Abstract
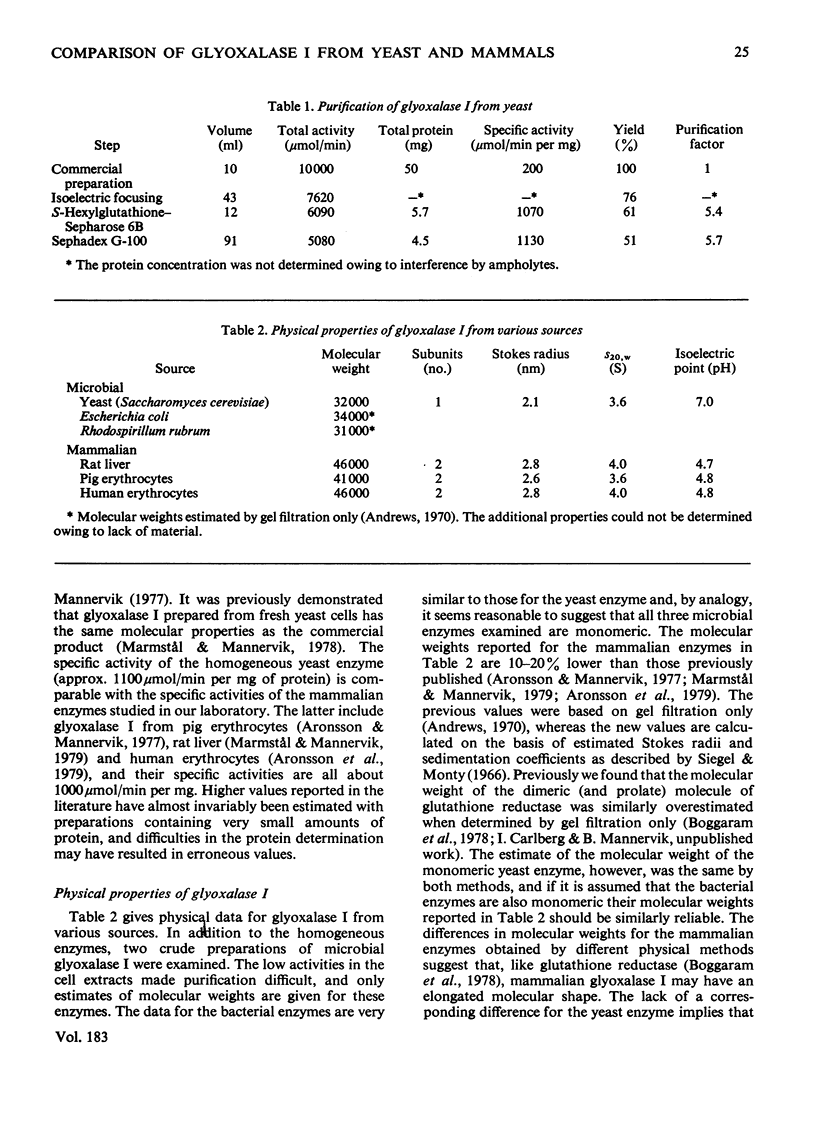
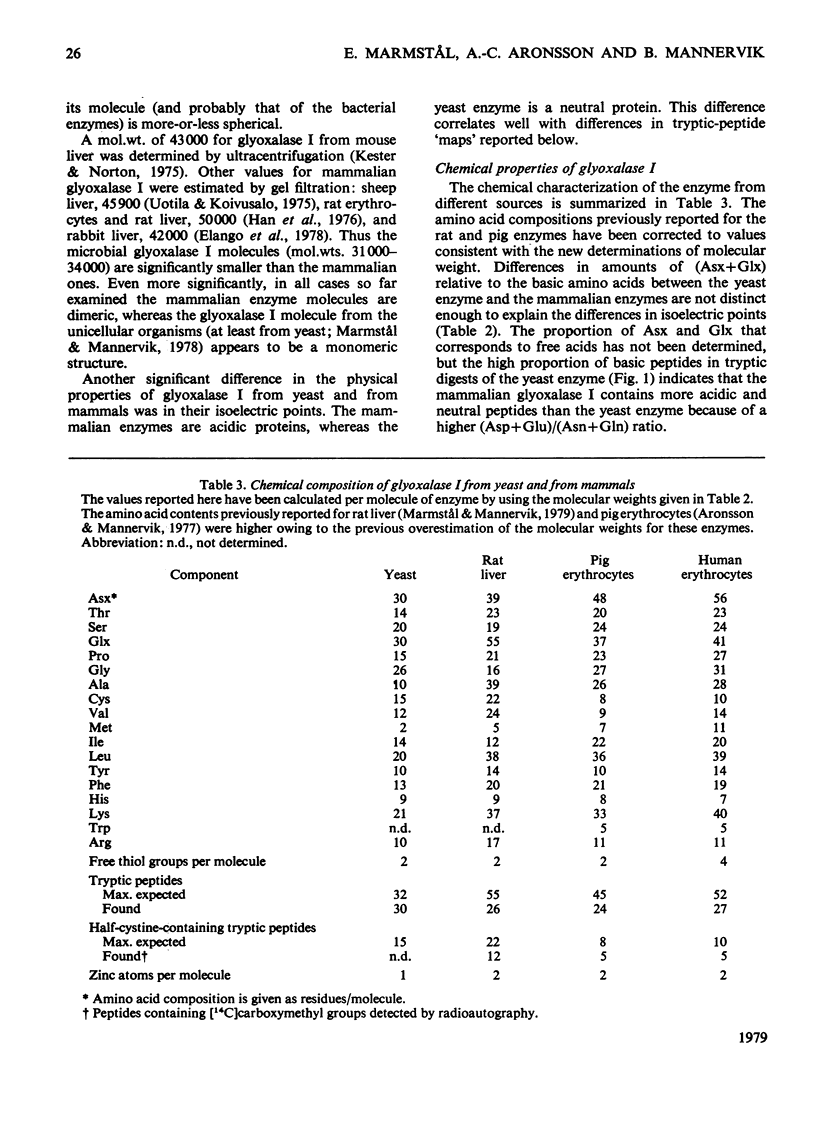
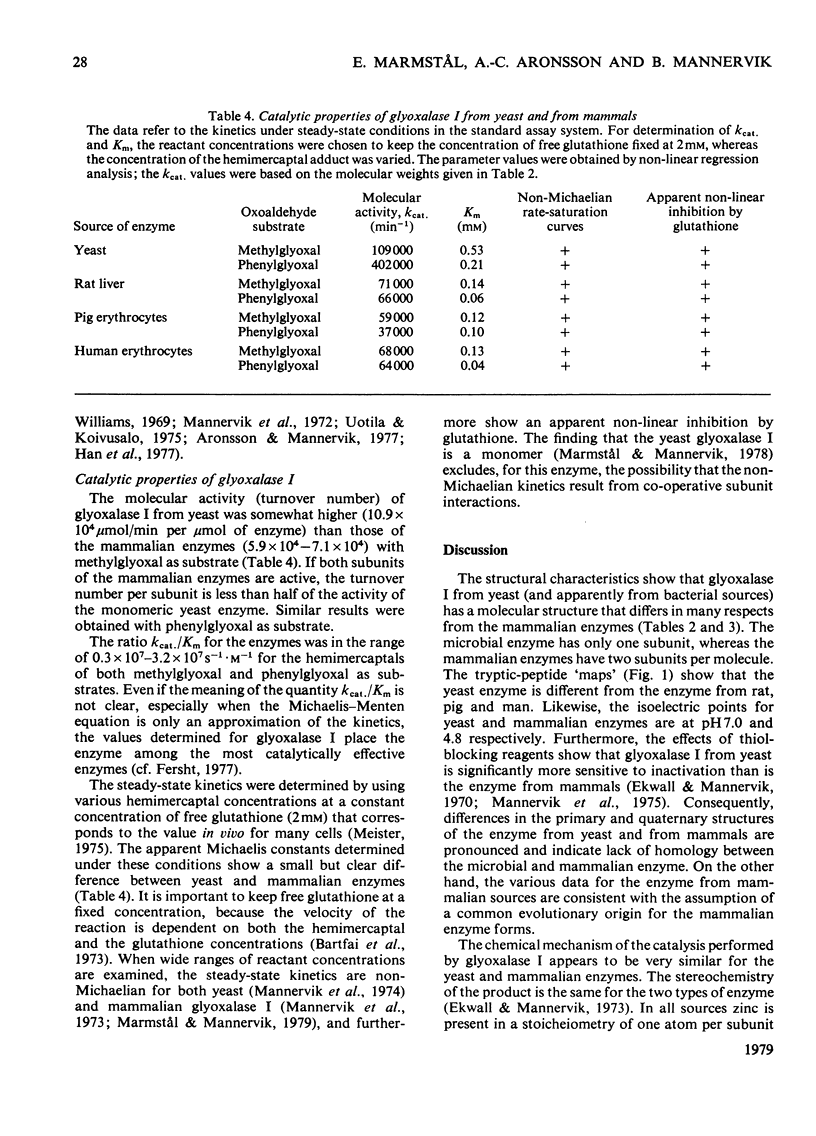
Glyoxalase I from yeast (Saccharomyces cerevisiae) purified by affinity chromatography on S-hexylglutathione–Sepharose 6B was characterized and compared with the enzyme from rat liver, pig erythrocytes and human erythrocytes. The molecular weight of glyoxalase I from yeast was, like the enzyme from Rhodospirillum rubrum and Escherichia coli, significantly less (approx. 32000) than that of the enzyme from mammals (approx. 46000). The yeast enzyme is a monomer, whereas the mammalian enzymes are composed of two very similar or identical subunits. The enzymes contain 1Zn atom per subunit. The isoelectric points (at 4°C) for the yeast and mammalian enzymes are at pH7.0 and 4.8 respectively; tryptic-peptide `maps' display corresponding dissimilarities in structure. These and some additional data indicate that the microbial and the mammalian enzymes may have separate evolutionary origins. The similarities demonstrated in mechanistic and kinetic properties, on the other hand, indicate convergent evolution. The kcat. and Km values for the yeast enzyme were both higher than those for the enzyme from the mammalian sources with the hemimercaptal adduct of methylglyoxal or phenylglyoxal as the varied substrate and free glutathione at a constant and physiological concentration (2mm). Glyoxalase I from all sources investigated had a kcat./Km value near 107s−1·m−1, which is close to the theoretical diffusion-controlled rate of enzyme–substrate association. The initial-velocity data show non-Michaelian rate saturation and apparent non-linear inhibition by free glutathione for both yeast and mammalian enzyme. This rate behaviour may have physiological importance, since it counteracts the effects of fluctuations in total glutathione concentrations on the glyoxalase I-dependent metabolism of 2-oxoaldehydes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Aronsson A. C., Mannervik B. Characterization of glyoxalase I purified from pig erythrocytes by affinity chromatography. Biochem J. 1977 Sep 1;165(3):503–509. doi: 10.1042/bj1650503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsson A. C., Marmstål E., Mannervik B. Glyoxalase I, a zinc metalloenzyme of mammals and yeast. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1235–1240. doi: 10.1016/0006-291x(78)91268-8. [DOI] [PubMed] [Google Scholar]
- Aronsson A. C., Tibbelin G., Mannervik B. Purification of glyoxalase I from human erythrocytes by the use of affinity chromatography and separation of the three isoenzymes. Anal Biochem. 1979 Jan 15;92(2):390–393. doi: 10.1016/0003-2697(79)90676-6. [DOI] [PubMed] [Google Scholar]
- Boggaram V., Larson K., Mannervik B. Characterization of glutathione reductase from porcine erythrocytes. Biochim Biophys Acta. 1978 Dec 8;527(2):337–347. doi: 10.1016/0005-2744(78)90348-0. [DOI] [PubMed] [Google Scholar]
- Bártfai T., Ekwall K., Mannervik B. Discrimination between steady-state kinetic models of the Mechanism of action of yeast glyoxalase I. Biochemistry. 1973 Jan 30;12(3):387–391. doi: 10.1021/bi00727a004. [DOI] [PubMed] [Google Scholar]
- Bártfai T., Mannervik B. A procedure based on statistical criteria for discrimination between steady state kinetic models. FEBS Lett. 1972 Oct 1;26(1):252–256. doi: 10.1016/0014-5793(72)80585-4. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. Methylglyoxal formation during glucose catabolism by Pseudomonas saccharophila. Identification of methylglyoxal synthase. Eur J Biochem. 1974 May 2;44(1):81–86. doi: 10.1111/j.1432-1033.1974.tb03459.x. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Williams G. R. Glyoxalase I, a lyase or an oxidoreductive isomerase? Can J Biochem. 1969 May;47(5):553–556. doi: 10.1139/o69-086. [DOI] [PubMed] [Google Scholar]
- Ekwall K., Mannervik B. Inhibition of yeast S-lactylglutathione lyase (glyoxalase I) by sulfhydryl reagents. Arch Biochem Biophys. 1970 Mar;137(1):128–132. doi: 10.1016/0003-9861(70)90419-4. [DOI] [PubMed] [Google Scholar]
- Ekwall K., Mannervik B. The stereochemical configuration of the lactoyl group of S-lactoylglutathionine formed by the action of glyoxalase I from porcine erythrocytes and yeast. Biochim Biophys Acta. 1973 Feb 28;297(2):297–299. doi: 10.1016/0304-4165(73)90076-7. [DOI] [PubMed] [Google Scholar]
- Elango N., Janaki S., Rao A. R. Two affinity chromatography methods for the purification of glyoxalase I from rabbit liver. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1388–1395. doi: 10.1016/0006-291x(78)91375-x. [DOI] [PubMed] [Google Scholar]
- Han L. P., Davison L. M., Vander Jagt D. L. Purification and kinetic study of glyoxalase-I from rat liver, erythrocytes, brain and kidney. Biochim Biophys Acta. 1976 Sep 14;445(2):486–499. doi: 10.1016/0005-2744(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Han L. P., Schimandle C. M., Davison L. M., Vander Jagt D. L. Comparative kinetics of Mg2+-, Mn2+-, Co2+-, and Ni2+-activated glyoxalase I. Evaluation of the role of the metal ion. Biochemistry. 1977 Dec 13;16(25):5478–5484. doi: 10.1021/bi00644a013. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Cooper R. A. The purification and properties of Escherichia coli methylglyoxal synthase. Biochem J. 1972 Jun;128(2):321–329. doi: 10.1042/bj1280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J., Binkley F. Glutathione dependent control of protein disulfide-sulfhydryl content by subcellular fractions of hepatic tissue. Biochim Biophys Acta. 1977 Mar 29;497(1):192–204. doi: 10.1016/0304-4165(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Kester M. V., Norton S. J. The isolation and characterization of mouse liver glyoxalase I. Biochim Biophys Acta. 1975 May 23;391(1):212–221. doi: 10.1016/0005-2744(75)90168-0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Bartfai T., Górna-Hall B. Random pathway mechanism involving parallel one- and two- substrate branches for glyoxalase I from yeast. J Biol Chem. 1974 Feb 10;249(3):901–903. [PubMed] [Google Scholar]
- Mannervik B., Bártfai T. Discrimination between mathematical models of biological systems exemplified by enzyme steady state kinetics. Acta Biol Med Ger. 1973;31(2):203–215. [PubMed] [Google Scholar]
- Mannervik B., Górna-Hall B., Bártfai T. The steady-state kinetics of glyoxalase I from porcine erythrocytes. Evidence for a random-pathway mechanism involving one- and two-substrate branches. Eur J Biochem. 1973 Aug 17;37(2):270–281. doi: 10.1111/j.1432-1033.1973.tb02985.x. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Lindström L., Bártfai T. Partial purification and characterization of glyoxalase I from porcine erythrocytes. Eur J Biochem. 1972 Sep 18;29(2):276–281. doi: 10.1111/j.1432-1033.1972.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Marmstål E., Ekwall K., Górna-Hall B. Inactivation of glyoxalase I from porcine erythrocytes and yeast by amino-group reagents. Eur J Biochem. 1975 May 6;53(2):327–333. doi: 10.1111/j.1432-1033.1975.tb04072.x. [DOI] [PubMed] [Google Scholar]
- Marmstål E., Mannervik B. Purification, characterization and kinetic studies of glyoxalase I from rat liver. Biochim Biophys Acta. 1979 Feb 9;566(2):362–370. doi: 10.1016/0005-2744(79)90040-8. [DOI] [PubMed] [Google Scholar]
- Marmstål E., Mannervik B. Subunit structure of glyoxalase I from yeast. FEBS Lett. 1978 Jan 15;85(2):275–278. doi: 10.1016/0014-5793(78)80472-4. [DOI] [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Tsai P. K., Gracy R. W. Isolation and characterization of crystalline methylglyoxal synthetase from Proteus vulgaris. J Biol Chem. 1976 Jan 25;251(2):364–367. [PubMed] [Google Scholar]
- Uotila L., Koivusalo M. Purification and properties of glyoxalase I from sheep liver. Eur J Biochem. 1975 Apr 1;52(3):493–503. doi: 10.1111/j.1432-1033.1975.tb04019.x. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Han L. P., Lehman C. H. Kinetic evaluation of substrate specificity in the glyoxalase-I-catalyzed disproportionation of -ketoaldehydes. Biochemistry. 1972 Sep 26;11(20):3735–3740. doi: 10.1021/bi00770a011. [DOI] [PubMed] [Google Scholar]
- Vince R., Daluge S. Glyoxalase inhibitors. A possible approach to anticancer agents. J Med Chem. 1971 Jan;14(1):35–37. doi: 10.1021/jm00283a009. [DOI] [PubMed] [Google Scholar]
- Vince R., Daluge S., Wadd W. B. Studies on the inhibition of glyoxalase I by S-substituted glutathiones. J Med Chem. 1971 May;14(5):402–404. doi: 10.1021/jm00287a006. [DOI] [PubMed] [Google Scholar]


