Abstract
Purpose
The role of abdominal compliance in pneumoperitoneum is not fully understood. This study aimed to clarify the association between abdominal wall stretching tendency and surgical stress in laparoscopic groin hernia repair.
Methods
We conducted a retrospective single-center cohort study, evaluating 51 patients who underwent elective transabdominal preperitoneal groin hernia repair. Abdominal compliance was assessed using the abdominal compliance index (ACI; insufflated intra-abdominal volume [L] / body surface area [m²]) at 8 mmHg intra-abdominal pressure. Surgical stress and recovery were evaluated with patient-reported outcome measures (PROMs), including QOR-15 and pain visual analog scale (VAS) scores. Associations between ACI, PROMs, and clinical outcomes were analyzed.
Results
The median ACI was 1.229 L/m² (0.369–2.091). Eleven patients (21.6%) above the 75th percentile cutoff (1.576 L/m²) were categorized as high ACI. While body constitution was similar between groups, the high ACI group had significantly greater insufflated intra-abdominal volume (2.88 L vs. 1.89 L, P < 0.0001). Pre-operative QOR-15 scores were similar. However, on postoperative day 1, the high ACI group had significantly lower QOR-15 scores (90.2 vs. 110.1, P = 0.017), with subcategory analysis showing reduced physical well-being. Multivariate analysis indicated that high ACI was a significant predictor of poorer QOR. The high ACI group also reported higher, though not statistically significant, postoperative pain.
Conclusion
Abdominal walls with greater elasticity, which stretch excessively under pneumoperitoneum, were more susceptible to surgical stress. Further studies are warranted to evaluate the efficacy of tailored pneumoperitoneum pressure adjustment based on abdominal compliance to mitigate surgical stress.
Keywords: Abdominal compliance, Transabdominal preperitoneal groin hernia repair, Patient-reported outcome measure, Postoperative recovery, Quality of recovery
Introduction
Abdominal compliance (AC) is the measure of ease of abdominal expansion and is defined as the change in intra-abdominal volume (IAV) per change in intra-abdominal pressure (IAP) [1]. In laparoscopic surgery, AC is pivotal in determining the extent to which the abdominal cavity expands at a given insufflation pressure. While higher insufflation pressure provides a larger laparoscopic workspace, the maximum expansion capacity varies between patients due to individual body composition and abdominal rigidity [2]. Although the understanding of AC may optimize IAP management in laparoscopic surgery, the precise measurement of AC requires specific instruments or imaging devices [3–5]. Recognizing the challenges of real-time AC evaluation in clinical settings, we introduced a surrogate index, namely, the abdominal compliance index (ACI), easily calculated using the gas volume in the initial pneumoperitoneum insufflation [6]. ACI serves as a predictor of abdominal wall stretching tendency.
In recent studies, we demonstrated that lower insufflation pressure was associated with reduced postoperative pain in laparoscopic groin hernia repair [7]. However, a subset of patients with high ACI experienced higher postoperative pain and longer hospital stay despite low-pressure settings [6]. The higher tendency of the abdominal wall to extend at a given IAP was suspected to cause increased muscular and nervous stress. These investigations have unveiled a broader role for AC, extending beyond its original scope of assessing abdominal wall stretching capacity. Our studies indicated that AC may be intricately linked with and potentially serve as a predictor of postoperative outcomes in laparoscopic surgery.
In this study, we further investigated the influence of AC on surgical stress and postoperative recovery. To this end, we employed a patient-reported outcome measure (PROM) to evaluate the patient’s subjective quality of recovery. The QOR-15 is a valid, reliable, and responsive PROM that assesses emotional state, psychological support, physical independence, physical comfort, and pain through a 15-item questionnaire [8]. QOR-15 is a clinically feasible modification of the more extensive QOR-40 questionnaire [9].
We aimed to investigate the association between AC and postoperative patient subjective outcomes and elucidate the role of abdominal compliance in surgical stress after laparoscopic surgery.
Patients and methods
Study design and setting
This retrospective cohort study was conducted at Yamato Takada Municipal Hospital. We reviewed all patients who underwent groin hernia surgery from June 2023 to May 2024. Data were extracted from electronic medical records.
Patients
During the study period, 98 groin hernia surgeries were performed. Among the 79 patients who underwent laparoscopic groin hernia repair, ACI was measured in 63.
Patients included in the final analysis met the following criteria: (1) Underwent a standard transabdominal preperitoneal (TAPP) laparoscopic procedure, (2) had the IAV measured during the procedure, and (3) completed the QOR-15 questionnaire on the day after surgery.
Exclusion criteria included: Conversion to open surgery due to intra-abdominal adhesions (n = 3), emergency surgery for incarcerated hernia (n = 2), unconventional TAPP procedures such as additional trocar placement, modified IPOM, or very low pneumoperitoneum pressure (n = 4), and inability to complete or cooperate with the QOR-15 questionnaire (n = 3).
The final analysis included 51 patients who underwent elective TAPP (Fig. 1).
Fig. 1.
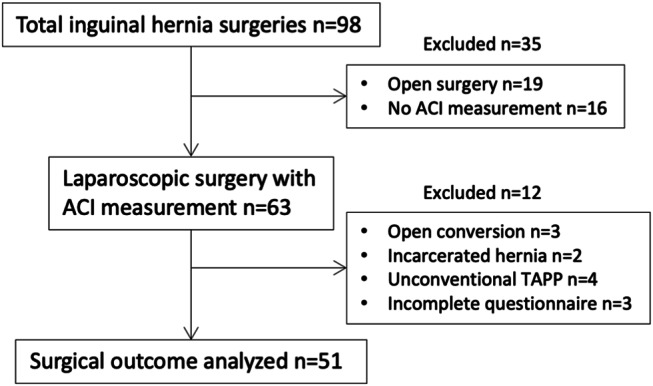
Flow chart diagram of patient inclusion criteria
Surgical procedures
Laparoscopic groin hernia repairs were performed using the TAPP technique. Pneumoperitoneum was generally maintained at an IAP of 8 mmHg, with adjustments made according to the surgeon’s preference. Dissection extended medially over the midline, more than 3 cm beyond Hesselbach’s triangle ventrally, laterally over the anterior superior iliac spine, and more than 5 cm below the iliopubic tract dorsally. A single type of lightweight 3D mesh was used in all cases. The standard mesh size was 10 × 15 cm, but medium-sized meshes were used when adjustments were needed for body size or dissection plane. Fixation was performed using five absorbable tacks, with variations based on surgeon preference. At the end of the surgery, CO2 evacuation was visually confirmed under the laparoscope before removing the trocars, without using any additional manual gas evacuation techniques. General anesthesia with deep neuromuscular blockade was routinely administered, with the train-of-four (TOF) monitored and maintained at a count of 0 throughout the surgery. Neuromuscular blockade was reversed using sugammadex at the end of the procedure. Additionally, bilateral rectus sheath blocks were administered pre-incision, using 60 ml of 0.2% ropivacaine (40 ml for patients weighing less than 40 kg).
Postoperative pain management
Routine postoperative and post-discharge adjuvant analgesia was not administered. Postoperative rescue analgesia with NSAIDs was provided upon the patient’s request at appropriate intervals.
Data collection
ACI was used as a surrogate measure of AC [6]. ACI reflects the degree of abdominal expansion during pneumoperitoneum, adjusted for the patient’s body constitution. Pneumoperitoneum was established at 8 mmHg IAP, and once equilibrium was reached, the insufflated IAV was measured using the cumulative CO2 volume gauge on the insufflation unit. ACI was calculated as follows:
 |
Among the 63 patients who had IAV measured during the laparoscopic approach, the median ACI was 1.229 L/m² (range, 0.369–2.091 L/m²). ACI was categorized into high and low groups using a 75th percentile cutoff of 1.576 L/m². This cutoff was selected because it was an objective threshold that approximately aligned with a significant value identified in our prior research [6], suggesting its potential relevance in categorizing abdominal compliance levels.
Patient demographics included age, gender, height, weight, body mass index, body surface area, and American Society of Anesthesiologists (ASA) score. Intraoperative variables included insufflated intra-abdominal volume, insufflated intra-abdominal pressure, affected side, operation time, Japanese Hernia Society (JHS) groin hernia classification, implanted mesh size, and mesh fixating tacks count. Type of hospital room was also documented to evaluate the environmental influencing factors of postoperative recovery.
Surgical outcomes
Surgical outcomes were measured using PROMs. Surveys were administered on three occasions: the day before surgery, postoperative day 1 (POD1), and at the first post-hospital discharge visit. The primary outcome was the patient’s subjective surgical stress and postoperative recovery, as measured by the QOR-15 scale [8], which ranges from 0 to 150 points, with higher scores indicating better recovery.
The QOR-15 consists of 15 questions that assess various aspects of recovery: (1) able to breathe easily, (2) able to enjoy food, (3) feeling rested, (4) having had a good sleep, (5) able to look after personal toilet and hygiene unaided, (6) able to communicate with family or friends, (7) receiving support from hospital doctors and nurses, (8) able to resume work or usual home activities, (9) feeling comfortable and in control, (10) having a sense of general well-being, (11) experiencing moderate pain, (12) experiencing severe pain, (13) nausea or vomiting, (14) feeling worried or anxious, and (15) feeling sad or depressed. These questions can be grouped to represent the physical and mental aspects of postoperative recovery [8]. Questions 11 and 12 reflect pain, questions 1, 2, 3, 4, and 13 reflect physical comfort, and questions 5 and 8 reflect physical independence. Psychological support is assessed by questions 6 and 7, while the emotional state is measured by questions 9, 10, 14, and 15. Physical well-being is represented by the combination of pain, physical comfort, and physical independence, while mental well-being comprises psychological support and emotional state. These subgroups of QOR-15 were also documented and analyzed.
Secondary outcomes included postoperative pain, assessed using a 10-point visual analog scale (VAS), and length of hospital stay.
Statistical analysis
Categorical variables were compared using the chi-square or Fisher’s exact test, as appropriate. Continuous variables were analyzed using Student’s t-test. A multivariate linear regression analysis was conducted to identify potential predictors of QOR-15 scores on POD1. Variables included in the analysis were selected based on borderline to significant differences between the groups (P < 0.1). Additionally, linear regression analysis was performed to assess the correlation between post-discharge days and the increase in QOR-15 scores, and the correlation between tack fixation count and QOR-15 scores. Statistical significance was considered for P values < 0.05. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics (Version 28).
Results
Patient demographics of the final dataset of 51 patients are detailed in Table 1. The median ACI and range were consistent with those of the total data set, at 1.229 L/m2 (0.369–2.091). Eleven patients (21.6%) were classified into the high ACI group. Comparison between the high and low ACI groups revealed no differences in characteristics such as age, gender, ASA classification, height, and weight. Although BMI trended to be lower in the high ACI group, both groups had similar body surface area and overall body constitution. However, the high ACI group exhibited a significantly larger insufflated intra-abdominal volume than the low ACI group. (2.88 L vs. 1.89 L, P < 0.0001). Conversely, the average pneumoperitoneum pressure was significantly higher in the low ACI group (8.18 mmHg vs. 8.85 mmHg, P = 0.04).
Table 1.
Patient demographics and background
| High ACI (n = 11) |
Low ACI (n = 40) |
P value | ||
|---|---|---|---|---|
| Age (years) | Median (range) | 73 (69–82) | 67 (36–84) | 0.108 |
| Gender | (%) | |||
| Male | 9 (81.8) | 35 (87.5) | 0.635 | |
| Female | 2 (18.2) | 5 (12.5) | ||
| ASA classification | (%) | |||
| 2 or less | 11 (100) | 37 (92.5) | 1.000 | |
| 3 or more | 0 (0) | 3 (7.5) | ||
| Height (cm) | mean (SD) | 165 (8.1) | 165 (8.5) | 0.866 |
| Weight (kg) | Mean (SD) | 57 (9.8) | 62 (12.4) | 0.189 |
| Body mass index (kg/m2) | Mean (SD) | 20.8 (2.6) | 22.6 (2.8) | 0.067 |
| Body surface area (m2) | Mean (SD) | 1.61 (0.16) | 1.68 (0.19) | 0.296 |
| Intra-abdominal volume (L) | Mean (SD) | 2.88 (0.50) | 1.89 (0.41) | < 0.0001 |
| Intra-abdominal pressure (mmHg) | Mean (SD) | 8.18 (0.60) | 8.85 (1.00) | 0.040 |
| Abdominal compliance index (L/m2) | Mean (SD) | 1.77 (0.19) | 1.13 (0.24) | < 0.0001 |
ACI abdominal compliance index, ASA American Society of Anesthesiologists
In terms of surgical outcomes, it is noteworthy that bilateral lesions had significantly lower QOR scores compared to unilateral lesions (80.8 vs. 109.2, P = 0.007). However, the proportion of bilateral cases was similar between the two ACI groups, and there were no differences in the affected side. No other differences were observed between the groups regarding JHS classification, unilateral operation time, implanted mesh size, tack fixation count, or hospital room type (Table 2). For unilateral cases, the number of tacks used for mesh fixation ranged from 3 to 8, but a higher tack count did not correlate with lower QOR scores (R2 = 0.001, P = 0.78). Additionally, neither larger hernia lesions (97.2 vs. 112.1, P = 0.09) nor larger mesh sizes (107.9 vs. 115.1, P = 0.44) were associated with worse recovery outcomes. No patients experienced postoperative complications that required additional interventions.
Table 2.
Surgical results and outcomes
| High ACI (n = 11) |
Low ACI (n = 40) |
P value | ||
|---|---|---|---|---|
| Affected side | (%) | |||
| Right | 4 (36.7) | 27 (67.5) | 0.231*1 | |
| Left | 4 (36.7) | 10 (25.0) | ||
| Bilateral | 3 (27.3) | 3 (7.5) | 0.105*2 | |
| JHS classification (in unilateral cases) | (%) | |||
| M 1–2 | 1 (12.5) | 6 (16.2) | 0.651*3 | |
| M 3 | 1 (12.5) | 3 (8.1) | ||
| L 1–2 | 5 (62.5) | 22 (59.5) | ||
| L 3 | 1 (12.5) | 4 (10.8) | ||
| F 1–3 | 0 (0) | 2 (5.4) | ||
| Unilateral operation time (minutes) | Mean (SD) | 78 (22.6) | 84 (21.0) | 0.534 |
| Mesh size (in unilateral cases) | (%) | |||
| Medium | 2 (25.0) | 6 (16.2) | 0.617 | |
| Large | 6 (75.0) | 31 (83.8) | ||
| Tack fixation count (in unilateral cases) | Mean (SD) | 5.5 (1.06) | 5.6 (0.92) | 0.799 |
| Hospital room type | (%) | |||
| Shared | 7 (63.6) | 28 (70.0) | 0.723 | |
| Private | 4 (36.4) | 12 (30.0) | ||
| VAS pain scale on POD1 | Mean (SD) | 6.4 (2.31) | 5.2 (3.00) | 0.258 |
| Postoperative hospital stay (days) | Mean (SD) | 2.1 (0.53) | 2.1 (0.63) | 0.965 |
*1 Right vs. Left, *2 Unilateral vs. Bilateral, *3 L3/M3 vs. L1-2/M1-2/F
ACI abdominal compliance index, JHS Japanese Hernia Society, VAS visual analogue scale, POD postoperative day
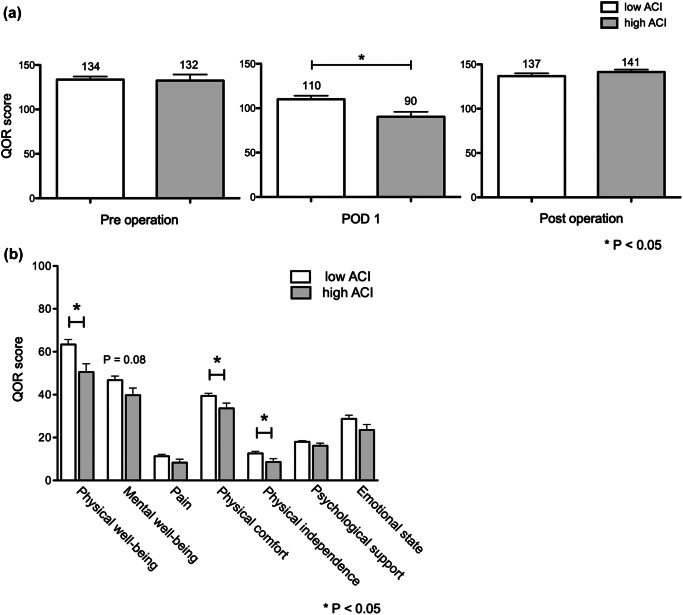
Pre-operative QOR-15 scores were similar between both groups. However, on POD1, the high ACI group demonstrated significantly lower QOR-15 scores compared to the low ACI group (90.2 vs. 110.1, P = 0.017). Specifically, the high ACI group reported lower, but not significant scores in pain (8.2 vs. 11.3, P = 0.108), significantly lower physical comfort (33.6 vs. 39.4, P = 0.035), and physical independence (8.6 vs. 12.6, P = 0.043), resulting in significantly lower overall physical well-being than the low ACI group (50.5 vs. 63.3, P = 0.012). There were no significant differences in psychological support or emotional state between the groups, although overall mental well-being tended to be lower in the high ACI group (39.7 vs. 46.7, P = 0.08) (Fig. 2).
Fig. 2.
(a) Transition of QOR-15 scores. The high ACI group demonstrated significantly lower scores on POD1. Both groups recovered to preoperative standards by the first post-hospital discharge visit (b) Sub-categories of QOR-15 on POD1. Overall physical well-being was significantly lower in the high ACI group, while differences in mental aspects were not significant
A multivariate linear regression analysis was conducted with high ACI, BMI, IAV, and IAP as independent variables (R = 0.154). The results revealed that high ACI and IAV were significant independent predictors of QOR on POD1 (Table 3).
Table 3.
Multivariate analysis of predictors of QOR-15 score on POD1
| Variant | Beta Coefficient (95% CI) | Standard Error | P value | VIF |
|---|---|---|---|---|
| High ACI | -36.3 (-60.1 and -12.4) | 11.83 | 0.004 | 1.86 |
| BMI | 0.65 (-2.09–3.40) | 1.36 | 0.632 | 1.32 |
| IAV | 17.80 (1.49–34.1) | 8.08 | 0.033 | 1.90 |
| IAP | 3.11 (-5.20–11.43) | 4.12 | 0.454 | 1.39 |
POD1 Postoperative day 1, ACI Abdominal compliance index, BMI Body mass index, IAV intra-abdominal volume, IAP intra-abdominal pressure, CI confidence interval, VIF Variance inflation factor
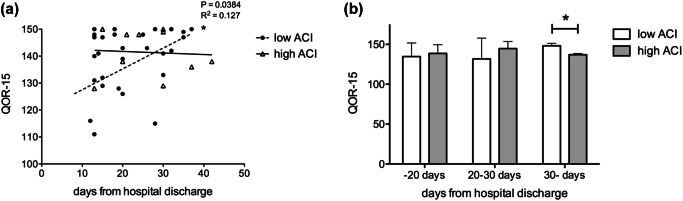
After a mean follow-up of 22.7 ± 9.21 days, both groups showed similar average QOR-15 scores and exhibited excellent recovery at the first post-hospital discharge visit (Fig. 2). In the low ACI group, a significantly positive but weak correlation (R2 = 0.127, P = 0.038) was observed between days after discharge and QOR-15 score recovery. When comparing QOR scores across three post-discharge intervals, the low ACI group demonstrated significantly higher scores (137.0 vs. 148.3, P = 0.001), nearing the maximum of 150 points, from day 30 onwards. The high ACI group, meanwhile, maintained a good to excellent level of recovery (Fig. 3).
Fig. 3.
(a) Correlation between QOR recovery and days after hospital discharge. The low ACI group exhibited a significantly positive but weak correlation, whereas the high ACI group did not show a significant correlation. (b) Comparison of QOR scores across post-discharge intervals. The low ACI group demonstrated significantly higher scores from day 30 onward
Regarding secondary outcomes, the high ACI group exhibited higher, though not statistically significant, levels of postoperative pain as measured by VAS pain scales and comparable hospital stays compared to the low ACI group (Table 2).
Discussion
Our results indicate that patients with a heightened tendency for abdominal stretching experience greater surgical stress and poorer postoperative recovery during the acute period following surgery. This finding is particularly relevant in groin hernia surgery, given the global prevalence of ambulatory procedures. Indeed, surgical stress factors, such as inadequate pain control, have been reported as critical reasons for converting day surgeries to hospital stays [10, 11]. Consequently, AC may be a crucial factor to consider in ambulatory laparoscopic procedures. Additionally, we examined recovery over the longer postoperative period up to the first hospital visit after discharge. Although the sample size was limited for conclusive analysis, the data indicated a trend suggesting that lower ACI may be associated with more consistent improvements in recovery. In contrast, the high ACI group showed a stable but less pronounced upward trend, implying a different recovery trajectory potentially influenced by abdominal compliance. These findings highlight the need for further investigation into the association between ACI and long-term postoperative outcomes.
Various perioperative factors [12–14] were examined to determine their contribution to differences in surgical stress between the high and low ACI groups. Factors such as the extent of dissection and the size of the hernia could involve more extensive tissue handling, potentially leading to increased surgical stress. Larger hernia lesions and larger mesh sizes were presumed to reflect the extent of dissection; however, neither of these factors was associated with poorer QOR scores. Similarly, since mesh fixation is known to contribute to postoperative pain, the number of tacks used was assessed, but no correlation was found between tack numbers and QOR scores. Notably, bilateral lesions, which involve twice the dissection area and higher tack numbers, were associated with significantly lower QOR scores, suggesting that these factors play a role in postoperative recovery. Nonetheless, these factors did not explain the differences observed between the high and low ACI groups. The QOR-15 questionnaire, as a generic measure of postoperative recovery, captures both physical and mental aspects, which means that environmental factors, such as bed quality, room conditions, noise levels, and shared accommodation, could influence scores related to sleep, restfulness, and comfort. We documented the hospital room types to account for these potential influences, and most patients were accommodated in shared rooms with similar environments, minimizing variability. Additionally, subgroup analysis revealed no significant differences in the mental well-being components of the QOR-15 between the two groups, indicating that the observed differences in surgical stress were more likely due to physical factors. Finally, multivariate analysis demonstrated that a high ACI was independently associated with poorer QOR scores, supporting the notion that the tendency for increased abdominal stretch during pneumoperitoneum is a significant contributor to surgical stress.
In the context of pneumoperitoneum, AC plays a pivotal role in determining the extent to which the abdomen expands at a given insufflation pressure. While higher insufflation pressure ensures a larger laparoscopic workspace, as a trade-off the high IAP affects physiological function. In high-pressure settings such as bariatric surgery, where the IAP can rise to 15 mmHg, pneumoperitoneum is known to enhance venous stasis, reduce intraoperative urinary output, increase airway pressure, and impair cardiac function [15]. Understanding the interplay between AC, IAP, and postoperative outcomes is therefore essential in laparoscopic surgery.
In our institution, the standard protocol involves maintaining low pneumoperitoneum pressure. However, adequate workspace is the priority and IAP was increased when necessary. In this study, the low ACI group had significantly higher IAP than the high ACI group, presumably increased by the surgeon due to constraints associated with a confined laparoscopic working space. Paradoxically, despite the significantly lower IAP, the high ACI group showed significantly increased physical surgical stress and higher postoperative pain. In other words, patients with low ACI demonstrated a more rigid abdominal wall, displaying greater tolerance to pneumoperitoneum stress but operating within a limited laparoscopic workspace. Conversely, high ACI patients with more elastic abdominal walls were susceptible to surgical stress even under low-pressure conditions. These findings are consistent with the results of our previous study conducted at another institution [6], further supporting the hypothesis that mechanical stretching of the abdominal wall by pneumoperitoneum is a major contributor to surgical stress, likely due to muscular and nerve strain. Moreover, this highlights the ongoing clinical importance of AC in laparoscopic surgery.
Various studies have shown the benefits of low-pressure pneumoperitoneum, particularly in reducing postoperative pain and enhancing recovery [16–18]. However, our findings suggest that a uniform low-pressure setting may not effectively alleviate surgical stress across all patients. Specifically, patients with low ACI may tolerate pneumoperitoneum stress well and may not benefit from lower pressures due to the associated constraints on the surgical working space. A more effective approach could involve identifying patients with high ACI, who are more susceptible to excessive abdominal wall extension, and adjusting pressure settings accordingly. Tailored low-pressure IAP may potentially improve patient outcomes, but at present, this involves a trial-and-error method adjusting to the lowest pressure based on the surgeon’s subjective satisfactory surgical view [19]. ACI may serve as a clearer reference for adjusting intra-abdominal pressure to achieve an optimal balance between minimizing surgical stress and maintaining adequate laparoscopic working space. Easily calculated during surgery, it offers a practical means to individualize pneumoperitoneum pressure management based on the patient’s characteristics. Future studies are warranted to assess the feasibility and efficacy of selectively utilizing very low-pressure pneumoperitoneum (6 mmHg) in groin hernia patients with high ACI, to mitigate postoperative surgical stress.
PROMs have become an essential tool in evaluating the quality of groin hernia surgery, as modern expectations extend beyond successful hernia repair to ensure a less painful and more satisfactory postoperative experience [20]. While the advent of prosthetic meshes has reduced hernia recurrence rates, the focus of surgical outcomes has shifted to patient-centered concerns, such as chronic postoperative pain and quality of life. These subjective experiences are now critical indicators, measured through various generic and disease-specific PROMs. The SF-36, a gold standard in generic PROMs, has limitations in capturing changes in quality of life following hernia surgery [21, 22], while more comprehensive scales like the QOR-40 can be cumbersome [9]. Though several hernia-specific PROMs have been developed [23–26], their reliability and responsiveness remain in question [27].
In this study, we opted for the QOR-15, a clinically feasible and reliable PROM applicable across various surgical settings, including preoperative, postoperative, ambulatory, and elective surgery scenarios [28, 29]. The mean QOR-15 score difference between the high and low ACI groups on POD1 was more than three times the minimum clinically significant difference [30], underscoring the underestimated impact of AC on the patient’s subjective experience of the postoperative course. QOR-15 questions can be grouped into sub-categories to evaluate the physical and mental burden on patients [8]. Notably, the high ACI group exhibited lower scores in physical aspects, emphasizing the impact of abdominal wall extension on surgical stress. The use of the QOR-15 in this study was crucial, providing valuable insights into the relationship between AC, IAP, and postoperative outcomes. By effectively capturing both the physical and mental aspects of patients’ experiences, it highlighted the important role of AC in surgical stress and postoperative recovery.
One limitation of this study was the incomplete feasibility of the QOR-15 questionnaire. Although the QOR-15 is relatively simple and can be completed in approximately 2.7 min [29], three elderly participants (4.8%) found it challenging, leading to incomplete data collection. This highlights a crucial aspect: in the context of inguinal hernia surgery, where a significant portion of the population is elderly, evaluation using PROMs may sometimes be difficult due to cognitive impairment. This consideration is particularly important given that elderly patients are often more frail and susceptible to surgical stress. Therefore, scales designed for patients with cognitive impairment, such as the DQoL [31], or scales that can be completed by a caregiver, such as the QOL-AD [32], may be considered alternative assessments for elderly patients. Further studies are required to assess the feasibility and reliability of these scales in the postoperative setting. Secondly, while some studies suggest that muscle relaxants do not affect abdominal wall elasticity [33, 34], other reports indicate that deep neuromuscular blockade can improve the surgical workspace and view [35], suggesting that neuromuscular blockade may promote abdominal expansion. Although all patients in this study underwent surgery using deep neuromuscular blockade, our standard protocol maintained a low IAP at 8 mmHg, which, when combined, has been shown to provide adequate surgical visualization while potentially reducing surgical stress [19, 36]. However, we cannot entirely rule out the possibility that muscle relaxants may have contributed to muscular strain in patients with weaker abdominal walls. Lastly, this study is limited by its retrospective, single-institution design and relatively small sample size. To address these limitations, we identified and accounted for as many potential confounding variables as possible, conducting a multivariate analysis to better clarify the factors contributing to the observed differences in surgical stress between the ACI groups. A prospective study with standardized surgical protocols, such as consistent pneumoperitoneum pressure, would provide clearer insights into the role of ACI in laparoscopic groin hernia surgery. Additionally, evaluating ACI across a wider spectrum of diseases and laparoscopic procedures could offer a more comprehensive understanding of the clinical significance of AC in the pneumoperitoneum setting.
In conclusion, abdominal expansion by pneumoperitoneum was a crucial factor of surgical stress and postoperative recovery in laparoscopic groin hernia repair. High ACI represented a heightened tendency of abdominal wall extension, which was less tolerant to pneumoperitoneum-induced surgical stress. Future studies are warranted to investigate the efficacy of selectively adjusting insufflation pressure to mitigate surgical stress in such patients susceptible to excessive abdominal expansion by pneumoperitoneum.
Acknowledgements
The authors express their gratitude to the operating room staff at Yamatotakada Municipal Hospital for their assistance with the documentation of on-site intra-abdominal volume measurements.
Author contributions
Study conception and design were performed by Shoichi Kinoshita. Material preparation, data collection, and analysis were performed by Shoichi Kinoshita, Chisato Hara, Yayoi Matsumoto, Kohei Fukuoka, Kenji Nakagawa, Daisuke Hokuto, and Hiroyuki Kuge. The first draft of the manuscript was written by Shoichi Kinoshita and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was not funded.
Declarations
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This study was approved by the institutional review board (Yamatotakada Municipal Hospital Ethics Committee, No. R5-5).
Informed consent
Written informed consent was obtained from all patients before treatment.
Conflict of interest
All authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malbrain ML, Roberts DJ, De Laet I et al (2014) The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 1: definitions and pathophysiology. Anaesthesiol Intensive Ther 46:392–405. 10.5603/AIT.2014.0062 [DOI] [PubMed] [Google Scholar]
- 2.Blaser AR, Björck M, De Keulenaer B, Regli A (2015) Abdominal compliance: a bench-to-bedside review. J Trauma Acute Care Surg 78:1044–1053. 10.1097/TA.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 3.Malbrain ML, De Laet I, De Waele JJ et al (2014) The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 2: measurement techniques and management recommendations. Anaesthesiol Intensive Ther 46:406–432. 10.5603/AIT.2014.0063 [DOI] [PubMed] [Google Scholar]
- 4.Song C, Alijani A, Frank T, Hanna GB, Cuschieri A (2006) Mechanical properties of the human abdominal wall measured in vivo during insufflation for laparoscopic surgery. Surg Endosc 20:987–990. 10.1007/s00464-005-0676-6 [DOI] [PubMed] [Google Scholar]
- 5.Sterke F, van Weteringen W, Ventura L, Milesi I, Wijnen RMH, Vlot J, Dellacà RL (2022) A novel method for monitoring abdominal compliance to optimize insufflation pressure during laparoscopy. Surg Endosc 36:7066–7074. 10.1007/s00464-022-09406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinoshita S, Kawaguchi C, Takagi T, Ohyama T (2022) Proposal of a Novel Index of Abdominal Compliance and the Association with Postoperative Pain after laparoscopic inguinal hernia repair. Surg Laparosc Endosc Percutan Tech 32:182–187. 10.1097/SLE.0000000000001033 [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita S, Ohyama T, Kawaguchi C, Ikeda N, Sho M (2021) Significance of umbilical trocar size and intra-abdominal pressure on postoperative pain after transabdominal preperitoneal repair for inguinal hernia. Asian J Endosc Surg 14:63–69. 10.1111/ases.12813 [DOI] [PubMed] [Google Scholar]
- 8.Stark PA, Myles PS, Burke JA (2013) Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology 118:1332–1340. 10.1097/ALN.0b013e318289b84b [DOI] [PubMed] [Google Scholar]
- 9.Myles PS, Weitkamp B, Jones K, Melick J, Hensen S (2000) Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 84:11–15. 10.1093/oxfordjournals.bja.a013366 [DOI] [PubMed] [Google Scholar]
- 10.Joyner J, Ayyaz FM, Cheetham M, Briggs TWR, Gray WK (2024) Factors associated with conversion from day-case to in-patient elective inguinal hernia repair surgery across England: an observational study using administrative data. Hernia 28:555–565. 10.1007/s10029-023-02949-y [DOI] [PubMed] [Google Scholar]
- 11.Awan FN, Zulkifli MS, McCormack O, Manzoor T, Ravi N, Mehigan B, Reynolds JV (2013) Factors involved in unplanned admissions from general surgical day-care in a modern protected facility. Ir Med J 106:153–154 [PubMed] [Google Scholar]
- 12.Mouton WG, Bessell JR, Otten KT, Maddern GJ (1999) Pain after laparoscopy. Surg Endosc 13:445–448. 10.1007/s004649901011 [DOI] [PubMed] [Google Scholar]
- 13.Nienhuijs S, Staal E, Strobbe L, Rosman C, Groenewoud H, Bleichrodt R (2007) Chronic pain after mesh repair of inguinal hernia: a systematic review. Am J Surg 194:394–400. 10.1016/j.amjsurg.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 14.Taylor C, Layani L, Liew V, Ghusn M, Crampton N, White S (2008) Laparoscopic inguinal hernia repair without mesh fixation, early results of a large randomised clinical trial. Surg Endosc 22:757–762. 10.1007/s00464-007-9510-7 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen NT, Wolfe BM (2005) The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg 241:219–226. 10.1097/01.sla.0000151791.93571.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Taji O, Howell-Etienne J, Taktak S, Hanchanale V (2023) Lower vs standard pressure pneumoperitoneum in robotic-assisted radical prostatectomy: a systematic review and meta-analysis. J Robot Surg 17:303–312. 10.1007/s11701-022-01445-2 [DOI] [PubMed] [Google Scholar]
- 17.Park SE, Hong TH (2023) Effects of extremely low-pressure pneumoperitoneum on postoperative recovery after single site robot-assisted cholecystectomy: a randomized controlled trial. Langenbecks Arch Surg 408:242. 10.1007/s00423-023-02988-0 [DOI] [PubMed] [Google Scholar]
- 18.Celarier S, Monziols S, Célérier B, Assenat V, Carles P, Napolitano G, Laclau-Lacrouts M, Rullier E, Ouattara A, Denost Q (2021) Low-pressure versus standard pressure laparoscopic colorectal surgery (PAROS trial): a phase III randomized controlled trial. Br J Surg 108:998–1005. 10.1093/bjs/znab069 [DOI] [PubMed] [Google Scholar]
- 19.Díaz-Cambronero O, Mazzinari G, Flor Lorente B et al (2020) Effect of an individualized versus standard pneumoperitoneum pressure strategy on postoperative recovery: a randomized clinical trial in laparoscopic colorectal surgery. Br J Surg 107:1605–1614. 10.1002/bjs.11736 [DOI] [PubMed] [Google Scholar]
- 20.Quality of life (2022) Is the most important outcome measure of hernia repair. [editorial] Hernia 26(3):685 [DOI] [PubMed] [Google Scholar]
- 21.Velanovich V (1998) Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. J Gastrointest Surg 2:141–145. 10.1016/s1091-255x(98)80004-8 [DOI] [PubMed] [Google Scholar]
- 22.Burney RE, Jones KR, Coon JW, Blewitt DK, Herm A, Peterson M (1997) Core outcomes measures for inguinal hernia repair. J Am Coll Surg 185:509–515. 10.1016/s1072-7515(97)00108-7 [DOI] [PubMed] [Google Scholar]
- 23.Heniford BT, Walters AL, Lincourt AE, Novitsky YW, Hope WW, Kercher KW (2008) Comparison of generic versus specific quality-of-life scales for mesh hernia repairs. J Am Coll Surg 206:638–644. 10.1016/j.jamcollsurg.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 24.Kehlet H, Bay-Nielsen M, Kingsnorth A (2002) Chronic postherniorrhaphy pain–a call for uniform assessment. Hernia 6:178–181. 10.1007/s10029-002-0082-0 [DOI] [PubMed] [Google Scholar]
- 25.Muysoms FE, Vanlander A, Ceulemans R, Kyle-Leinhase I, Michiels M, Jacobs I, Pletinckx P, Berrevoet F (2016) A prospective, multicenter, observational study on quality of life after laparoscopic inguinal hernia repair with ProGrip laparoscopic, self-fixating mesh according to the European Registry for Abdominal Wall Hernias Quality of Life Instrument. Surgery 160:1344–1357. 10.1016/j.surg.2016.04.026 [DOI] [PubMed] [Google Scholar]
- 26.Huang CC, Tai FC, Chou TH, Lien HH, Jeng JY, Ho TF, Huang CS (2017) Quality of life of inguinal hernia patients in Taiwan: the application of the hernia-specific quality of life assessment instrument. PLoS ONE 12:e0183138. 10.1371/journal.pone.0183138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gram-Hanssen A, Christophersen C, Rosenberg J (2022) Results from patient-reported outcome measures are inconsistently reported in inguinal hernia trials: a systematic review. Hernia 26:687–699. 10.1007/s10029-021-02492-8 [DOI] [PubMed] [Google Scholar]
- 28.Kleif J, Waage J, Christensen KB, Gögenur I (2018) Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth 120:28–36. 10.1016/j.bja.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 29.Myles PS, Shulman MA, Reilly J, Kasza J, Romero L (2022) Measurement of quality of recovery after surgery using the 15-item quality of recovery scale: a systematic review and meta-analysis. Br J Anaesth 128:1029–1039. 10.1016/j.bja.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 30.Myles PS, Myles DB (2021) An updated minimal clinically important difference for the QoR-15 scale. [letter] Anesthesiology 135(5):934–935 [DOI] [PubMed] [Google Scholar]
- 31.Brod M, Stewart AL, Sands L, Walton P (1999) Conceptualization and measurement of quality of life in dementia: the dementia quality of life instrument (DQoL). Gerontologist 39:25–35. 10.1093/geront/39.1.25 [DOI] [PubMed] [Google Scholar]
- 32.Logsdon RG, Gibbons LE, McCurry SM, Teri L (2002) Assessing quality of life in older adults with cognitive impairment. Psychosom Med 64:510–519. 10.1097/00006842-200205000-00016 [DOI] [PubMed] [Google Scholar]
- 33.Chassard D, Berrada K, Tournadre J, Boulétreau P (1996) The effects of neuromuscular block on peak airway pressure and abdominal elastance during pneumoperitoneum. Anesth Analg 82:525–527. 10.1097/00000539-199603000-00017 [DOI] [PubMed] [Google Scholar]
- 34.Kopman AF, Naguib M (2015) Laparoscopic surgery and muscle relaxants: is deep block helpful. Anesth Analg 120:51–58. 10.1213/ANE.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 35.Bruintjes MH, van Helden EV, Braat AE, Dahan A, Scheffer GJ, van Laarhoven CJ, Warlé MC (2017) Deep neuromuscular block to optimize surgical space conditions during laparoscopic surgery: a systematic review and meta-analysis. Br J Anaesth 118:834–842. 10.1093/bja/aex116 [DOI] [PubMed] [Google Scholar]
- 36.Madsen MV, Istre O, Staehr-Rye AK, Springborg HH, Rosenberg J, Lund J, Gätke MR (2016) Postoperative shoulder pain after laparoscopic hysterectomy with deep neuromuscular blockade and low-pressure pneumoperitoneum: a randomised controlled trial. Eur J Anaesthesiol 33:341–347. 10.1097/EJA.0000000000000360 [DOI] [PubMed] [Google Scholar]