Abstract
Effects of the initial peritoneal dialysis (PD) prescription on clinical outcomes are unknown in Japan. We conducted a cohort study using data from Peritoneal Dialysis Outcomes and Practice Patterns Study. The patients were divided into two groups by the volume of the initial PD prescription (≤ 4 L/day or > 4 L/day). Cause-specific Cox proportional hazards survival models were used to model the association between different PD prescriptions and the clinical outcomes. The outcomes included transfer to HD, mortality, the composite of mortality and transfer to HD, peritonitis, hospitalization, and the patient-reported outcomes (PROs). Of the 342 patients, 98 were prescribed ≤ 4 L/day, and 244 were prescribed > 4 L/day. Patients prescribed ≤ 4 L/day were older with a lower percentage being male, had more cardiovascular and cerebrovascular disease but lower diabetes prevalence, were more likely to be receiving CAPD, used more assisted PD, and had lower BMI and mean serum creatinine levels. There were no significant differences between groups in terms of transfer to HD, mortality, transfer to HD or mortality, hospitalization, incidence of peritonitis, and PROs. Patients with initial PD prescriptions of ≤ 4 L/day compared to > 4 L/day had similar clinical outcomes. This practice may provide health economic benefits in Japan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81934-6.
Keywords: Peritoneal dialysis prescription, Mortality, Peritonitis, Transfer to hemodialysis, Incremental peritoneal dialysis
Subject terms: Renal replacement therapy, End-stage renal disease
Introducton
The number of Japanese chronic kidney disease patients has reached 14.8 million, and it is increasingly recognized as a common disease among the elderly1. According to the annual survey of the Japanese Society for Dialysis Therapy Renal Data Registry, the number of dialysis patients is also on the rise; there were reportedly 9445 peritoneal dialysis (PD) patients in 2018, with PD patients accounting for 2.8% of 339,841 dialysis patients2.
The Japanese survey conducted at the end of 2016 investigating PD prescriptions found that 80% of patients with PD vintage of less than 1 year were prescribed a PD volume of < 8 L/day, with the prescribed PD volume increasing with increasing PD vintage. In addition, longer PD vintage was associated with longer PD treatment time3. In other words, low volume PD prescription is initially applied, and a gradual increase in the PD volume is widely practiced in Japan.
The strategy of gradually increasing the prescription according to residual kidney function (RKF) is widely known as incremental PD (incrPD)4. The International Society for Peritoneal Dialysis (ISPD) practice recommendations have emphasized the importance of quality of life (QOL), and indicate that incrPD should be considered in PD patients to minimize treatment burden5. The potential clinical implications of incrPD include not only improved QOL for patients and their families due to reduction in the number of exchanges, but also reduced risk of peritonitis due to fewer connections, peritoneal membrane preservation due to reduced glucose exposure, preservation of RKF, reduced systemic glucose complications, and reduced financial costs and environmental waste6. Furthermore, lower dwell volumes result in lower intraperitoneal pressure, which might lead to fewer mechanical side effects, such as back pain, abdominal fullness, and heartburn7. IncrPD might also allow for less anxiety and stress when starting PD, and increase life participation through better use of time. On the other hand, there are concerns about reduced small solute clearance, increased risk of fluid overload, and poorer patient survival6.
At this time, the optimal incrPD prescription has not been established, and the potential clinical implications of initial low PD prescription in Japanese PD patients are unclear. Thus, we investigated the effects of different initial PD prescriptions on clinical outcomes in Japanese PD patients using longitudinal Japanese PD patient data from a national sample of Japanese PD facilities that have participated in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS).
Methods
Data source
PDOPPS is an international, prospective cohort study of adults (≥ 18 years of age) receiving chronic, maintenance PD for kidney failure8. Patients were enrolled randomly from national samples of randomly selected PD facilities treating a minimum of 20 PD patients in participating countries. All methods were carried out in accordance with relevant guidelines and regulations. Study approval was obtained from a central Institutional Review Board in the United States as well as all 30 Japanese PD centers participating in PDOPPS recruitment. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations. Written informed consent was obtained from all patients eligible for study participation in accordance with the Declaration of Helsinki.
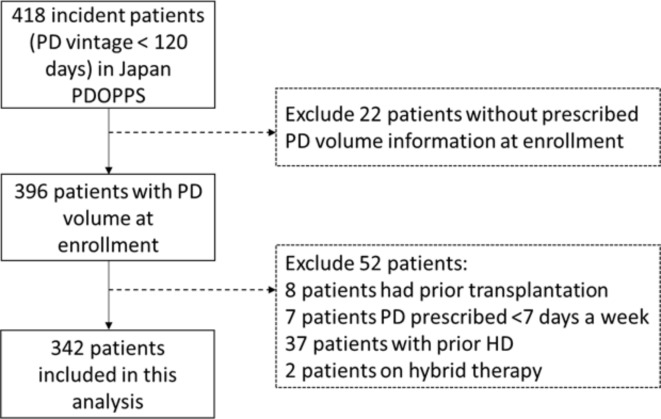
The present study was restricted to incident patients enrolled in PDOPPS phase 1–2 from Japan in 2014–2022. A total of 418 incident patients with PD vintage < 120 days at study enrollment were included. Patients who were missing information on prescribed PD volume at enrollment (n = 22), or who had prior transplantation (n = 8), had PD prescribed for < 7 days a week (n = 7), had prior hemodialysis (HD) (n = 37), or were receiving hybrid therapy (n = 2) were excluded. Finally, 342 patients were included (Fig. 1).
Fig. 1.
Study flow chart. PD; peritoneal dialysis, HD; hemodialysis, PDOPPS; Peritoneal Dialysis Outcomes and Practice Patterns Study.
Data on demographics, comorbid conditions, laboratory values, and prescriptions were abstracted from medical records at study entry (baseline), and clinical outcomes including transfer to HD, mortality, peritonitis, and hospitalization were collected continuously during study follow-up using uniform and standardized data collection tools.
Study design
In the study conducted by Lee et al.9 incrPD was defined as two or fewer exchanges per day (≤ 4 L/day). Therefore, we divided patients into two groups according to the volume of PD prescription dose at the start of PD; ≤ 4 L/day or > 4 L/day. The outcomes of interest in the present study included transfer to HD, mortality, the composite of mortality and transfer to HD, peritonitis, hospitalization, and patient-reported outcomes (PROs): physical component summary score (PCS), mental component summary score (MCS) and the Center for Epidemiologic Studies Depression Rating Scale (CES-D) ≥ 10 indicative of depression symptoms. Transfer to HD was defined as a planned modality switch (clinician-reported), or transfers from PD to HD in which the patient did not return to PD within 12 weeks (84 days). Hybrid therapy switches (the addition of any HD to continued PD therapy) were counted as an HD transfer event. An infection worksheet capturing the first presentation was completed for each resolved peritonitis episode during PDOPPS follow-up. Peritonitis episodes were additionally ascertained from facility-reported hospitalizations with a peritonitis diagnosis. Hospitalization was defined as any inpatient event. The PCS and MCS derived from the Kidney Disease Quality of Life Short Form (KDQOL-36) and CES-D were taken from a patient’s first reported patient questionnaire.
The causes of transfer from PD were classified into suboptimal dialysis due to problems with solute and water clearance, risk or diagnosis of encapsulating peritoneal sclerosis (EPS), psychosocial medical reasons, PD access-related mechanical dysfunction, peritoneal leak and hernia, peritonitis, and other PD catheter infection.
Statistical analysis
Event rates were calculated as number of patients with events over total study time at risk. Peritonitis time at risk for each patient started at study baseline and continued until the earliest of the following: first peritonitis episode during the study or the end of data for a given patient (e.g., due to end of data collection, patient transfer to another facility or kidney transplantation, permanent transfer to HD or temporary transfer to HD lasting more than 84 days [censored at the date of transfer], or death). For all-cause mortality, follow-up started at study baseline and ended at whichever came first: death, 60 days after modality switch, loss to follow-up, transplantation, or study end. For mortality and transfer to HD, follow-up started at study enrollment and ended with death, transfer to HD, loss to follow-up, transplantation, or study end, whichever occurred earliest. In the analyses for mortality and the composite outcome of mortality and transfer to HD, patients were followed for an additional 60 days after transfer to HD, with mortality within this window attributed to PD. Hospitalization time at risk started at study baseline and continued until the earliest of first hospitalization or the end of data for a given patient, as stated above.
Cause-specific Cox proportional hazards survival models were used to model the association between different PD prescriptions and time to first peritonitis, hospitalization, mortality, transfer to HD, and the composite of mortality and transfer to HD; the competing events were treated as censored observations in the analysis. All models accounting for facility clustering using robust sandwich covariance estimators, were stratified by PDOPPS phase and PD modality (automated PD [APD]/ continuous ambulatory PD [CAPD]). Linear mixed models with a random intercept were used to estimate the mean difference in MCS and PCS scores between the two different PD prescriptions whereas Generalized Estimating Equations were used to assess the relationship of PD prescriptions with the odds of a patient having a CES-D score ≥ 10 vs < 10. The point estimate and 95% confidence intervals (CIs) are reported in models stratified by PD modality with increasing levels of stepwise adjustment for potential confounders: model 1, unadjusted; model 2, adjusted for age, sex, body mass index (BMI); model 3, further adjusted for modified Charlson comorbidity index previously validated in DOPPS research10, plus adjusted for albumin, potassium, and hemoglobin; model 4, further adjusted for estimated glomerular filtration rate (eGFR) just before starting PD, calculated based on the formula of Matsuo et al11; and model 5, further adjusted for icodextrin and assisted PD (assisted PD was defined as caregiver involvement in PD exchange). A separate model adjusting for a propensity score for ≤ 4 L/day only was also performed. The propensity score for ≤ 4 L/day was estimated using a multivariable logistic regression model that included the covariates in model 4. Causes of death and reasons for transfer to HD were analyzed.
Missing data were imputed by the chained-equations method as implemented with IVEware for SAS12. Twenty imputations each were performed for patient-level variables and merged by replicate number. Analyses were performed separately on each imputed dataset, with results combined using the Rubin method13. Data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Descriptive data
Of the 342 patients on PD < 120 days in Japan at study enrollment included in this analysis, 98 (29%) were prescribed a volume of ≤ 4 L/day, and 244 (71%) were prescribed > 4L/day. Patients with ≤ 4 L/day were older with a lower percentage being male, had more cardiovascular and cerebrovascular disease, had lower diabetes prevalence, were slightly more likely to be on CAPD, and used more assisted PD; these patients had lower BMI and mean serum creatinine levels, and higher eGFR before the start of PD. Mean ± standard deviation (SD) PD volume was 2.9 ± 0.8 L/day for patients with ≤ 4 L/day group and 6.7 ± 1.9 L/day for patients in > 4L/day group. Other average laboratory values and comorbidities were similar between the two groups (Table 1).
Table 1.
Patients’ characteristics in patients with ≤ 4 L/day and patients with > 4 L/day.
| Characteristic | ≤ 4 L/day | > 4 L/day |
|---|---|---|
| (n = 98) | (n = 244) | |
| Demographics | ||
| Age, y | 67 ± 14 | 61 ± 13 |
| Sex (% male) | 63% | 74% |
| Comorbidity history | ||
| Diabetes | 42% | 49% |
| Hypertension | 95% | 95% |
| Coronary artery disease | 16% | 12% |
| Heart failure | 19% | 16% |
| Cerebrovascular disease | 15% | 8% |
| Peripheral vascular disease | 9% | 6% |
| Other cardiovascular disease | 20% | 10% |
| Others | 19% | 15% |
| Charlson comorbidity index | 6.3 ± 1.9 | 5.5 ± 1.8 |
| Dialysis treatments | ||
| PD volume, L per day | 2.9 ± 0.8 | 6.7 ± 1.9 |
| APD | 40% | 44% |
| CAPD number of exchanges | ||
| < 4 | 88% | 51% |
| 4 | 12% | 49% |
| Assisted PDa | 17% | 11% |
| Icodextrin | 30% | 35% |
| Lab/biometric marker | ||
| Body mass index, kg/m2 | 21.9 ± 3.4 | 23.9 ± 4.0 |
| Serum albumin, g/dL | 3.3 ± 0.4 | 3.3 ± 0.5 |
| Hemoglobin, g/dL | 10.8 ± 1.1 | 10.9 ± 1.4 |
| Serum phosphorus, mg/dL | 4.9 ± 1.4 | 4.9 ± 1.2 |
| Serum calcium, mg/dL | 8.3 ± 0.8 | 8.2 ± 0.7 |
| Serum magnesium, mg/dL | 2.0 ± 0.4 | 2.1 ± 0.4 |
| Serum potassium, mEq/L | 4.4 ± 0.8 | 4.2 ± 0.7 |
| 24-h urine volume, Lb | 1.1 ± 0.5 | 1.0 ± 0.5 |
| Bicarbonate, mEq/L | 23.6 ± 3.5 | 24.6 ± 3.6 |
| Creatinine, mg/dL | 6.6 ± 2.0 | 8.0 ± 2.7 |
| Intact PTH, pg/mL | 302 ± 235 | 338 ± 232 |
| Sodium, mEq/L | 138.0 ± 4.1 | 139.2 ± 3.6 |
| Total cholesterol, mg/dL | 181.6 ± 37.9 | 174.1 ± 37.8 |
| Triglycerides, mg/dL | 131.7 ± 69.2 | 137.6 ± 84.4 |
| Residual Kt/V urea | 1.2 ± 0.4 | 0.8 ± 0.3 |
| Peritoneal Kt/V urea | 0.7 ± 0.3 | 1.0 ± 0.3 |
| eGFR before PD start, mL/min/1.73 m2 | 7.3 ± 2.3 | 6.4 ± 2.2 |
| Medications | ||
| Diuretic | 66% | 64% |
| RAASi | 59% | 59% |
The values expressed as means ± SD or the proportions of patients.
PD: peritoneal dialysis, ESKD: end-stage kidney disease, APD: automated peritoneal dialysis, PTH: parathyroid hormone, eGFR: estimated glomerular filtration rate, RAASi: renin–angiotensin–aldosterone system inhibitor.
aAssisted PD was defined as caregiver involvement in PD exchange.
bAmong patients with 24 h urine volume measured at study enrollment (~ 50%); in phase 2, anuria was specifically queried, and only 1 patient was recorded as anuric.
The average daily PD volume in all patients was 5.6 L, with 89% of patients using 8 L or less; 30% of CAPD patients and 27% of APD patients were prescribed PD volume ≤ 4L/ day (Supplemental Figure S1).
Figure 2 shows the PD volume trajectories for ≤ 4 L/day and > 4 L/day. The PD volume of > 4 L/day was stable during the study period, whereas the PD volume of ≤ 4 L/day increased over time, but was still lower than the PD volume of > 4 L/day on average after two years of follow-up. The median PD volume at the 0-, 1- and 2-year time points is 3 L, 3 L and 4.25 L for the ≤ 4L/ day group compared to 6 L, 7.25 L and 7.9 L for the > 4L/day. Group, respectively.
Fig. 2.
PD volume trajectories for ≤ 4 L/day and > 4 L/day in incident Japanese PD patients. PD; peritoneal dialysis.
Associations between different PD prescriptions at start and clinical outcomes
Overall, the median follow-up was 21 months [IQR 13–30 months], and 79 patients transferred to HD. Among these HD transfers, 3 of 18 patients (17%) prescribed ≤ 4 L/day, and 13 of 61 (21%) prescribed > 4 L/day transferred to hybrid (PD + HD) therapy. A lower HR for transfer to HD was observed for ≤ 4 L/day, even after full adjustment (HR 0.70, 95% CI (0.35–1.41)), although the CI was wide (Table 2, model 5). Infection-related complications were the most common reason reported for transfer to HD in patients in both ≤ 4 L/day and > 4 L/day, followed by water problems and solute clearance (Supplemental Table S1). Solute clearance as the primary cause for transferring to HD was > threefold more common in the ≤ 4L/day group (17%) than for the > 4L/day group (5%). Conversely, water problems as the cause for transferring to HD was 1.4-fold more common in the > 4L/day group (31%) than for the ≤ 4L/day group (22%). Similar results were observed for the composite outcome of transfer to HD and mortality (HR 0.94, 95% CI (0.58–1.53)) (Table 2).
Table 2.
Associations between initial peritoneal dialysis prescription at start and clinical outcomes.
| Outcome | PD Volume | N outcomes | Hazard Ratio (95% CI), PD volume ≤ 4 L/day (N pts = 98) vs. > 4 L/day (N pts = 244) | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | Model PSf | |||
| Transfer to HD | > 4 L/day | 61 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 18 |
0.70 (0.37, 1.34) |
0.76 (0.39, 1.50) |
0.69 (0.34, 1.38) |
0.69 (0.34, 1.41) |
0.70 (0.36, 1.56) |
0.81 (0.41, 1.59) |
|
| Mortality | > 4 L/day | 16 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 14 |
2.41 (1.31, 4.44) |
1.72 (0.96, 3.10) |
1.65 (0.84,3.23) |
1.56 (0.80, 3.06) |
1.60 (0.77, 3.34) |
1.87 (0.97, 3.62) |
|
| Transfer to HD or mortality | > 4 L/day | 77 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 32 |
1.02 (0.65, 1.60) |
1.06 (0.66, 1.72) |
0.97 (0.59, 1.58) |
0.96 (0.59, 1.58) |
0.94 (0.58, 1.53) |
1.05 (0.65, 1.71) |
|
| Peritonitis | > 4 L/day | 63 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 22 |
0.74 (0.49, 1.11) |
0.73 (0.48, 1.12) |
0.80 (0.51, 1.26) |
0.78 (0.48, 1.26) |
0.77 (0.47, 1.25) |
0.76 (0.51, 1.14) |
|
| Hospitalization | > 4 L/day | 142 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 68 |
1.17 (0.86, 1.64) |
1.18 (0.81, 1.56) |
1.18 (0.87, 1.67) |
1.16 (0.87, 1.66) |
1.19 (0.87, 1.63) |
1.11 (0.81, 1.52) |
|
PD: peritoneal dialysis, HD: hemodialysis, CAPD: continuous ambulatory peritoneal dialysis, APD: automated peritoneal dialysis, eGFR: estimated glomerular filtration rate, BMI: body mass index, N pts: number of patients.
a. Stratified by PD modality (CAPD/APD);
b. Model 1 + adjusted for age, sex, BMI.
c. Model 2 + Charlson comorbidity index, albumin, potassium, hemoglobin.
d. Model 3 + eGFR.
e. Model 4 + icodextrin, assisted PD.
f. Stratified by PD modality (CAPD/APD); adjusted for propensity score only.
Thirty patients died. The unadjusted HR for ≤ 4 L/day was 2.41 (95% CI 1.31–4.44) with > 4 L/day as the reference, with this HR in part possibly reflecting the much older mean age and comorbidity burden for patients in the ≤ 4 L/day group. The HR tended to decrease with each adjustment for confounding factors, but remained high, at 1.60 (95% CI 0.77–3.34), in the fully adjusted model and no significant difference was observed between the two groups. Cardiovascular deaths were the main cause of death in both ≤ 4 L/day and > 4 L/day (Supplemental Table S2).
Peritonitis events occurred in 85 patients. The incidence of first peritonitis in all patients was 0.24 (95% CI 0.18–0.27) episodes/patient-year. The incidence of peritonitis for ≤ 4 L/day was 0.20 (95% CI 0.13–0.30) episodes/patient-year, and for > 4 L/day it was 0.25 (95% CI 0.20–0.32) episodes/patient-year. The adjusted HR for the association of ≤ 4 L/day and peritonitis was 0.77 (95% CI 0.47–1.25) in the fully adjusted model with > 4 L/day as the reference and no significant difference was observed between the two groups. Model results were also consistent across different levels of adjustment.
A total of 210 patients were hospitalized. The first hospitalization rate in all patients was 0.85 (95% CI 0.79–0.95) episodes/patient-year. The hospitalization rate for ≤ 4 L/day was 0.82 (95% CI 0.78–0.85) episodes/patient-year, and that for > 4 L/day was 0.88 (95% CI 0.70–0.92) episodes/patient-year. A slightly higher risk of hospitalization was seen in ≤ 4 L/day (HR 1.19, 0.87–1.63) in the most fully adjusted model (Table 2).
An additional modeling approach—adjusting for the propensity score for being prescribed ≤ 4 L/day—was also performed and was shown to have similar results to those prescribed above.
Associations between different PD prescriptions at start and PROs
The mean ± SD PCS (43.3 ± 8.9 vs 43.9 ± 8.5) and MCS (45.7 ± 10.3 vs 46.6 ± 11.4) were similar in patients with PD volume ≤ 4 L/day (vs > 4 L/day) (Table 3). After adjustment, patients with PD volume ≤ 4 L/day (vs > 4 L/day) had 0.18 lower PCS (95% CI: −2.56, 2.21) and 0.21 lower MCS (95% CI: −3.44, 3.01). However, no significant difference was observed between the two groups.
Table 3.
Associations between initial peritoneal dialysis prescription at start and patient-reported outcomes.
| Outcome | PD Volume | QoL score, mean ± SD | Score difference (95% CI), PD volume ≤ 4 L/day (N pts = 98) vs. > 4 L/day (N pts = 244) | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | Model PSf | |||
| PCS | > 4 L/day | 43.9 ± 8.5 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 43.3 ± 8.9 |
-0.68 (-3.07,1.71) |
-0.21 (-2.62,2.20) |
-0.56 (-2.89,1.78) |
-0.38 (-2.81,2.06) |
-0.18 (-2.56,2.21) |
-0.35 (-2.93,2.23) |
|
| MCS | > 4 L/day | 46.6 ± 11.4 | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 45.7 ± 10.3 |
-0.31 (-3.30,2.67) |
0.00 (-3.07,3.06) |
-0.28 (-3.37,2.81) |
-0.26 (-3.47,2.94) |
-0.21 (-3.44,3.01) |
-0.50 (-3.74,2.75) |
|
| Outcome | PD Volume | Outcome, % | Odds ratio (95% CI), PD volume ≤ 4 L/day vs. > 4 L/day | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | Model PSf | |||
| CESD 10 + | > 4 L/day | 41% | ref | ref | ref | ref | ref | ref |
| ≤ 4 L/day | 39% |
0.81 (0.47,1.40) |
0.87 (0.49,1.53) |
0.97 (0.54,1.76) |
0.90 (0.49,1.67) |
0.90 (0.49,1.67) |
0.93 (0.51,1.69) |
|
PD: peritoneal dialysis, HD: hemodialysis, CAPD: continuous ambulatory peritoneal dialysis, APD: automated peritoneal dialysis, eGFR: estimated glomerular filtration rate, BMI: body mass index, N pts: number of patients
a. adjusted for PD modality (CAPD/APD);
b. Model 1 + adjusted for age, sex, BMI
c. Model 2 + Charlson comorbidity index, albumin, potassium, hemoglobin
d. Model 3 + eGFR
e. Model 4 + icodextrin, assisted PD
f. Stratified by PD modality (CAPD/APD); adjusted for propensity score only
The prevalence of patient-reported symptoms of depression (CES-D score ≥ 10) was slightly lower (39% vs 41%) in patients with PD volume ≤ 4 L/day (vs > 4 L/day). The adjusted OR of CES-D ≥ 10 vs < 10 was 0.90 (95% CI: 0.49, 1.67) in patients with PD volume ≤ 4 L/day (vs > 4 L/day).
Discussion
This multicenter, prospective, cohort study is the first to examine the association between different initial PD prescriptions and clinical and patient-reported outcomes in Japanese PD patients. We found no significant differences in transfer to HD, mortality, transfer to HD or mortality, incidence of peritonitis, hospitalization, and PROs between initial PD volumes of ≤ 4 L/day and > 4 L/day. Interestingly, although the > 4 L/day group had approximately twice the PD volume as the ≤ 4 L/day group during the first 2 years on PD, there were no differences in various clinical outcomes.
As two randomized control trials have shown that there is no survival advantage in aiming for high dialysis efficiency in PD patients14,15, incrPD is currently attracting attention in PD therapy. In the 2000s, there were several reports on the incrPD, mainly from Italy, although those were single-arm studies with a small number of patients16–18. In recent years, however, large-scale clinical studies comparing incrPD and standard PD (or full-dose PD) have been conducted around the world. Since 2016, one clinical study each on incrPD has been conducted in the United States19, United Kingdom20, and Portugal21, two studies each have been conducted in Italy22,23, Canada24,25, and South Korea9,26, three studies were in Australia27–29, and five in China30–34. Of these 17 studies, 13 studies were conducted at a single center (11 were single center retrospective cohort studies, one was a single center randomized controlled trial, and one was a single center cross-sectional study), and four studies were conducted using registry data. The single-center retrospective cohort studies had a minimum of 87 patients and a maximum of 1315 patients, which was significantly higher than previous observational studies. The definitions of incrPD and standard PD were different in each study. To divide incrPD and standard PD, nine studies applied the number of exchanges per day, one study applied the number of exchanges per week, five studies applied PD volume per day, and two studies applied PD volume per week. These studies compared patient survival, technique survival, peritonitis, hospitalization, RKF and QOL as outcomes.
Thirteen studies investigated patient survival9,19–23,26,28,29,31–34, with three of them showing a survival advantage for incrPD (one of which had a survival advantage of up to 6 years)21,32,33, nine studies showing no difference between the two groups9,19,20,22,23,26,28,29,31, and one study reporting that standard PD had a survival advantage34. Although there was no significant difference in patient survival between the two groups in the present study, the unadjusted HR for death was higher in patients with a PD volume ≤ 4 L/day. However, this could be because patients with a PD volume ≤ 4 L/day on average were 6 years older than patients with a PD volume > 4 L/day, and had greater comorbidity burden—especially for cardiovascular and cerebrovascular comorbidities, and possibly -more advanced frailty in view of all of this, and especially given that the mortality difference between groups was not significant after adjustment for other factors.
Technique survival was previously investigated in 12 studies9,19–21,23,26,28–33, with three studies showing the superiority of incrPD (one of which demonstrated superior technique survival in the low PD intensity group)20,21,28, and nine studies showing no difference between the two groups9,19,23,26,29–33. Surprisingly, no study has reported that incrPD was inferior to standard PD in terms of technical survival. However, since the definition of technique survival varied between studies, it is difficult to directly compare them. Additionally, it is already known that there is substantial heterogeneity in the definition of technique survival among clinical studies35, suggesting that there is need for international unification of the definition. In the present study, no significant difference in transfer to HD was observed between the two groups, as in many other studies.
Of the thirteen studies that compared the incidence of peritonitis9,20–23,26–33, six studies showed the superiority of incrPD (one of which showed a nominally longer peritonitis-free survival time)23,26,28,29,31,33, In these six studies, the peritonitis rates were 0.06–0.23 episode/patient-year in the incrPD patients, compared to 0.14–0.32 peritonitis episodes/patient-year in the standard PD patients. On the other hand, seven studies found no difference between the two groups9,20–22,27,30,32. Initiating PD with a reduced number of daily changes could theoretically be expected to reduce the incidence of peritonitis due to the reduced risk of contamination. However, several studies that reduced the number of exchanges showed no advantage in terms of peritonitis. In the present study, the HR for the association between a PD volume of ≤ 4 L/day and peritonitis was consistently lower in various adjusted models, although no significant differences were observed, with the incidence rates in both groups seen to be below the new ISPD guideline targets36.
Six studies examined hospitalization19,21–23,26,29, with three studies showing an advantage with incrPD21–23 and three studies showing no difference between the two groups19,26,29. However, the definition of a hospitalization event also differed between studies, with one study comparing hospitalization-free survival and another comparing hospitalization rates. Although there was no difference in the first hospitalization rates between the two groups in our study, the rates were higher in our study compared to previous studies. While the reason for this observation is not clear, we believe it could be related to the older age of patients in the present study and differences in the admission criteria.
Preservation of RKF is an issue of high concern to medical professionals managing PD patients. Twelve of the 17 studies investigated longitudinal changes in RKF with incrPD and standard PD9,19,20,22,23,26,27,29–33. Four of them showed the superiority of incrPD9,22,32,33, and the remaining eight studies reported no difference between the two groups19,20,23,26,27,29–31. We were unable to include RKF in the analysis because there was a large amount of missing data regarding RKF.
Naljayan et al. reported on the effect of incrPD on QOL19. They evaluated QOL separately for APD patients and CAPD patients, and reported the superiority of incrPD only in CAPD patients. Their results suggest that an increase in the number of manual bag changes affects QOL. In our study, we investigated the relationship between PD volume and QOL, but found no significant difference between the two groups.
The present study has some limitations. First, it was an observational study, the sample size was relatively small, and residual confounding factors (e.g., activities of daily living, socioeconomic factors, mental status, reason for choosing PD, other unmeasured confounders) could not be excluded. Therefore, the present study lacked statistical power, and hence, one should be cautious when attempting to generalize the results of this study. However, the number of patients was larger than some single-center, retrospective, cohort studies previously reported, and it is noteworthy that the study was prospectively conducted in a multicenter setting. In addition, we collected several variables as possible confounders for investigation, and performed the analysis taking them into account. Second, it was not possible to compare the change of RKF, which has a significant impact on the prognosis of PD patients, because there were many missing data. It is well-known that RKF is a critical factor that affects the survival of PD patients. However, we showed that 24-h urine volumes which are a reflection of RKF were similar for the two groups. A large, randomized, controlled trial comparing defined outcome measures is needed to resolve confounding biases and clarify causality. Third, an important study limitation is that it is not clear why the lower PD prescription was chosen in the patients. Finally, no data were provided regarding dwell time, an important factor that may impact solute or excess water removal.
In conclusion, the present study showed that patients with a PD volume of ≤ 4 L/day have similar outcomes with respect to patient survival, transfer to HD, hospitalization and peritonitis, as those receiving dialysis with a PD volume of > 4 L/day. In addition, patients with a PD volume of ≤ 4 L/day may reduce medical costs compared with patients with a PD volume of > 4 L/day.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the contributions of the staffs, nurses, and all investigators who work at participating Japanese PD Outcomes and Practice Patterns Study (PDOPPS-Japan); PDOPPS-Japan committee member: Mizuya Fukasawa, Yasuhiko Ito, Hideki Kawanishi, Ikuto Masakane, Jun Minakuchi, Hidetomo Nakamoto, Kosaku Nitta, Munekazu Ryuzaki, Ken Tsuchiya, Tadashi Tomo, Susumu Takahashi, Kenji Tsuchida, and Akihiro C Yamashita We acknowledge and thank the following individuals for their contributions. PDOPPS Steering Committee members: David Johnson (Australia); Jeffrey Perl (Canada); Hideki Kawanishi (Japan); Yong-Lim Kim (South Korea); Talerngsak Kanjanabuch (Thailand); Simon Davies (United Kingdom); Angelito Bernardo, Ron Pisoni, Bruce Robinson, Jenny Shen (United States). Additional PDOPPS Research Group members: Sunil Badve, Neil Boudville, Fiona Brown, Josephine Chow, John Collins, Rachael Morton, Scott Wilson (Australia); Andreas Vychytil (Austria); Wim Van Biesen (Belgium); Ana Figueiredo, Thyago de Moraes (Brazil); Gillian Brunier, Arsh Jain, Vanita Jassal, Sharon Nessim, Matthew Oliver, Valerie Price, Rob Quinn (Canada); Wei Fang (China); CC Szeto, Angela Wang (Hong Kong); Mizuya Fukasawa, Yasuhiko Ito, Munekazu Ryuzaki, Tadashi Tomo (Japan); Alfonso Cueto Manzano (Mexico); Mark Marshall (New Zealand); Susanne Ljungman (Sweden); Sarinya Boongird, Chanchana Boonyakrai, Areewan Cheawchanwattana, Guttiga Halue, Suchai Sritippayawan, Sajja Tatiyanupanwong, Kriang Tungsanga (Thailand); Elaine Bowes, Edwina Brown, Richard Fluck, Bak Leong Goh, Helen Hurst, Martin Wilkie, Graham Woodrow (United Kingdom); Filitsa Bender, Judith Bernardini, Dinesh Chatoth, John Crabtree, Fred Finkelstein, Arshia Ghaffari, Rajnish Mehrotra, Beth Piraino, Martin Schreiber, Isaac Teitelbaum (United States). Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This manuscript was funded in part by Baxter Ltd (Japan). Global support for the ongoing DOPPS Programs is provided without restriction on publications (see https://www.dopps.org/AboutUs/Support.aspx for more information). Local support is provided by the Japanese Society for Peritoneal dialysis (JSPD).
Author contributions
T. S., J. Z., C. T., B. B., M. C., R, L. P., J. P., K. T., H. K. and J. M. made a substantial contribution to the concept or design of the work; or acquisition, analysis or interpretation of data. T. S., J. Z., C. T., B. B., M. C., R, L. P., J. P., K. T., H. K. and J. M. drafted the article or revised it critically for important intellectual content. T. S., J. Z., C. T., B. B., M. C., R, L. P., J. P., K. T., H. K. and J. M. approved the version to be published. T. S., J. Z., C. T., B. B., M. C., R, L. P., J. P., K. T., H. K. and J. M. should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. The authors accept direct responsibility for the manuscript on behalf of the study group.
Data availability
The data that support the findings of this study are available from Arbor Research Collaborative for Health, but restrictions apply to the availability of these data which were used for the current study, and so are not publicly available. However, the data underlying this article will be shared on reasonable request to the corresponding author.
Competing interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors have read and understood Peritoneal Dialysis International’s policy on conflicts of interest disclosures. T. S. has received speaker honorarium from Baxter Healthcare and received research grant from Terumo Corporation. J. Z., C. T., B.B. and R, L. P. are employees of Arbor Research Collaborative for Health, which administers the DOPPS programs. M. C. has received travel support from Amgen and is supported by a Queensland Advancing Clinical Research Fellowship. J. P. has received speaking honoraria from AstraZeneca, Baxter Healthcare, DaVita Healthcare Partners, Fresenius Medical Care, Dialysis Clinics Incorporated and Satellite Healthcare. He has served as a consultant for Baxter Healthcare, DaVita Healthcare Partners, Fresenius Medical Care and LiberDi. K. T., H. K. and J. M. declare that they have no relevant financial interest.
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. Study approval was obtained from a central institutional review board. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations. Written informed consent was obtained from all patients eligible for study participation in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagai, K. et al. Estimating the prevalence of definitive chronic kidney disease in the Japanese general population. Clin Exp Nephrol.25, 885–892 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Nitta, K. et al. Annual dialysis data report 2018, JSDT Renal Data Registry: Dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Renal Replacement Therapy.6, 51 (2020). [Google Scholar]
- 3.Masakane, I. et al. Annual dialysis data report 2016, JSDT renal data registry. Renal Replacement Therapy.4, 45 (2018). [Google Scholar]
- 4.Mehrotra, R., Nolph, K. D. & Gotch, F. Early initiation of chronic dialysis: Role of incremental dialysis. Perit Dial Int.17, 426–430 (1997). [PubMed] [Google Scholar]
- 5.Brown, E. A. et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int.40, 244–253 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Cheetham, M. S. et al. Incremental versus standard (full-dose) peritoneal dialysis. Kidney Int Rep.7, 165–176 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake, P. G., Dong, J. & Davies, S. J. Incremental peritoneal dialysis. Perit Dial Int.40, 320–326 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Perl, J. et al. The peritoneal dialysis outcomes and practice patterns study (PDOPPS): Unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int.36, 297–307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, Y. et al. Incremental peritoneal dialysis may be beneficial for preserving residual renal function compared to full-dose peritoneal dialysis. Sci Rep.9, 10105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikbov, B. et al. Hemodialysis practice patterns in the Russia Dialysis Outcomes and Practice Patterns Study (DOPPS), with international comparisons. Hemodial Int.21, 393–408 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Matsuo, S. et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 5, 982–992 (2009). [DOI] [PubMed]
- 12.Raghunathan, T. E., Lepkowski, J. E., Hoewyk, J. V. & Solenberger, P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology.27, 85–96 (2001). [Google Scholar]
- 13.Little, R. J. A. & Rubin, D. B. Statistical analysis with missing data (Wiley, 1987). [Google Scholar]
- 14.Paniagua, R. et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol.13, 1307–1320 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Lo, W. K. et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int.64, 649–656 (2003). [DOI] [PubMed] [Google Scholar]
- 16.De Vecchi, A. F, Scalamogna, A., Finazzi, S., Colucci, P. & Ponticelli, C. Preliminary evaluation of incremental peritoneal dialysis in 25 patients. Perit Dial Int. 20, 412–417 (2000). [PubMed]
- 17.Neri, L., Viglino, G., Cappelletti, A., Gandolfo, C. & Barbieri, S. Incremental dialysis with automated peritoneal dialysis. Adv Perit Dial.19, 93–96 (2003). [PubMed] [Google Scholar]
- 18.Viglino, G., Neri, L. & Barbieri, S. Incremental peritoneal dialysis: Effects on the choice of dialysis modality, residual renal function and adequacy. Kidney Int Suppl.108, S52–S55 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Naljayan, M. et al. Use of incremental peritoneal dialysis: impact on clinical outcomes and quality of life measure. J Nephrol.36, 1897–1905 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Navaratnarajah, A. et al. Flexibility in peritoneal dialysis prescription: Impact on technique survival. Perit Dial Int.41, 49–56 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Fernandes, A., Matias, P. & Branco, P. Incremental peritoneal dialysis: Is it better for preservation of residual kidney function and clinical outcomes?. Clin Nephrol.99, 11–17 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Sandrini, M. et al. Incremental peritoneal dialysis: a 10 year single-centre experience. J Nephrol.9, 871–879 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardelli, L. et al. Relationship between number of daily exchanges at CAPD start with clinical outcomes. Perit Dial Int.44, 98–108 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Ankawi, G. A., Woodcock, N. I., Jain, A. K., Garg, A. X. & Blake, P. G. The use of incremental peritoneal dialysis in a large contemporary peritoneal dialysis program. Can J Kidney Health Dis.3, 2054358116679131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan, H., Abreu, Z. & Bargman, J. M. Incremental peritoneal dialysis in incident end-stage kidney disease patients. Perit Dial Int.42, 387–393 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Lee, S. M., Min, Y. S., Son, Y. K., Kim, S. E. & An, W. S. Comparison of clinical outcome between incremental peritoneal dialysis and conventional peritoneal dialysis: a propensity score matching study. Ren Fail.43, 1222–1228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayat, A. et al. Association of Incremental peritoneal dialysis with residual kidney function decline in patients on peritoneal dialysis: The balANZ trial. Perit Dial Int.43, 374–382 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Cheetham, M. S. et al. Multicentre registry analysis of incremental peritoneal dialysis incidence and associations with patient outcomes. Perit Dial Int2023(43), 383–394 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Huang, L. L., Mah, J. Y., Howard, J., Roberts, M. A. & McMahon, L. P. Incremental peritoneal dialysis is a safe and feasible prescription in incident patients with preserved residual kidney function. Nephrology (Carlton).27, 74–81 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Zhang, W., Lv, J., Li, Y., Liang, Y. & Jiping, S. Are USPD patients suitable for incremental peritoneal dialysis: Yes or no?. Clin Nephrol.97, 215–225 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Yan, H. et al. Three versus 4 daily exchanges and residual kidney function decline in incident CAPD patients: A randomized controlled trial. Am J Kidney Dis.69, 506–513 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Liu, R. et al. Incremental peritoneal dialysis and survival outcomes: A propensity-matched cohort study. J Nephrol.36, 1907–1919 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Liu, R. et al. The feasibility of incremental peritoneal dialysis for older patients on peritoneal dialysis. Kidney Res Clin Pract.10.23876/j.krcp.22.202 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Yu, X. et al. Number of daily peritoneal dialysis exchanges and mortality risk in a Chinese population. Perit Dial Int.38(suppl 2), S53–S63 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Elphick, E. et al. Outcome measures for technique survival reported in peritoneal dialysis: A systematic review. Perit Dial Int.42, 279–287 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Li, P. K. et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int.42, 110–153 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Arbor Research Collaborative for Health, but restrictions apply to the availability of these data which were used for the current study, and so are not publicly available. However, the data underlying this article will be shared on reasonable request to the corresponding author.