Abstract
Background
Reishi, a medicinal mushroom, is increasingly used for symptom control by cancer patients worldwide. However, data around patients' experiences with Reishi in oncology are lacking, limiting safe, effective clinical applications. We thus sought to evaluate patient reported benefits and harms of using Reishi.
Methods
We conducted a cross-sectional survey among Chinese cancer patients using Reishi products, probing for symptom improvements and/or adverse events (AEs) after taking Reishi. Multivariable logistic regression models assessed whether socio-demographic or clinical factors, as well as duration of Reishi use or combination with other TCM herbs, were associated with being a “responder” – reporting “quite a bit” or “very much” symptom improvement.
Results
Among 1374 participants, more than half of participants reported that nausea (55 %), fatigue (52 %), poor appetite (51 %), and depression (50 %) improved quite a bit or very much after taking Reishi. In multivariate analyses, age <65 years (adjusted odds ratios [AOR] = 1.76, p = 0.001), diagnosis ≥ 10 years (AOR = 1.78, p = 0.018), and duration of Reishi use ≥ 1 year (1–3 years: AOR = 1.53, p = 0.045; 3–5 years: AOR = 2.04, p = 0.001; >5 years: AOR = 2.07, p < 0.001) were significantly associated with higher responder rates for symptom improvement. However, 125 (9.1 %) also reported a range of AEs, including dry mouth (5 %), constipation (4 %), insomnia (3 %), pruritus (3 %) and vertigo (3 %).
Conclusion
While majority of cancer patients using Reishi reported symptom improvements, some reported adverse effects. This information can assist clinicians in advising cancer patients on safe and effective use of Reishi and help identify specific outcomes for assessment in future prospective clinical trials.
Keywords: Reishi mushroom, Cross-sectional survey, Symptom management, Cancer survivors, Adverse events
1. Introduction
Despite significant advances in conventional cancer treatments, patients and survivors still commonly experience persistent side effects and symptoms, such as fatigue, pain, and nausea, that are often poorly controlled.1,2 To ameliorate these, more than half of cancer patients worldwide use complementary and alternative medicine (CAM) as part of their cancer treatment or recovery, with the highest prevalence among developing countries.3,4 Herbal medicine in particular is often used by patients with the hopes of preventing cancer metastasis or recurrence, enhancing the immune system, managing comorbid symptoms, improving overall quality of life, and addressing side effects and other needs unmet by conventional care.5,6 For many patients, herbal medicine also offers a sense of personal autonomy and choice, with locally available, culturally appropriate, and familiar, easy-to follow-regimens.6,7
Reishi is one of the most popular herbal medicines among cancer patients worldwide.8, 9, 10 Epidemiological research in China has shown that use among breast cancer survivors increased from 18.9 % in the 1990s to 58.4 % by 2006.10 Scientifically known as Ganoderma Lucidum (G. Lucidum), Reishi is a type of medical mushroom that has been used in Traditional Chinese Medicine (TCM) for over 2000 years for “promoting vivacity and longevity” .11,12 Beyond China, Reishi is also cultivated in Japan, Korea, Malaysia, North America, and in the tropical and warm temperate regions of India. It has been listed in the American Herbal Pharmacopoeia and Therapeutic Compendium as well as in the Chinese Pharmacopoeia.13 It typically grows on deciduous trees, especially those that are dead or dying, and is commonly found on species such as oak, pyrus, and maple.14,15 Globally, a variety of Reishi-derived products are available as dietary supplements or other over the counter (OTC) products. However, in many Asian countries such as China, Japan, and Korea, preparations of Reishi may be classified as drugs requiring a physician's prescription.16
Research on the therapeutic potential of Reishi indicates that the basidiocarp, mycelia, and spores of Reishi contain around 400 different bioactive compounds,17 among which triterpenoids and polysaccharides are the two major active anti-cancer constituents.18 Triterpenoids are recognized for their anti-inflammatory, anti-tumor, and cytotoxic activities, inhibiting tumor invasion and metastasis.13,19 Polysaccharides, particularly beta-glucans, activate crucial immune cells like macrophages and natural killer cells, supporting immune surveillance and tumor elimination.20,21 Moreover, these constituents offer symptom relief from cancer and its treatments, impacting various bodily functions including muscle function, antioxidant capacities, cardiovascular and hepatic functions, immunomodulation, hormonal regulation, and blood glucose control.22
Despite these promising biochemical findings, rigorous clinical evidence is limited. Promising data suggest Reishi may be a safe, effective complementary therapy alongside conventional cancer treatments,23,24 although most findings originate from in vitro studies.23 While several small clinical trials have investigated Reishi use in oncology settings, their outcomes mainly focused on overall quality of life and therefore lack specificity.25, 26, 27 To our knowledge, no studies have specifically evaluated patients' experiences with integrating Reishi into their conventional cancer care. As a result, it is difficult to advise patients on the symptoms for which Reishi may be beneficial, or the potential adverse effects.
To address this critical gap, we conducted a cross-sectional survey of Chinese cancer patients using Reishi products. Our study aimed to identify patients-reported benefits and harms, as well as clinical and demographic factors associated with symptom improvement. Our findings offer novel insights into the effective, safe integration of herbal medicine into oncology care and can also help researchers focus on appropriate, patient-centered outcomes in future trial design, ultimately improving quality of life among patients and survivors.
2. Methods
2.1. Sample and study design
We conducted a cross-sectional survey of cancer patients and survivors using Reishi products in China from October 2022 to December 2022. Participants were recruited from a database of customers purchasing Reishi products from Zhongke Health International LLC. Eligible participants included individuals diagnosed with cancers of all types and stages who were 18 years of age or older, previously or currently using Reishi products, and fluent in both written and spoken Mandarin. Trained research staff contacted potential participants by phone or WeChat (a popular messaging tool in China) to confirm eligibility, explain the study's aims and procedures, and obtain oral or written informed consent. Participants had the option of completing surveys online or by phone, with data was collected by WJX (Changsha Ranxing IT Ltd.), a Chinese platform with functions equivalent to SurveyMonkey. In appreciation for participation, participants received gifts with an approximate value of $2 upon survey completion. This study was approved by Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (2022-XLA129–1).
2.2. Study variables
2.2.1. Outcomes
Symptom improvements: We used items from the Edmonton Symptom Assessment System (ESAS) to evaluate symptom improvements after taking Reishi. ESAS is a brief instrument widely used to evaluate patient-reported symptoms in cancer care.28 It measures ten symptoms frequently reported by the cancer population: pain, tiredness, nausea, depression, anxiety, drowsiness, poor appetite, shortness of breath, feeling of not well-being, and distress.29 It has been validated in Chinese, with a Cronbach's alpha coefficient of 0.72.30 To focus on Reishi's potential impact on specific symptoms, we excluded “feeling of not well-being”. Guided by the current literature and our clinical experience with Reishi,24,31,32 we included 5 additional relevant symptoms: pruritus, constipation, diarrhea, hot flash, and insomnia. A five-point Likert scale prompted participants to identify and rate any improvements in each symptom after taking Reishi (0 = do not have this symptom, 1 = no improvement, 2 = a little improvement, 3 = quite a bit improvement, and 4 = very much improvement).
Adverse events: Our survey also probed participants about a range of potential AEs after using Reishi. Participants were first asked if they had experienced any AEs after using Reishi. If they answered yes, a list of AEs was then presented: poor appetite, dizziness, insomnia, dry month, headache, constipation, facial flushing, diarrhea, and pruritus. These items were chosen based on current literature and our clinical experience with Reishi.24,31, 32, 33 Participants then used a four-point Likert scale to indicate the occurrence and severity (1 = none, 2 = a little 3 = quite a bit, and 4 = very much) of each AE experienced after using Reishi. Participants also had the opportunity to specify any other AEs experienced after using Reishi.
2.2.2. Co-variables
Sociodemographic factors included age, gender, location, education, and employment status. We also collected data on cancer-related variables, such as cancer type, cancer stage, years since cancer diagnosis, and current cancer treatment status (diagnosis, post-surgery, chemotherapy/radiation, survivorship/endocrine, palliative care, and unknown). Additionally, given that variations in Reishi use may influence its effects, we asked patients to provide information regarding their Reishi utilization, including the types of Reishi used in the past month, the duration of Reishi usage, and whether they combined it with other TCM herbs.
2.3. Statistical analysis
Descriptive statistics were used to assess symptom improvements, AEs, as well as co-variables (e.g., age, gender, and cancer type), using frequencies, proportions and means (standard deviation [SD]). For each symptom, we calculated the proportion of participants who reported improvement, excluding those who reported “do not have this symptom”. We categorized those who reported improving “a little”, “quite a bit” and “very much” as showing “some” improvement and those who reported “quite a bit” and “very much” as demonstrating “clinically meaningful” improvement. Similarly, the proportion of participants who experienced each AE was calculated. We combined those who reported experiencing each AE “a little”, “quite a bit” and “very much” as indicating “some” AEs and those who reported “quite a bit” and “very much” as having “clinical meaningful” AEs.
To explore factors that may influence symptom improvements, we dichotomized participants into “non-responders” and “responders” – those reporting clinically meaningful improvement in at least one symptom. We only included participants who reported having at least one symptom in this analysis (N = 1120, 81.5 %). First, we conducted Chi square tests to assess whether responder status differed by participant characteristics (demographic, clinical, duration of Reishi utilization, and combination with other TCM herbs). Then characteristics with a significance level of P < 0.2 were included as independent variables in multivariable logistic regression models, with responder status as the dependent variable. All analyses were two-sided with a p-value of <0.05 for statistical significance. Statistical analyses were conducted using SPSS (version 26; IBM Corp).
3. Results
3.1. Characteristics of participants
Of the 1600 cancer patients and survivors approached, 1374 (85.9 %) agreed to participate and completed the survey. The mean age was 68.4 years (SD, 10.3 years), ranging from 25 to 100 years. The majority were female (891, 64.8 %), had less than college education (81.7 %), and were currently unemployed or retired (93.4 %). Participants were from 24 provincial-level administrative regions (70.6 % of 34 regions total), with the majority from the East (51.4 %) and South (17.3 %) regions.
Participants represented a diverse range of cancer types, with the most common being breast (27.1 %) and lung (20.1 %). The mean time since diagnosis was 8.2 years (SD, 6.3 years). Over two-thirds of participants (67.7 %) were classified as stage I-III, and 40.5 % had completed surgical treatment at the time of the survey (Table 1).
Table 1.
Characteristics of participants.
| Characteristics | Total No. | No. | % |
|---|---|---|---|
| Mean age in years (SD) | 1369 | 68.4 (10.3) | |
| <65 | 421 | 27.1 | |
| 65–75 | 598 | 43.8 | |
| >75 | 350 | 29.1 | |
| Gender | 1374 | ||
| Male | 483 | 35.2 | |
| Female | 891 | 64.8 | |
| Education | 1374 | ||
| Less than college | 1122 | 81.7 | |
| College or higher | 252 | 18.3 | |
| Employment status | 1374 | ||
| Working | 91 | 6.6 | |
| Unemployed or retired | 1283 | 93.4 | |
| Cancer type | 1374 | ||
| Breast | 373 | 27.1 | |
| Lung | 276 | 20.1 | |
| Colorectal | 197 | 14.3 | |
| Gynecologic | 109 | 7.9 | |
| Gastric | 104 | 7.8 | |
| Prostate | 65 | 4.7 | |
| Other* | 250 | 18.2 | |
| Years since cancer diagnosis, mean years (SD) | 1373 | 8.22 (6.3) | |
| <2 | 163 | 11.9 | |
| 2–5 | 283 | 20.6 | |
| 5–10 | 452 | 32.9 | |
| ≥10 | 475 | 34.6 | |
| Cancer stage | 1374 | ||
| l-lll | 930 | 67.7 | |
| lV | 82 | 6.0 | |
| Unknown | 362 | 26.4 | |
| Current cancer treatment status | 1374 | ||
| Diagnosis | 6 | 0.4 | |
| Post-surgery | 557 | 40.5 | |
| Chemotherapy/radiation | 45 | 3.3 | |
| Survivorship/endocrine | 425 | 30.9 | |
| Palliative care | 136 | 9.9 | |
| Unknown | 205 | 14.9 | |
Abbreviations: SD, Standard deviation.
Other cancer types include brain, bone, head and face, esophagus, and other.
3.2. Reishi utilization
Among the 1374 participants, nearly half (49.1 %) had been taking Reishi for over 5 years, 20.5 % for <1 year, 15.9 % for 1 to 3 years, and 14.5 % for 3 to 5 years. More than half (55.9 %) combined Reishi products with other TCM herbs.
3.3. Patient perceived symptom improvement after using Reishi
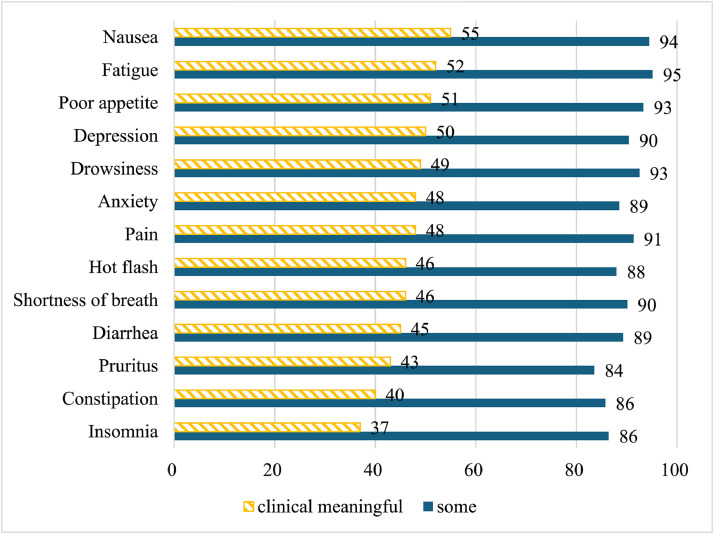
Fig. 1 shows symptom improvements reported by participants after using Reishi. Among 1120 (81.5 %) participants who had experienced symptoms, over 80 % reported improvement in at least one symptom. Participants were most likely to report clinically meaningful improvement for nausea (55 %), fatigue (52 %), poor appetite (51 %), depression (50 %), and drowsiness (49 %).
Fig. 1.
Proportion (%) of Participants Experienced Symptom Improvement After Reishi Use For each symptom, we calculated the proportion of participants who reported improvement among participants who experienced the symptom (excluding those who reported “do not have this symptom”). We categorized those who reported improving “a little”, “quite a bit” and “very much” as showing “some” improvement and those who reported “quite a bit” and “very much” as demonstrating “clinically meaningful” improvement.
3.4. Factors associated with clinically meaningful response to Reishi
Among participants with survey-specified symptoms (N = 1120), 629 (56.2 %) were responders. Chi-square tests (Table 2a) found associations between responder status and age, years since cancer diagnosis, and years taking Reishi (all p < 0.2). Compared to participants under 65 years old, those aged 65–75 years and older than 75 years had lower responder rates (60.5% vs. 56.0 % and 50.9 %, respectively, p = 0.056). Longer times since diagnosis were associated with higher responder rates (≥10 years, 5–10 years, 2–5 years, and <2 years: 64.7% vs. 58.6% vs. 48.6 %, and 39.9 % respectively, p = 0.000). Similarly, longer Reishi usage was related to higher responder rates (≥5 years, 3–5 years, 1–3 years, and <1 year: 63.5% vs. 59.9% vs. 50.8 %, and 40.3 % respectively, p = 0.000).
Table 2.
Factors associated with patient-reported symptom response.
| Characteristics | Total No.† | Responder (%) | P-value |
|---|---|---|---|
| Mean age in years (SD) | 0.056 | ||
| <65 | 354 | 214 (60.5) | |
| 65–75 | 486 | 272 (56.0) | |
| >75 | 277 | 141 (50.9) | |
| Gender | 0.49 | ||
| Male | 389 | 213 (54.8) | |
| Female | 731 | 416 (56.9) | |
| Cancer stage | 0.31 | ||
| l-lll | 751 | 434 (57.8) | |
| lV | 68 | 35 (51.5) | |
| Cancer type | 0.40 | ||
| Breast | 310 | 179 (57.7) | |
| Lung | 231 | 119 (51.5) | |
| Colorectal | 164 | 92 (56.1) | |
| Gynecologic | 93 | 57 (61.3) | |
| Gastric | 75 | 36 (48.0) | |
| Prostate | 53 | 31 (58.5) | |
| Other* | 194 | 115 (59.3) | |
| Years since cancer diagnosis | 0.000 | ||
| <2 | 138 | 55 (39.9) | |
| 2–5 | 243 | 118 (48.6) | |
| 5–10 | 362 | 212 (58.6) | |
| ≥10 | 377 | 244 (64.7) | |
| Years since taking Reishi | 0.000 | ||
| <1 year | 226 | 91 (40.3) | |
| 1–3 years | 185 | 94 (50.8) | |
| 3–5 years | 167 | 100 (59.9) | |
| ≥5 years | 542 | 344 (63.5) | |
| Other TCM herbs | 0.70 | ||
| No | 468 | 266 (56.8) | |
| Yes | 652 | 363 (55.7) |
Other cancer types include brain, bone, head and face, esophagus, and other.
The Total No of each characteristic was calculated excluding participants who reported “do not have this symptom”.
Characters with p-value < 0.2 were included into multivariate logistic regression.
Based on these Chi square results, three factors with univariate p value of <0.2–age, years since diagnosis, and years taking Reishi–were included in the multivariable logistic regression model (Table 3). This analysis showed participants under 65 years old were 1.76 times more likely to be responders than those over 75 years old (95 % CI 1.26–2.46, p = 0.001). Additionally, participants at least 10 years since diagnosis were 1.78 times more likely to be responders than those newly diagnosed (<2 years) (95 % CI 1.10–2.89, p = 0.018). Compared to those using Reishi for <1 year, those using it for 1–3 years, 3–5 years, and >5 years were 1.53, 2.04, and 2.07 times more likely to be responders, respectively (1–3 years: 95 % CI 1.01–2.31, p = 0.045; 3–5 years: 95 % CI 1.32–3.17, p = 0.001; >5 years: 95 % CI 1.39–3.08, p = 0.000).
Table 3.
Multivariate logistic regression: factors associated with patient-perceived symptom response.
| AOR | 95 % CI | P-value | |
|---|---|---|---|
| Age (years) | |||
| >75 | – | ||
| 65–75 | 1.29 | 0.95–1.75 | 0.098 |
| <65 | 1.76 | 1.26–2.46 | 0.001 |
| Diagnosis (years) | |||
| <2 | – | ||
| 2–5 | 1.09 | 0.69–1.72 | 0.72 |
| 5–10 | 1.42 | 0.90–2.26 | 0.14 |
| ≥10 | 1.78 | 1.10–2.89 | 0.018 |
| Time since taking Reishi (years) | |||
| <1 | – | ||
| 1–3 | 1.53 | 1.01–2.31 | 0.045 |
| 3–5 | 2.04 | 1.32–3.17 | 0.001 |
| >5 | 2.07 | 1.39–3.08 | 0.000 |
Abbreviations: CI, confidence interval; AOR, adjusted odds ratio.
p-value < 0.05 is statistically significant.
3.5. Adverse events
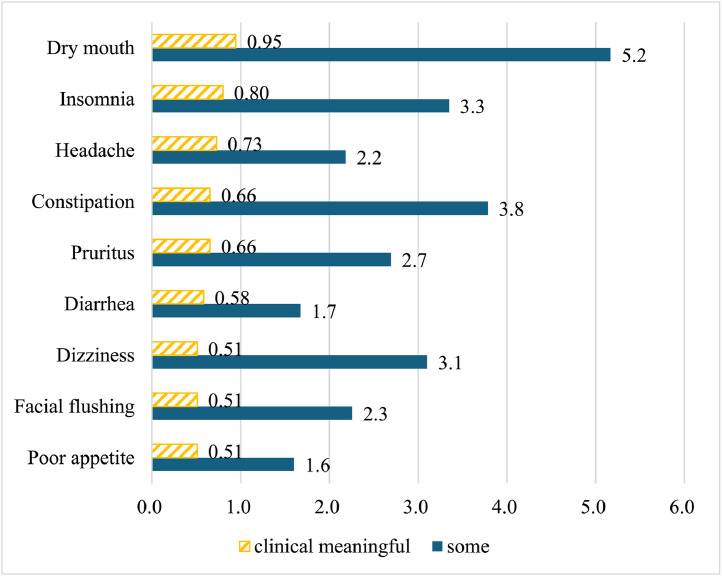
Among all participants (N = 1374), 125 (9.1 %) reported experiencing adverse effects (AEs) after using Reishi. The most common AE was dry month, experienced by 5 % of participants, followed by constipation (4 %), insomnia (3 %), and pruritus (3 %) (Fig. 2). Beyond the survey's prespecified AEs, 6 participants reported abdominal discomfort (e.g., bloating), and 1 described mild inflammatory reactions.
Fig. 2.
Proportion (%) of Participants Experienced Adverse Events After Reishi Use (N=1374). For each adverse event, we calculated the proportion of participants who reported experiencing each adverse event among all participants. We combined those who reported experiencing each AE “a little”, “quite a bit” and “very much” as indicating “some” AEs and those who reported “quite a bit” and “very much” as having “clinical meaningful” AEs.
4. Discussion
To our knowledge, no prior studies have evaluated patients' experiences integrating Reishi into their conventional cancer care. This cross-sectional study of 1374 cancer patients and survivors using Reishi products thus offers novel insights into the population's characteristics, Reishi usage patterns, and patient-reported benefits and risks. We also found that factors such as younger age (<65), longer duration since diagnosis (≥10 years), and extended Reishi usage (≥1 year) were significantly associated with higher responder rates for symptom improvement. These data can inform the design of future clinical trials focusing on patient-reported outcomes. Our findings can also assist clinician-patient communication regarding Reishi use.
In this large survey study, we identified the most commonly reported symptom improvements and adverse events associated with Reishi use. To our knowledge, only two studies have investigated Reishi's effects on patient-reported outcomes in oncology.25,33 One pilot randomized clinical trial33 with 48 breast cancer patients undergoing endocrine therapy showed that 4-week Reishi powder use significantly reduced fatigue, anxiety, depression, and appetite loss compared to the placebo. Similarly, around 50 % participants in our study reported clinically meaningful improvement in these same symptoms. Another study with 82 lung cancer patients undergoing chemotherapy revealed that a Reishi formula led to non-statistically-significant improvements in quality of life, general health, emotional well-being, and fatigue compared to placebo.25 Additionally, consistent with our study, both studies found Reishi to be safe with mild adverse events. However, the former study33 reported higher rates of dizziness (16.0 %), dry mouth (12.0 %), diarrhea (8.6 %) compared to our findings, potentially due to its small sample size or the endocrine therapy received by patients.
Our epidemiological examination of patient-reported benefits can inform future clinical trials to evaluate the specific effects of Reishi. In one large, population-based cohort study of breast cancer survivors. (n = 4149), Reishi use was associated with better social but worse physical well-being in breast cancer survivors.10 However, due to the study's design, it was impossible to determine whether Reishi negatively influenced patients’ physical well-being or if individuals with low physical well-being were more likely to use Reishi. In contrast, our study found that the most commonly reported benefits from Reishi were improved fatigue, depression, nausea, and poor appetite. Future prospective clinical trials should aim to target these specific symptoms or symptom clusters. Furthermore, existing literature supports the association between impaired immune function, inflammatory reactions, and the symptoms (e,g. fatigue, depression) highlighted in this study.34, 35, 36 Bioactive components in Reishi, such as fungal immunomodulatory proteins and polysaccharides, may enhance the host's immune response and inhibit pro-inflammatory cytokine expressions in invitro experiments.37,38 Therefore, future clinical trials should also evaluate immune or inflammation biomarkers, such as natural killer cells, T cells, and B cells26,37,39 to explore the underlining mechanisms of Reishi's effects on symptom control.
Until evidence from well-conducted clinical trials is available, our data can aid patient-centered clinician communication. Despite the widespread use of herbal medicines, there is very limited communication between physicians and patients about such use. A recent survey study revealed that 73 % of oncology professionals lack sufficient knowledge to assist patients with herbs and other CAM treatments,40 hindering their discussions with patients on appropriate use.41 Additionally, nearly half of cancer patients avoid disclosing their herbal use to oncologists,42 increasing the risk of significant side effects due to improper usage. Our study can inform and empower clinicians to raise these issues and communicate evidence-based information about Reishi's reported benefits (e.g. fatigue, depression, nausea, poor appetite) and potential side effects (e.g. dry mouth, insomnia, headaches).
Our study has several limitations. First, it was conducted in a Chinese cancer population, so the attitudes and usage patterns we observed might differ in other contexts. Second, most of our participants were older, retired, and had relatively low levels of education. Further research should explore Reishi usage among patients in other age, educational, and employment cohorts. Third, the long recall period may give rise to recall bias. Fourth, as the majority of participants in our study were long-term Reishi users, there may be selection bias favoring the benefits of Reishi. Fifth, as this is a cross-sectional survey study, all benefits and AEs were patient-reported and could be due to either placebo/nocebo effects or regression to the mean. Participants may also exaggerate benefits or downplay adverse events due to recall bias or a desire to conform to social expectations. Further research is essential to establish causality and explore the long-term effects of Reishi use. Findings from this study should be tested through rigorous clinical trials. Sixth, the absence of a control group in our study limits the ability to definitively attribute symptom improvements to Reishi, as they could also be influenced by concurrent therapies or the natural progression of the disease. To enable replication and comparison, future research should rigorously document the specifics of Reishi products used, including dosages, preparation methods, and standardization of active compounds. Lastly, our participants were limited to those who took Reishi products from Zhongke Health International LLC; results may not be generalizable to other Reishi products or preparations.
Despite these limitations, the current study is the first with a large sample size to investigate the experiences of people with diverse cancer types using Reishi in their cancer care. The findings identify specific potential benefits and harms, as well as factors related to these effects. Our data provides critical evidence from patients’ experiences that can be incorporated in shared decision making about the use of Reishi in cancer settings. Further high-quality clinical trials are needed to develop more robust evidence about Reishi's specific benefits, potential risks, and the underlying clinical mechanisms.
Conflict of interest
Dr. Mao reports grants from Tibet Cheezheng Tibetan Medicine Co Ltd and Zhongke Health International LLC outside the submitted work. Dr. Feng is a co-author of this paper and serves as the president of Zhongke Health Industry Group Corp., Ltd. The author's role in the company did not influence the design, execution, or interpretation of the study results. All other authors declare no conflicts of interest related to this research.
Funding
Laurance S. Rockefeller Fund supported the Herbal Research and Education in Oncology program at the MSK Integrative Medicine Service. The study was partly sponsored by Zhongke Health International LLC.
Ethics statement
This study was approved by Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (2022-XLA129–1). Informed consent was obtained from all participants.
Data availability
The data of this study will be available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Xiaotong Li: Conceptualization, Methodology, Formal analysis, Writing – original draft. Lingyun Sun: Methodology, Writing – review & editing. Susan Chimonas: Writing – review & editing. Susan Q. Li: Formal analysis, Writing – review & editing. Peng Feng: Writing – review & editing. Yufei Yang: Writing – review & editing. Jun J. Mao: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
References
- 1.Shi Q., Smith T.G., Michonski J.D., Stein K.D., Kaw C., Cleeland C.S. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American cancer society's studies of cancer survivors. Cancer. 2011;117(12):2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkett V.S., Cleeland C.S. Symptom burden in cancer survivorship. J Cancer Surviv. 2007;1(2):167–175. doi: 10.1007/s11764-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 3.Tuna S., Dizdar O., Calis M. The prevalence of usage of herbal medicines among cancer patients. J BUON. 2013;18(4):1048–1051. n/a. [PubMed] [Google Scholar]
- 4.Mao J.J., Palmer C.S., Healy K.E., Desai K., Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5(1):8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavakoli J., Miar S., Majid Zadehzare M., Akbari H. Evaluation of effectiveness of herbal medication in cancer care: a review study. Iran J Cancer Prev. 2012;5(3):144–156. n/a. [PMC free article] [PubMed] [Google Scholar]
- 6.Mao J.J., Palmer S.C., Straton J.B., Cronholm P.F., Keddem S., Knott K., et al. Cancer survivors with unmet needs were more likely to use complementary and alternative medicine. J Cancer Surviv. 2008;2(2):116–124. doi: 10.1007/s11764-008-0052-3. [DOI] [PubMed] [Google Scholar]
- 7.Astin J.A. Why patients use alternative medicine: results of a national study. JAMA. 1998;279(19):1548–1553. doi: 10.1001/jama.279.19.1548. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z., Gu K., Zheng Y., Zheng W., Lu W., Shu X.O. The use of complementary and alternative medicine among Chinese women with breast cancer. J Altern Complement Med. 2008;14(8):1049–1055. doi: 10.1089/acm.2008.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Y.N., Deng G., Mao JJ. Practical application of "about herbs" website: herbs and dietary supplement use in oncology settings. Cancer J. 2019;25(5):357–366. doi: 10.1097/PPO.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao P.P., Lu W., Cui Y., Zheng Y., Gu K., Chen Z., et al. Ginseng and ganoderma lucidum use after breast cancer diagnosis and quality of life: a report from the Shanghai breast cancer survival study. PLoS ONE. 2012;7(6):e39343. doi: 10.1371/journal.pone.0039343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hapuarachchi Kk K.K. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere. 2018;9(5):1025–1052. doi: 10.5943/mycosphere/9/5/6. [DOI] [Google Scholar]
- 12.Wachtel-Galor S., Yuen J., Buswell J.A., Benzie I.F.F. In: Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Benzie IFF, Wachtel-Galor S, editors. Boca Raton (FL): CRC Press/Taylor & Francis Copyright © 2011 by Taylor and Francis Group, LLC; 2011. Ganoderma lucidum (Lingzhi or Reishi): a Medicinal Mushroom. [Google Scholar]
- 13.Wu G.-S., Guo J.-J., Bao J.-L., Li X.-W., Chen X.-P., Lu J.-J., et al. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum–a review. Expert Opin Investig Drugs. 2013;22(8):981–992. doi: 10.1517/13543784.2013.805202. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A. Ganoderma Lucidum: A traditional chinese medicine used for curing tumors. Int J Pharm Pharm Sci. 2021;13:1–13. doi: 10.22159/ijpps.2021v13i3.40614. [DOI] [Google Scholar]
- 15.Upton R. U.S.A. Canada: Santa Cruz; 2000. Pharmacopoeia A. Reishi Mushroom: Ganoderma Lucidum: Standards of Analysis. [Google Scholar]
- 16.Lai T., Gao Y., Zhou S. Global marketing of medicinal Ling Zhi mushroom Ganoderma lucidum (W. Curt.: fr.) Lloyd (Aphyllophoromycetideae) products and safety concerns. Int J Med Mushrooms. 2004;6(2) doi: 10.1615/IntJMedMushr.v6.i2.100. [DOI] [Google Scholar]
- 17.Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10(8):717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 18.Yuen J.W., Gohel M.D. Anticancer effects of Ganoderma lucidum: a review of scientific evidence. Nutr Cancer. 2005;53(1):11–17. doi: 10.1207/s15327914nc5301_2. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J., Slivova V., Harvey K., Valachovicova T., Sliva D. Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappaB signaling. Nutr Cancer. 2004;49(2):209–216. doi: 10.1207/s15327914nc4902_13. [DOI] [PubMed] [Google Scholar]
- 20.Ekiz E., Oz E., Abd El-Aty A.M., Proestos C., Brennan C., Zeng M., et al. Exploring the potential medicinal benefits of ganoderma lucidum: from metabolic disorders to coronavirus infections. Foods. 2023;12(7):1512. doi: 10.3390/foods12071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y., Tang W., Dai X., Gao H., Chen G., Ye J., et al. Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J Med Food. 2005;8(2):159–168. doi: 10.1089/jmf.2005.8.159. [DOI] [PubMed] [Google Scholar]
- 22.Geng P., Siu K.C., Wang Z., Wu J.Y. Antifatigue functions and mechanisms of edible and medicinal mushrooms. Biomed Res Int. 2017;2017 doi: 10.1155/2017/9648496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y., Xu X., Liu S., Huang L., Gu J. Ganoderma: a cancer immunotherapy review. Front Pharmacol. 2018;9:1217. doi: 10.3389/fphar.2018.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X., Ruiz Beguerie J., Sze D.M., Chan G.C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2016;4(4) doi: 10.1002/14651858.CD007731.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Mao J.J., Li S.Q., Lin H. Preliminary efficacy and safety of reishi & privet formula on quality of life among non-small cell lung cancer patients undergoing chemotherapy: a randomized placebo-controlled trial. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420944491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S.N., Nan F.H., Liu M.W., Yang M.F., Chang Y.C., Chen S. Evaluation of immune modulation by beta-1,3; 1,6 D-glucan derived from ganoderma lucidum in healthy adult volunteers, a randomized controlled trial. Foods. 2023;12(3) doi: 10.3390/foods12030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan S., de Mores A.R., Cohen L., Anwar M.M., Lazar F., Hicklen R., et al. Medicinal mushroom supplements in cancer: a systematic review of clinical studies. Curr Oncol Rep. 2023;25(6):569–587. doi: 10.1007/s11912-023-01408-2. [DOI] [PubMed] [Google Scholar]
- 28.Hui D., Bruera E. The edmonton symptom assessment system 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017;53(3):630–643. doi: 10.1016/j.jpainsymman.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battaglia Y., Zerbinati L., Piazza G., Martino E., Provenzano M., Esposito P., et al. Screening Performance of edmonton symptom assessment system in kidney transplant recipients. J Clin Med. 2020;9(4) doi: 10.3390/jcm9040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Y., Chen H., Zheng Y., Guo Y., Kwon J.H., Liu E., et al. Psychometric validation of the edmonton symptom assessment system in chinese patients. J Pain Symptom Manage. 2015;50(5):e2. doi: 10.1016/j.jpainsymman.2015.05.018. 712-17. [DOI] [PubMed] [Google Scholar]
- 31.Unlu A., Nayir E., Kirca O., Ozdogan M. Ganoderma Lucidum (Reishi Mushroom) and cancer. J BUON. 2016;21(4):792–798. n/a. [PubMed] [Google Scholar]
- 32.Zhong L., Yan P., Lam W.C., Yao L., Bian Z. Coriolus versicolor and ganoderma lucidum related natural products as an adjunct therapy for cancers: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:703. doi: 10.3389/fphar.2019.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H., Zhang Q., Zhao L., Huang X., Wang J., Kang X. Spore powder of ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/809614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bovbjerg D.H., Redd W.H., Maier L.A., Holland J.C., Lesko L.M., Niedzwiecki D., et al. Anticipatory immune suppression and nausea in women receiving cyclic chemotherapy for ovarian cancer. J Consult Clin Psychol. 1990;58(2):153–157. doi: 10.1037/0022-006x.58.2.153. [DOI] [PubMed] [Google Scholar]
- 35.Bower J.E. The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer. 2019;125(3):353–364. doi: 10.1002/cncr.31790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiche E.M., Nunes S.O., Morimoto H.K. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q., Li Y., Pei G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J Neuroinflammation. 2017;14(1):63. doi: 10.1186/s12974-017-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia X., Ma B., Xue F., Xing Y., Wu P., Li T., et al. Structure characterization and anti-inflammatory activity of polysaccharides from Lingzhi or reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes) by microwave-assisted freeze-thaw extraction. Int J Med Mushrooms. 2022;24(11):49–61. doi: 10.1615/IntJMedMushrooms.2022045268. [DOI] [PubMed] [Google Scholar]
- 39.Cor Andrejc D., Knez Z., Knez Marevci M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: an overview. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.934982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trimborn A., Senf B., Muenstedt K., Buentzel J., Micke O., Muecke R., et al. Attitude of employees of a university clinic to complementary and alternative medicine in oncology. Ann Oncol. 2013;24(10):2641–2645. doi: 10.1093/annonc/mdt299. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Arye E., Samuels N., Goldstein L.H., Mutafoglu K., Omran S., Schiff E., et al. Potential risks associated with traditional herbal medicine use in cancer care: a study of Middle Eastern oncology health care professionals. Cancer. 2016;122(4):598–610. doi: 10.1002/cncr.29796. [DOI] [PubMed] [Google Scholar]
- 42.Pihlak R., Liivand R., Trelin O., Neissar H., Peterson I., Kivistik S., et al. Complementary medicine use among cancer patients receiving radiotherapy and chemotherapy: methods, sources of information and the need for counselling. Eur J Cancer Care (Engl) 2014;23(2):249–254. doi: 10.1111/ecc.12132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study will be available from the corresponding author upon reasonable request.