Abstract
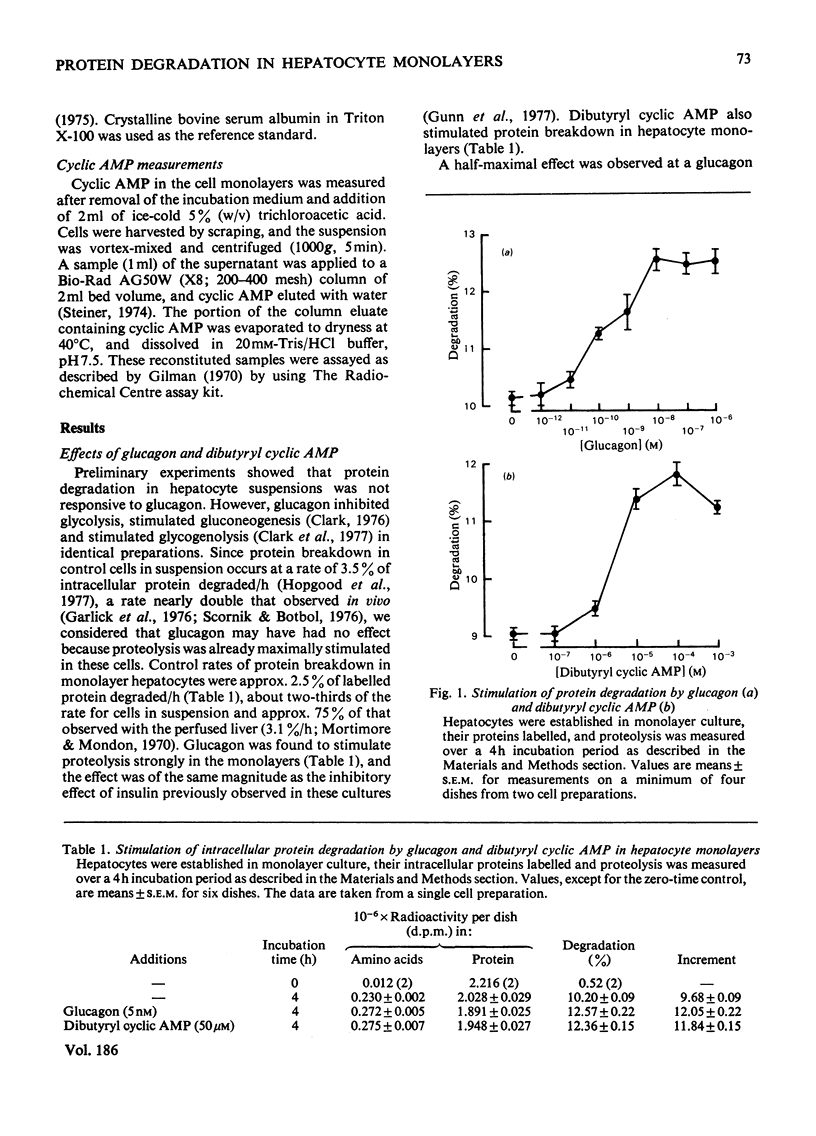
1. Hepatocytes were isolated by collagenase perfusion of livers from fed rats and established in stationary monolayer culture. 2. Degradation of intracellular protein was measured in these monolayers after labelling for 16h with [3H]leucine followed by a 3h chase period in medium containing 2mM-leucine. 3. Proteolysis in this system was stimulated by physiological concentrations of glucagon and also by added dibutyryl cyclic AMP. The effects of these two agents were not additive, which is consistent with the view that they act by the same mechanism. 4. A close correlation was found between intracellular cyclic AMP concentrations generated by glucagon and the degree of stimulation of proteolysis elicited by the hormone. 5. Insulin reduced glucagon-stimulated proteolysis, but not glucagon-elevated intracellular cyclic AMP concentrations. 6. The continual presence of either insulin or glucagon was necessary for the full expression of their effects on proteolysis. 7. In the presence of cycloheximide, proteolysis was normally responsive to glucagon but not to insulin. In contrast, proteolysis was not responsive to either hormone in the presence of ammonia, an agent that blocks the final lysosomal step of protein breakdown. 8. We propose that in hepatocyte monolayers glucagon may act via cyclic AMP to increase cellular autophagy and thus increase proteolysis, whereas insulin inhibits these processes independently of cyclic AMP.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Hlivko T. J., McBee A. G., Shinozuka H., Brocher S. Specific inhibition by NH4CL of autophagy-associated proteloysis in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):357–366. doi: 10.1016/0014-4827(78)90289-6. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila A. U., Shelburne J. D., Trump B. F. Studies on cellular autophagocytosis. A histochemical study on sequential alterations of mitochondria in the glucagon-induced autophagic vacuoles of rat liver. Lab Invest. 1972 Sep;27(3):317–323. [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Filsell O. H., Jarrett I. G. An effect of extracellular redox state on the glucagon-stimulated glucose release by rat hepatocytes and perfused liver. Horm Metab Res. 1977 May;9(3):213–217. doi: 10.1055/s-0028-1093539. [DOI] [PubMed] [Google Scholar]
- Clark M. G. Glucagon regulation of pyruvate kinase in relation to phosphenol pyruvate substrate cycling in isolated rat hepatocytes. Biochem Biophys Res Commun. 1976 Jan 12;68(1):120–126. doi: 10.1016/0006-291x(76)90018-8. [DOI] [PubMed] [Google Scholar]
- Claus T. H., Pilkis S. J. Regulation by insulin of gluconeogenesis in isolated rat hepatocytes. Biochim Biophys Acta. 1976 Feb 24;421(2):246–262. doi: 10.1016/0304-4165(76)90291-9. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. Reduced sensitivity of the hepatic adenylate cyclase-cyclic AMP system to glucagon during sustained hormonal stimulation. J Clin Invest. 1976 Feb;57(2):435–443. doi: 10.1172/JCI108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L., Baudhuin P., De Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol. 1967 Nov;35(2):C11–C16. doi: 10.1083/jcb.35.2.c11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dich J., Gluud C. N. Effect of glucagon on cyclic AMP, albumin metabolism and incorporation of 14C-leucine into proteins in isolated parenchymal rat liver cells. Acta Physiol Scand. 1976 Aug;97(4):457–469. doi: 10.1111/j.1748-1716.1976.tb10285.x. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Lewis S. B., Ho R. J., Robison G. A., Park C. R. The role of cyclic AMP in the interaction of glucagon and insulin in the control of liver metabolism. Ann N Y Acad Sci. 1971 Dec 30;185:85–100. doi: 10.1111/j.1749-6632.1971.tb45239.x. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Garlick P. J., Waterlow J. C., Swick R. W. Measurement of protein turnover in rat liver. Analysis of the complex curve for decay of label in a mixture of proteins. Biochem J. 1976 Jun 15;156(3):657–663. doi: 10.1042/bj1560657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsmann W. H., Mortimore G. E. Influence of glucagon and 3', 5'-AMP on insulin responsiveness of the perfused rat liver. Am J Physiol. 1968 Sep;215(3):553–559. doi: 10.1152/ajplegacy.1968.215.3.553. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Wong K. Y., Jones A. L. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1368–1372. doi: 10.1073/pnas.74.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammeltvedt R., Berg T. Influence of insulin on lysosomal activity and urea production in isolated parenchymal cells from rat liver. Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):977–981. doi: 10.1515/bchm2.1976.357.2.977. [DOI] [PubMed] [Google Scholar]
- Gunn J. M., Clark M. G., Knowles S. E., Hopgood M. F., Ballard F. J. Reduced rates of proteolysis in transformed cells. Nature. 1977 Mar 3;266(5597):58–60. doi: 10.1038/266058a0. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Gunn J. M., Hanson R. W., Ballard F. J. Increased degradation rates of protein synthesized in hepatoma cells in the presence of amino acid analogues. Biochem J. 1975 Mar;146(3):595–600. doi: 10.1042/bj1460595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Assimacopoulos-Jeannet F. D., Exton J. H., Park C. R. Stimulation of a low Km phosphodiesterase from liver by insulin and glucagon. J Biol Chem. 1978 Feb 10;253(3):746–757. [PubMed] [Google Scholar]
- Loten E. G., Sneyd J. G. An effect of insulin on adipose-tissue adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1970 Nov;120(1):187–193. doi: 10.1042/bj1200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER L. L. Glucagon: a protein catabolic hormone in the isolated perfused rat liver. Nature. 1960 Jan 23;185:248–248. doi: 10.1038/185248a0. [DOI] [PubMed] [Google Scholar]
- Mackrell D. J., Sokal J. E. Antagonism between the effects of insulin and glucagon on the isolated liver. Diabetes. 1969 Nov;18(11):724–732. doi: 10.2337/diab.18.11.724. [DOI] [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Neely A. N., Cox J. R., Fortney J. A., Schworer C. M., Mortimore G. E. Alterations of lysosomal size and density during rat liver perfusion. Suppression by insulin and amino acids. J Biol Chem. 1977 Oct 10;252(19):6948–6954. [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N., Toews C. J. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes. 1974 Sep;23(9):725–731. doi: 10.2337/diab.23.9.725. [DOI] [PubMed] [Google Scholar]
- Pfeifer U. Inhibition by insulin of the physiological autophagic breakdown of cell organelles. Acta Biol Med Ger. 1977;36(11-12):1691–1694. [PubMed] [Google Scholar]
- Pilkis S. J., Claus T. H., Johnson R. A., Park C. R. Hormonal control of cyclic 3':5'-AMP levels and gluconeogenesis in isolated hepatocytes from fed rats. J Biol Chem. 1975 Aug 25;250(16):6328–6336. [PubMed] [Google Scholar]
- Reijngoud D. J., Oud P. S., Kás J., Tager J. M. Relationship between medium pH and that of the lysosomal matrix as studied by two independent methods. Biochim Biophys Acta. 1976 Oct 5;448(2):290–302. doi: 10.1016/0005-2736(76)90243-1. [DOI] [PubMed] [Google Scholar]
- Rosa F. Ultrastructural changes produced by glucagon, cyclic 3'5'-AMP and epinephrine on perfused rat livers. J Ultrastruct Res. 1971 Feb;34(3):205–213. doi: 10.1016/s0022-5320(71)80068-0. [DOI] [PubMed] [Google Scholar]
- Scornik O. A., Botbol V. Role of changes in protein degradation in the growth of regenerating livers. J Biol Chem. 1976 May 25;251(10):2891–2897. [PubMed] [Google Scholar]
- Seglen P. O. Protein degradation in isolated rat hepatocytes is inhibited by ammonia. Biochem Biophys Res Commun. 1975 Sep 2;66(1):44–52. doi: 10.1016/s0006-291x(75)80292-0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976 Jul;100(2):276–280. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- Shelburne J. D., Arstila A. U., Trump B. F. Studies on cellular autophagocytosis. Cyclic AMP- and dibutyryl cyclic AMP-stimulated autophagy in rat liver. Am J Pathol. 1973 Sep;72(3):521–540. [PMC free article] [PubMed] [Google Scholar]
- Shikama H., Ui M. Attenuation of epinephrine-induced increase in liver cyclic AMP by endogeneous insulin in vivo. Biochim Biophys Acta. 1976 Sep 24;444(2):461–471. doi: 10.1016/0304-4165(76)90390-1. [DOI] [PubMed] [Google Scholar]
- Steiner A. L. Assay of cyclic nucleotides by radioimmunoassay methods. Methods Enzymol. 1974;38:96–105. doi: 10.1016/0076-6879(74)38016-0. [DOI] [PubMed] [Google Scholar]
- Woodside K. H., Ward W. F., Mortimore G. E. Effects of glucagon on general protein degradation and synthesis in perfused rat liver. J Biol Chem. 1974 Sep 10;249(17):5458–5463. [PubMed] [Google Scholar]


