education Science Planning Project (No. C/2022/01/36), Xuzhou Medical University Labor Education Special Project (No. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (No. 2023JSETKT136), and Xuzhou Medical University Research Topic of Higher Education Teaching Reform (No. Xjyzrd202304).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University. The studies were conducted in accordance with all local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or their legal guardians/next-of-kin because the data were retrospectively collected without patient identifiers.
Author contributions
XC: writing–review and editing, writing–original draft, visualization, validation, supervision, software, resources, project administration, methodology, investigation, funding acquisition, formal analysis, data curation, and conceptualization. YZ: writing–review and editing, supervision, software, methodology, investigation, data curation, and conceptualization. DY: writing–review and editing, validation, and methodology. Y-WJ: writing–review and editing, validation, and methodology. S-MH: writing–review and editing, writing–original draft, visualization, validation, supervision, software, resources, project administration, methodology, investigation, formal analysis, data curation, and conceptualization. C-XL: writing–review and editing, visualization, validation, supervision, software, resources, methodology, investigation, formal analysis, and conceptualization. CZ: writing–review and editing, visualization, validation, supervision, software, resources, project administration, methodology, investigation, funding acquisition, formal analysis, data curation, and conceptualization. D-DW: writing–review and editing, writing–original draft, visualization, validation, supervision, software, resources, project administration, methodology, investigation, funding acquisition, formal analysis, data curation, and conceptualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Anderson B. J., Holford N. H. (2008). Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48, 303–332. 10.1146/annurev.pharmtox.48.113006.094708 [DOI] [PubMed] [Google Scholar]
- Bai J., Wen A., Li Z., Li X., Duan M. (2024). Population pharmacokinetics and dosing optimisation of imipenem in critically ill patients. Eur. J. Hosp. Pharm. 31 (5), 434–439. 10.1136/ejhpharm-2022-003403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken G. V., Molden E., Knutsen K., Lunder N., Hermann M. (2012). Metabolism of the active metabolite of quetiapine, N-desalkylquetiapine in vitro . Drug Metab. Dispos. 40 (9), 1778–1784. 10.1124/dmd.112.045237 [DOI] [PubMed] [Google Scholar]
- Britz H., Hanke N., Volz A. K., Spigset O., Schwab M., Eissing T., et al. (2019). Physiologically-based pharmacokinetic models for CYP1A2 drug-drug interaction prediction: a modeling network of fluvoxamine, theophylline, caffeine, rifampicin, and midazolam. CPT Pharmacometrics Syst. Pharmacol. 8 (5), 296–307. 10.1002/psp4.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro T., Lopez-Rodriguez R., Roman M., Ochoa D., Novalbos J., Borobia A., et al. (2015). Pharmacogenetics of quetiapine in healthy volunteers: association with pharmacokinetics, pharmacodynamics, and adverse effects. Int. Clin. Psychopharmacol. 30 (2), 82–88. 10.1097/YIC.0000000000000047 [DOI] [PubMed] [Google Scholar]
- Charlson F. J., Ferrari A. J., Santomauro D. F., Diminic S., Stockings E., Scott J. G., et al. (2018). Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr. Bull. 44 (6), 1195–1203. 10.1093/schbul/sby058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer S. M., Wagstaff A. J. (2004). Quetiapine. A review of its use in the management of schizophrenia. CNS Drugs 18 (3), 173–199. 10.2165/00023210-200418030-00004 [DOI] [PubMed] [Google Scholar]
- Cinderella M. A., Morell B., Munjal S. (2021). Grapefruit juice cleanse mimicking quetiapine overdose: case report and review of literature. J. Clin. Psychopharmacol. 41 (6), 690–692. 10.1097/JCP.0000000000001469 [DOI] [PubMed] [Google Scholar]
- Deng J., Peng L., Wang Y., Li J., Tang L., Yu Y. (2024). Population pharmacokinetics and dose optimization of magnesium sulfate in Chinese preeclampsia population. BMC Pregnancy Childbirth 24 (1), 424. 10.1186/s12884-024-06620-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst B. A., Champion K. M., Pierre J. M., Wirshing D. A., Wirshing W. C. (2002). Possible association of QTc interval prolongation with co-administration of quetiapine and lovastatin. Biol. Psychiatry 51 (3), 264–265. 10.1016/s0006-3223(01)01333-6 [DOI] [PubMed] [Google Scholar]
- Guo W., Yu Z., Gao Y., Lan X., Zang Y., Yu P., et al. (2021). A machine learning model to predict risperidone active moiety concentration based on initial therapeutic drug monitoring. Front. Psychiatry 12, 711868. 10.3389/fpsyt.2021.711868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Zhang J., Yang L., Zhou C., Yu Z., Gao F., et al. (2023). A machine learning model for predicting blood concentration of quetiapine in patients with schizophrenia and depression based on real-world data. Br. J. Clin. Pharmacol. 89 (9), 2714–2725. 10.1111/bcp.15734 [DOI] [PubMed] [Google Scholar]
- Hojlund M., Lund L. C., Andersen K., Correll C. U., Hallas J. (2021). Association of low-dose quetiapine and diabetes. JAMA Netw. Open 4 (5), e213209. 10.1001/jamanetworkopen.2021.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth F., Schiller H., Jin Y., Poller B., Schuhler C., Weis W., et al. (2022). Novel Bruton's Tyrosine Kinase inhibitor remibrutinib: drug-drug interaction potential as a victim of CYP3A4 inhibitors based on clinical data and PBPK modeling. Clin. Transl. Sci. 15 (1), 118–129. 10.1111/cts.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhar S., Johnstone M., McKenna P. J. (2022). Schizophrenia. Lancet 399 (10323), 473–486. 10.1016/s0140-6736(21)01730-x [DOI] [PubMed] [Google Scholar]
- Kasper S., Muller-Spahn F. (2000). Review of quetiapine and its clinical applications in schizophrenia. Expert Opin. Pharmacother. 1 (4), 783–801. 10.1517/14656566.1.4.783 [DOI] [PubMed] [Google Scholar]
- Leegwater E., Baidjoe L., Wilms E. B., Visser L. G., Touw D. J., de Winter B. C. M., et al. (2024). Population pharmacokinetics of trimethoprim/sulfamethoxazole: dosage optimization for patients with renal insufficiency or receiving continuous renal replacement therapy. Clin. Pharmacol. Ther. 10.1002/cpt.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Mak W. Y., Ruan T., Dong F., Zheng N., Gu M., et al. (2023). Population pharmacokinetics of Amisulpride in Chinese patients with schizophrenia with external validation: the impact of renal function. Front. Pharmacol. 14, 1215065. 10.3389/fphar.2023.1215065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. Y., Li X., Cheng Z. N., Zhang B. K., Peng W. X., Li H. D. (2005). Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia. Eur. J. Clin. Pharmacol. 60 (11), 791–795. 10.1007/s00228-004-0853-x [DOI] [PubMed] [Google Scholar]
- Li Y., Fang Q., Wu Z., Huang S., Ge W., Shen J., et al. (2024). Population pharmacokinetics and dosage optimization of linezolid in Chinese older patients. Eur. J. Clin. Pharmacol. 80 (9), 1295–1304. 10.1007/s00228-024-03702-9 [DOI] [PubMed] [Google Scholar]
- Lin M., Zhang Y., Lv D., Xu N., Yang X., Liu X., et al. (2024). The impact of CYP3A5*3 on oral quetiapine: a population pharmacokinetic model in Chinese bipolar disorder patients. J. Affect Disord. 351, 309–313. 10.1016/j.jad.2024.01.170 [DOI] [PubMed] [Google Scholar]
- Liu T. L., Fang L. S., Liou J. R., Dai J. S., Chen Y. L. (2021). Determination of quetiapine and its metabolites in plasma by field-enhanced sample stacking. J. Food Drug Anal. 29 (4), 709–716. 10.38212/2224-6614.3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S. P., Tsai C. J., Chang C. C., Hsu W. Y. (2017). Delirium associated with concomitant use of duloxetine and bupropion in an elderly patient. Psychogeriatrics 17 (2), 130–132. 10.1111/psyg.12202 [DOI] [PubMed] [Google Scholar]
- Margraff T., Schoretsanitis G., Neuner I., Haen E., Gaebler A. J., Paulzen M. (2023). Discovering interactions in augmentation strategies: impact of duloxetine on the metabolism of aripiprazole. Basic Clin. Pharmacol. Toxicol. 133 (1), 73–81. 10.1111/bcpt.13875 [DOI] [PubMed] [Google Scholar]
- Patel P., Leeder J. S., Piquette-Miller M., Dupuis L. L. (2017). Aprepitant and fosaprepitant drug interactions: a systematic review. Br. J. Clin. Pharmacol. 83 (10), 2148–2162. 10.1111/bcp.13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohail M. U., Khan A., Pflaum R. M., Patel M., Moody M. A. (2023). An atypical case of neuroleptic malignant syndrome associated with ciprofloxacin and quetiapine. Cureus 15 (3), e36178. 10.7759/cureus.36178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Du Bois C., Schulz-Du Bois A. C., Bewig B., Gerstner I., Aldenhoff J. B., Cascorbi I., et al. (2008). Major increase of quetiapine steady-state plasma concentration following co-administration of clarithromycin: confirmation of the pharmacokinetic interaction potential of quetiapine. Pharmacopsychiatry 41 (6), 258–259. 10.1055/s-0028-1082071 [DOI] [PubMed] [Google Scholar]
- Seggio M., Contino A., Maccarrone G., Parenti C., Merlo S., Pappalardo G., et al. (2019). Preclinical evidence of enhanced analgesic activity of duloxetine complexed with succinyl-β-cyclodextrin: a comparative study with cyclodextrin complexes. Int. J. Pharm. 566, 391–399. 10.1016/j.ijpharm.2019.05.077 [DOI] [PubMed] [Google Scholar]
- Shen X., Li X., Lu J., Zhu J., He Y., Zhang Z., et al. (2024). Population pharmacokinetic analysis for dose regimen optimization of vancomycin in Southern Chinese children. CPT Pharmacometrics Syst. Pharmacol. 13 (7), 1201–1213. 10.1002/psp4.13151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaruno S., Chumin T., Ngamkitpamot Y., Boonchu W., Setthawatcharawanich S. (2024). Population pharmacokinetics and loading dose optimization of intravenous valproic acid in hospitalized Thai patients. J. Clin. Pharmacol. 64, 1343–1350. 10.1002/jcph.6102 [DOI] [PubMed] [Google Scholar]
- Siwek M., Woron J., Gorostowicz A., Wordliczek J. (2020). Adverse effects of interactions between antipsychotics and medications used in the treatment of cardiovascular disorders. Pharmacol. Rep. 72 (2), 350–359. 10.1007/s43440-020-00058-6 [DOI] [PubMed] [Google Scholar]
- Stauble C. K., Lampert M. L., Mikoteit T., Hatzinger M., Hersberger K. E., Meyer Zu Schwabedissen H. E. (2021). Severe adverse drug reactions to quetiapine in two patients carrying CYP2D6*4 variants: a case report. Int. J. Mol. Sci. 22 (12), 6480. 10.3390/ijms22126480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara H., Maebara C., Ohtani H., Handa M., Ando K., Mine K., et al. (2009). Effect of smoking and CYP2D6 polymorphisms on the extent of fluvoxamine-alprazolam interaction in patients with psychosomatic disease. Eur. J. Clin. Pharmacol. 65 (7), 699–704. 10.1007/s00228-009-0629-4 [DOI] [PubMed] [Google Scholar]
- Wang Y., Harlin M., Larsen F., Wang X., Park W., Rich B., et al. (2024). Population pharmacokinetics and dosing simulations for aripiprazole 2-month ready-to-use long-acting injectable in adult patients with schizophrenia or bipolar I disorder. Clin. Pharmacol. Drug Dev. 13 (6), 631–643. 10.1002/cpdd.1397 [DOI] [PubMed] [Google Scholar]
- Xu Q., Wu X., Li M., Huang H., Minica C., Yi Z., et al. (2016). Association studies of genomic variants with treatment response to risperidone, clozapine, quetiapine and chlorpromazine in the Chinese Han population. Pharmacogenomics J. 16 (4), 357–365. 10.1038/tpj.2015.61 [DOI] [PubMed] [Google Scholar]
- Yang P., Liu W., Ying Y., Zhao L., Xiong X., Zhang X., et al. (2024). Population pharmacokinetics of nirmatrelvir in Chinese patients with COVID-19: therapeutic drug monitoring and dosing regimen selection in clinical practice. Int. J. Antimicrob. Agents 64 (2), 107199. 10.1016/j.ijantimicag.2024.107199 [DOI] [PubMed] [Google Scholar]
- Yau K., McArthur E., Jeyakumar N., Tsobo Muanda F., Kim R. B., Clemens K. K., et al. (2023). Adverse events with quetiapine and clarithromycin coprescription: a population-based retrospective cohort study. Health Sci. Rep. 6 (6), e1375. 10.1002/hsr2.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Jiang L., Hu K., Chen L., Zhang Y. J., Shi H. Z., et al. (2024a). Effects of aripiprazole on olanzapine population pharmacokinetics and initial dosage optimization in schizophrenia patients. Neuropsychiatr. Dis. Treat. 20, 479–490. 10.2147/NDT.S455183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Jiang L., Hu K., Zhang Y. J., Han J., Chen J., et al. (2024b). Drug-drug interaction and initial dosage optimization of aripiprazole in patients with schizophrenia based on population pharmacokinetics. Front. Psychiatry 15, 1377268. 10.3389/fpsyt.2024.1377268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Bui K. H., Li J., Al-Huniti N. (2015). Population pharmacokinetic modeling of quetiapine after administration of seroquel and seroquel XR formulations to Western and Chinese patients with schizophrenia, schizoaffective disorder, or bipolar disorder. J. Clin. Pharmacol. 55 (11), 1248–1255. 10.1002/jcph.544 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.