Abstract
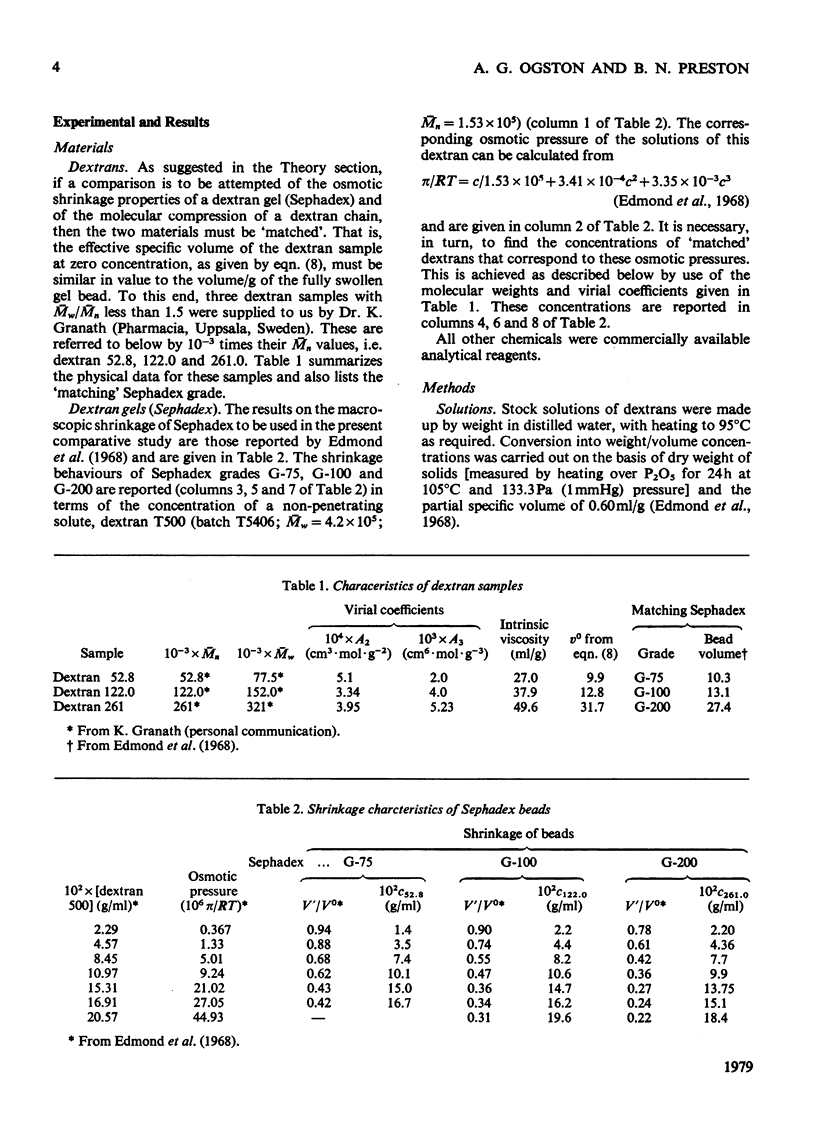
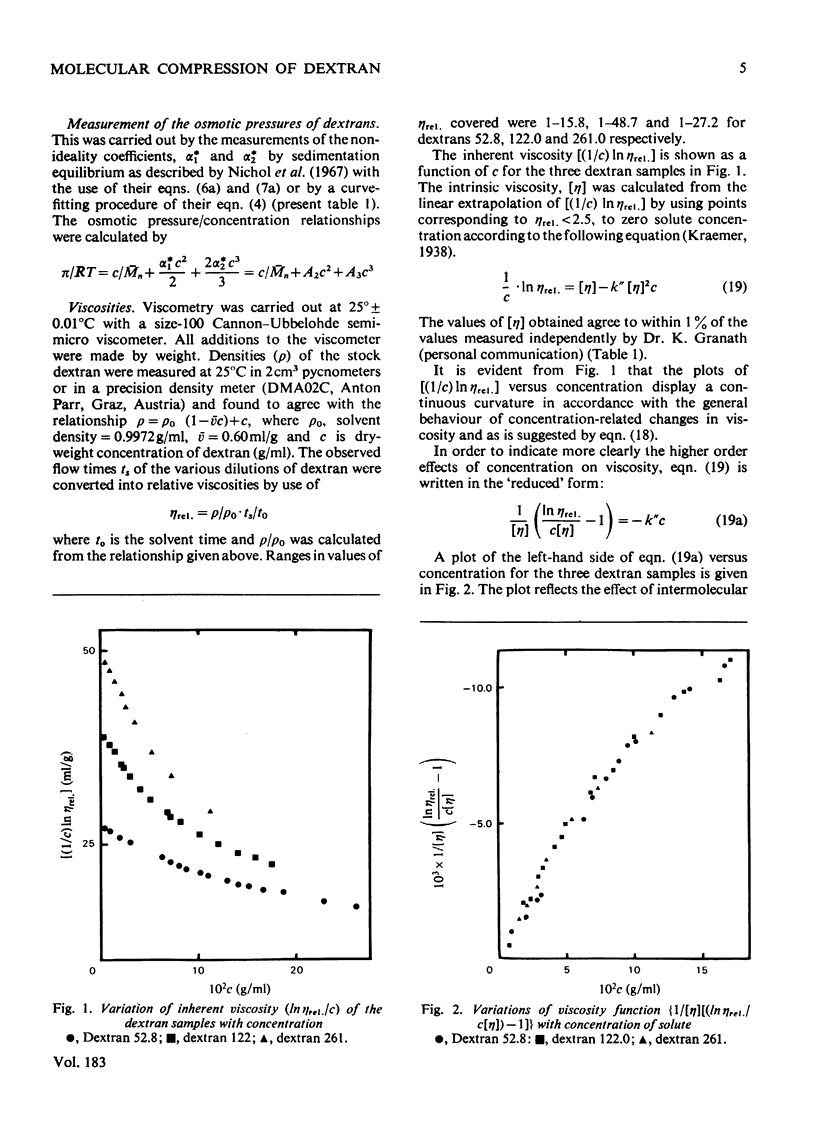
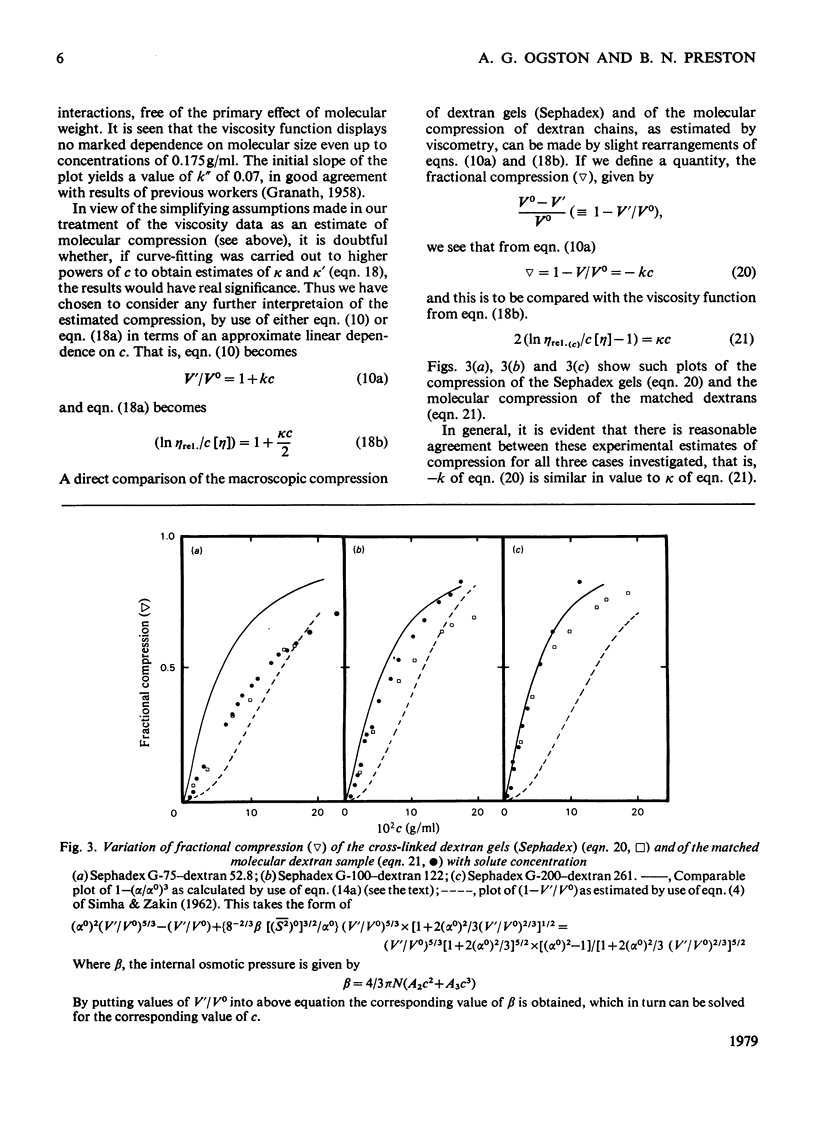
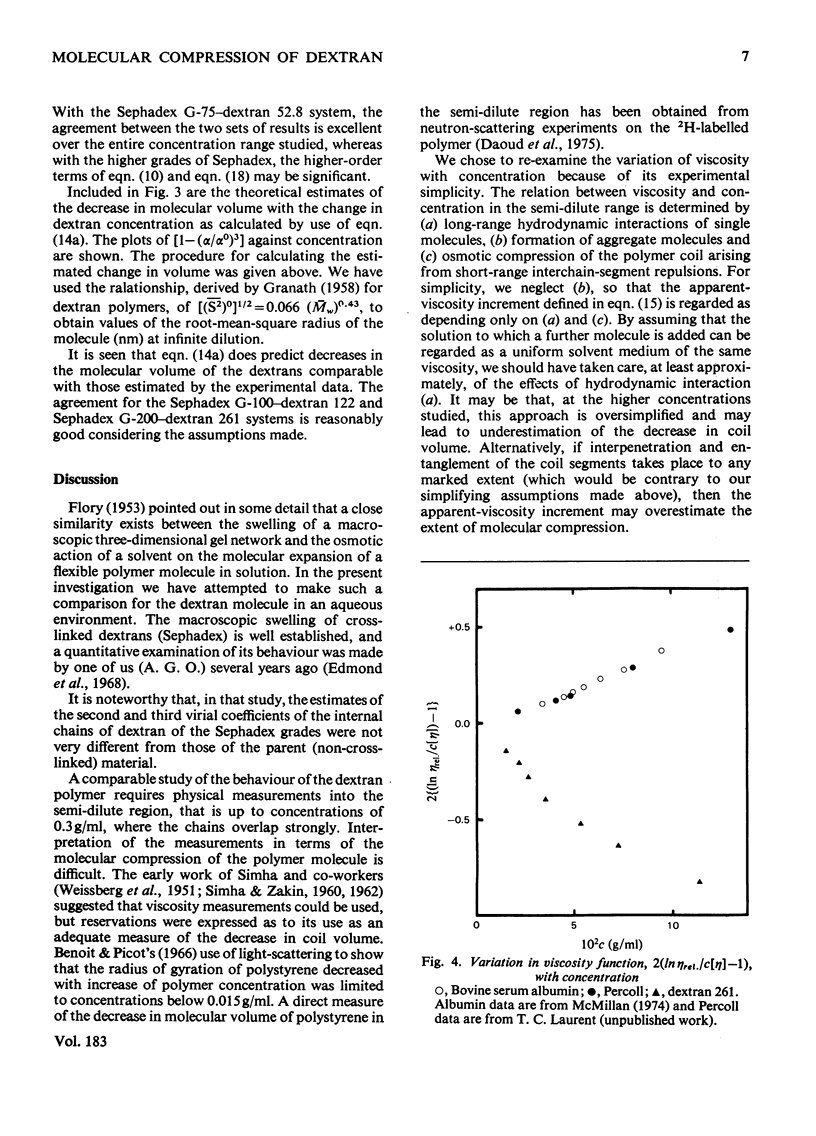
The suggestion is made that, in solution, the flexible-chain molecules of dextran can undergo an osmotic compression as concentration is increased. Approaches are developed described the molecular shrinkage (i) as arising from intra- and inter-molecular forces, (ii) based on the molecular characteristics of the dextran, and (iii) as estimated by viscosity measurements. Comparison with the macroscopic shrinkage of cross-linked dextran (Sephadex) beads [Edmond, Farquhar, Dunstone & Ogston (1968) Biochem. J. 108, 755-763] is made. In all systems studied, the experimental estimates of compression, both from gel-shrinkage and viscosity measurements were in reasonable agreement with theoretical predictions. The interpretation of the viscosity concentration-dependence was applied to compact structures (albumin and Percoll). Their behaviour was in marked contrast with that of dextran. It is noted that molecular compression may be important in considering transport processes in and thermodynamic properties of concentrated systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edmond E., Farquhar S., Dunstone J. R., Ogston A. G. The osmotic behaviour of Sephadex and its effects on chromatography. Biochem J. 1968 Aug;108(5):755–763. doi: 10.1042/bj1080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., BJOERK I., PIETRUSZKIEWICZ A., PERSSON H. ON THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. II. THE TRANSPORT OF GLOBULAR PARTICLES THROUGH HYALURONIC ACID SOLUTIONS. Biochim Biophys Acta. 1963 Oct 29;78:351–359. doi: 10.1016/0006-3002(63)91645-7. [DOI] [PubMed] [Google Scholar]
- Laurent T. C. In vitro studies on the transport of macromolecules through the connective tissue. Fed Proc. 1966 May-Jun;25(3):1128–1134. [PubMed] [Google Scholar]
- Laurent T. C., Sundelöf L. O., Wik K. O., Wärmegård B. Diffusion of dextran in concentrated solutions. Eur J Biochem. 1976 Sep;68(1):95–102. doi: 10.1111/j.1432-1033.1976.tb10767.x. [DOI] [PubMed] [Google Scholar]
- McMillan D. E. A comparison of five methods for obtaining the intrinsic viscosity of bovine serum albumin. Biopolymers. 1974;13(7):1367–1376. doi: 10.1002/bip.1974.360130708. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Ogston A. G., Preston B. N. The equilibrium sedimentation of hyaluronic acid and of two synthetic polymers. Biochem J. 1967 Feb;102(2):407–416. doi: 10.1042/bj1020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G. Some thermodynamic relationships in ternary systems, with special reference to the properties of systems containing hyaluronic acid and protein. Arch Biochem Biophys. 1962 Sep;Suppl 1:39–51. [PubMed] [Google Scholar]
- Ogston A. G. On water binding. Fed Proc. 1966 May-Jun;25(3):986–989. [PubMed] [Google Scholar]


