Abstract
Background
Segmented self-expanding metal stents (SEMS) are an alternative to conventional unsegmented SEMS in the treatment of esophageal strictures. Due to their segmented design, they may adapt better to the surrounding structures making them less likely to migrate or cause trauma. We examined if there are clinically relevant differences between segmented and conventional esophageal SEMS in benign and malignant stenosis in terms of their functionality and safety.
Patients and methods
We performed a multicenter, retrospective case–control study of segmented and conventional SEMS implantations in esophageal stenosis. Outcome parameters were adverse events such as migration, occlusion, and severe complications (i.e., bleeding and perforation).
Results
79 segmented SEMS were identified and compared to 79 conventional SEMS implantations. Groups were similar in terms of age, gender, and etiology. We observed 13.9% severe complications (SEMS-associated clinically significant bleeding or perforation) in the conventional SEMS group compared to 3.8% in the segmented SEMS group. This difference was statistically significant (p = 0.025). Rates of migration and occlusion were similar between both groups. Likewise, there was no significant difference in terms of short-term (30 days) clinical success.
Conclusion
In this first controlled analysis, segmented SEMS were associated with fewer severe clinical complications compared to conventional SEMS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-024-11262-3.
Keywords: Esophageal stent, SEMS, Esophageal stricture, Segmented esophageal stent, Esophageal cancer, Tracheo-esophageal fistula, Esophageal perforation, Upper GI bleeding
Several benign and malignant conditions may cause strictures of the esophageal lumen resulting in dysphagia, food bolus impaction, odynophagia, and/or loss of weight. Clinical relevant dysphagia usually occurs, if the esophageal lumen is restricted by more than 50% [1]. Endoscopic intervention aims to restore the esophageal passage, ensure oral intake, and preserve quality of life. Endoscopic treatment options include bouginage, balloon dilation, and the placement of self-expanding metal stents (SEMS). The 2021 European Guideline on esophageal stenting recommends partially or fully covered SEMS (fcSEMS) as the first line treatment of malignant stenosis in the palliative setting [2]. In the curative setting, it advises against SEMS as bridge to surgery since retrospective data suggest this approach may be associated with lower R0 resection rates and increases mortality [3]. However, other retrospective studies did not confirm this observation [4, 5]. In benign strictures, the guideline recommends SEMS placement as an option in cases that are refractory to simpler alternatives such as bouginage or balloon dilatation [2].
Esophageal SEMS placement may cause different adverse events. These include minor events such as cervical or retrosternal pain or gastroesophageal reflux [6]. Recurrent dysphagia may be due to stent migration or occlusion by tumor ingrowth or food impaction. Less frequently, severe adverse events such as stent-associated bleeding or perforation can occur [6]. These latter events are relatively rare, but often have devastating consequences.
SEMS are usually composed of a nitinol mesh that is partially or fully surrounded by a silicon cover to prevent tissue ingrowth. Partially covered SEMS have the advantage of being less likely to migrate since the uncovered ends quickly become embedded in tissue. fcSEMS have the advantage of being removable. A variant of fcSEMS, segmented fcSEMS (s-fcSEMS) have been introduced. They consist of several short nitinol mesh segments that are linked by connective loops made of polytetrafluoroethylene (PTFE) and surrounded by one silicon cover that spans the entire length of the stent. Figure 1 shows an example for a c-fcSEMS and a s-fcSEMS. The connective loops allow for a considerable degree of mobility between the segments. It is conceivable that s-fcSEMS compared to conventional (i.e., non-segmented) fcSEMS (c-fcSEMS) might be less prone to migrate or cause patient discomfort or tissue injury, because they adapt better to the surrounding anatomy. However, data on s-fcSEMS are currently limited to two uncontrolled case series [7, 8]. The aim of the retrospective analysis reported here was to compare clinically relevant outcomes between s-fcSEMS and c-fcSEMS in the treatment of esophageal strictures.
Fig. 1.
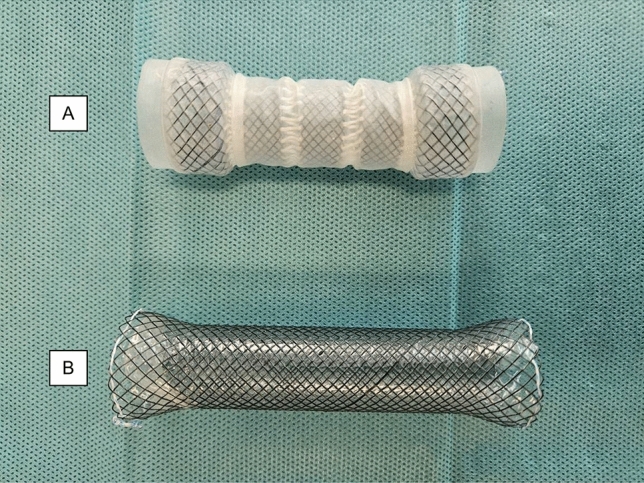
Example of the s-fcSEMS and c-fcSEMS; A s-fcSEMS. This illustrated model has a diameter of 22 mm at the center, 26 mm at the flange, and a length of 80 mm. It consists of 5 nitinol segments connected by a nylon cord. The cover is made of silicon and covers the whole stent. B c-fcSEMS with a diameter of 20 mm at the center, 26 mm at the flanges, and length of 100 mm. It consists of one meshwork of nitinol with a silicon cover. All stents have retraction cords at both ends
Methods
Study design
In this retrospective, multi-centered, pair-matched case–control study, we analyzed all patients that received s-fcSEMS for the treatment of esophageal strictures between 2011 and 2023 at three tertiary centers in Northern Germany (Asklepios Hospital Barmbek in Hamburg, University of Schleswig–Holstein Campus Luebeck, Hannover Medical School). These were matched 1:1 with patients from the same time period who received c-fcSEMS. S-fcSEMS were introduced in the centers at different times. One center started segmented stent deployment in 2017, the other two centers in 2020. The type of stent used was at the discretion of the endoscopist.
Clinical data were extracted from available patient files. We included both benign and malignant lesions as well as unaltered and post-surgical anatomy, i.e., esophago-gastric anastomosis. We excluded patients younger than 18 years and those who treated for other indications such as leakage and perforation of fistula. Moreover, stents with a diameter ≥ 28 mm in the center portion and all uncovered or partially covered stent implantations were excluded from analysis. Matching of the s-fcSEMS and c-fcSEMS cases was performed primarily based on sex and secondarily age, while being blinded to outcomes, i.e., adverse events. Supplemental Table 1 shows basic characteristics of the s-fcSEMS cohort as well as the c-fcSEMS cohort before and after matching.
The indication for SEMS deployment was patient reported clinically significant dysphagia due to an esophageal stricture in all cases. The duration of SEMS therapy was determined by indication—generally temporary stenting for 6–8 weeks in benign strictures, permanent in malignant strictures—and by unplanned adverse events requiring explanation particularly SEMS migration.
The study was approved—without a formal vote because of its retrospective design—by the Ethics Committee of the Hamburg Chamber of Physicians (Filing Number: 2023-300411-WF). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committees. This trial has been registered in the German Clinical Trials Register with the identification number DRKS00034173.
Devices and endoscopic procedures
Stent placement was performed using standard technique after placing a stiff guidewire across the stricture with fluoroscopic and/or endoscopic control. S-fcSEMS were from Microtech Co. Ltd. (Nanjing, China) and had a diameter between 18 and 22 mm in the center portion of the stent. C-fcSEMS were from different manufacturers and had diameters ranging from 18 to 26 mm in the center portion. Endoscopic and radiological images of the typical SEMS deployment procedure are shown in supplemental Fig. 1.
Outcome parameters
Predefined outcome parameters were stent migration, stent occlusion, and severe clinical complications. Migration was defined as a change of primary stent position resulting in a need for endoscopic repositioning or removal of the stent. Stent occlusion was defined as blockage of the stent lumen by food or tissue ingrowth resulting in dysphagia and the need for reintervention. Severe clinical complications were defined as stent-associated bleeding necessitating endoscopic intervention or stent-associated esophageal perforation. In addition, we evaluated clinical risk factors potentially associated with the outcome parameters above such as age, stricture etiology, esophageal anatomy, and stent diameter.
Statistics
Anonymized patient data were collected in Microsoft Excel and statistical analysis was performed using IBM SPSS Statistics version 29.0.2.0. To test for statistical significance, we used the Mann–Whitney-U test, Pearson’s chi-square test, log-rank test, and binary logistic regression depending on the data set. Continuous data were examined using means, standard deviations, and medians with inter-quartile range. A p value of < 0.05 was considered statistically significant.
Results
We retrospectively identified 79 cases that received a s-fcSEMS. These were compared with the same number of c-fcSEMS deployments (Table 1). The groups were similar regarding age, sex, etiology of the stenosis and histological subtypes in those with malignant stenosis. There was a higher percentage of patients with post-surgical anatomy in the s-fcSEMS group (22.8% vs. 10.1%). The two cohorts showed a similar distribution of the location of the stenosis (upper, middle, and lower third of the esophagus). The length of the strictures was also comparable in both groups. Strictures of 2 cm or less were regarded as short and those of more than 2 cm as long (Supplement Table 2). Comparison of the continuous data for strictures lengths was also similar: the median of the s-fcSEMS was 4 cm with standard deviation 3.887 and the median of the c-fcSEMS was 5 cm with standard deviation 3.889. In the Mann–Whitney-U test of the two groups, there was no significant difference in the lengths of the strictures (p = 0.134). The mean follow-up time was 136 days (median 50 days) and did not differ significantly between the two groups.
Table 1.
Baseline characteristics
| All patients (n = 158) | c-fcSEMS (n = 79) | s-fcSEMS (n = 79) | p | |
|---|---|---|---|---|
| Age (years) | ||||
| Median (Q1, Q3) | 69 (60, 75) | 70 (60, 76) | 68 (60, 75) | 0.190 |
| Mean (SD) | 67.5 (11.8) | 68 (11) | 67 (12.8) | |
| Follow up (days) | ||||
| Median (Q1, Q3) | 50 (14, 159) | 54 (13, 162) | 49 (17, 145) | 0.770 |
| Mean (SD) | 136 (224) | 161 (277) | 111 (151) | |
| Sex | ||||
| Male % (n) | 69.6 (110) | 69.6 (55) | 69.6 (55) | 1.000 |
| Female % (n) | 30.4 (48) | 30.4 (24) | 30.4 (24) | |
| Etiology of stenosis | ||||
| Benign % (n) | 22.8 (36) | 21.5 (17) | 24.1 (19) | 0.704 |
| Malignant % (n) | 77.2 (122) | 78.5 (62) | 75.9 (60) | |
| EAC % (n) | 27.2 (43) | 25.3 (20) | 29.1 (23) | |
| ESCC % (n) | 25.3 (40) | 32.9 (26) | 17.7 (14) | |
| Other % (n) | 24.7 (39) | 20.3 (16) | 29.1 (23) | |
| Anatomy | ||||
| Native % (n) | 83.5 (132) | 89.9 (71) | 77.2 (61) | 0.032 |
| Altered % (n) | 16.5 (26) | 10.1 (8) | 22.8 (18) | |
A p-value <0.05 was considered significant
Mann–Whitney-U test or Pearson’s chi-square test was used to test for statistical significance.
c-fcSEMS conventional fully covered self-expanding metal stent, s-fcSEMS segmented self-expanding metals stent, EAC esophageal adenocarcinoma, ESCC esophageal squamous cell carcinoma, Q1 first quartile, Q3 third quartile, SD standard deviation
Technical success rate of stent deployment was 100% in both the c-fcSMES and s-fcSEMS groups. Overall complication rate was numerically lower in the s-fcSEMS group compared to the c-fcSEMS group, but this difference did not reach statistical significance (31.6% vs. 36.7%) (Table 2). However, we found severe clinical complications (stent-associated bleeding necessitating intervention and stent-associated perforation) with significantly lower frequency in the s-fcSEMS compared to the c-fcSEMS group (3.8% vs. 13.9%) (p = 0.025). We also noted numerically fewer bleeding events in the s-fcSEMS group (3.8% vs 10.1%), but this fell short of statistical significance (Table 2).
Table 2.
Adverse events
| All patients (n = 158) | c-fcSEMS (n = 79) | s-fcSEMS (n = 79) | p | |
|---|---|---|---|---|
| Any complication | 34.2 (54) | 36.7 (29) | 31.6 (25) | 0.502 |
| Severe complication | 8.9 (14) | 13,9 (11) | 3,8 (3) | 0.025 |
| Perforation | 1.9 (3) | 3.8 (3) | 0 (0) | 0.080 |
| Bleeding | 7 (11) | 10.1 (8) | 3.8 (3) | 0.118 |
| SEMS occlusion | 4.4 (7) | 5.1 (4) | 3.8 (3) | 0.699 |
| SEMS migration | 24.7 (39) | 22.8 (18) | 26.6 (21) | 0.580 |
A p-value <0.05 was considered significant
Pearson’s chi-square test was used to test for statistical significance.
c-fcSEMS conventional fully covered self-expanding metal stent, s-fcSEMS segmented self-expanding metals stent, SEMS self-expanding metals stent
We evaluated potential risk factors for stent-associated bleeding. Seven out of the 11 patients with bleeding had at least one risk factor for bleeding (Supplement Table 3). However, the number of bleeding events was too low to perform a formal risk factor analysis. When evaluating only the subgroup with malignant etiology of stenosis, there were no statistically significant differences between the c-fcSEMS and the s-fcSEMS groups (Table 3).
Table 3.
Adverse events in patients with malignant stenosis
| All patients (n = 122) | c-fcSEMS (n = 62) | s-fcSEMS (n = 60) | p | |
|---|---|---|---|---|
| Any complication | 34.4 (42) | 37.1 (23) | 31.7 (19) | 0.528 |
| Severe complications | 9.8 (12) | 14.5 (9) | 5 (3) | 0.078 |
| Perforation | 1.6 (2) | 3.2 (2) | 0 (0) | 0.161 |
| Bleeding | 8.2 (10) | 11.3 (7) | 5 (3) | 0.205 |
| SEMS occlusion | 5.7 (7) | 6.5 (4) | 5 (3) | 0.730 |
| SEMS migration | 23.8 (29) | 22.6 (14) | 25 (15) | 0.754 |
A p-value <0.05 was considered significant
Pearson’s chi-square test was used to test for statistical significance.
c-fcSEMS conventional fully covered self-expanding metal stent, s-fcSEMS segmented self-expanding metals stent, SEMS self-expanding metals stent
Of note, there were no stent-associated perforations in the s-fcSEMS group compared to three such events in the c-fcSEMS group. The first patient had squamous cell carcinoma of the esophagus and presented 125 days after SEMS placement with pleural empyema and a perforation near the aboral flange. The initial c-fcSEMS was removed; a larger diameter c-fcSEMS was placed over the defect and a jejunostomy was established for enteral nutrition. The second patient had also had esophageal squamous cell carcinoma and presented 38 days after SEMS with new worsening dysphagia and cough. Bronchoscopy showed a trachea-esophageal fistula near the aboral flange of the c-fcSEMS; a tracheal stent was placed. The third patient had a bouginage-refractory anastomotic stricture following esophagectomy for cancer of the GE junction. A c-fcSEMS was placed. Thirteen days later a perforation near the aboral flange was detected. The stent was removed and vacuum therapy was initiated resulting in complete closure of the perforation. The three perforations occurred at two different centers; the c-fcSEMS had been placed by three different endoscopists.
The most frequent adverse event overall was stent migration. The frequency of stent migration did not differ between the groups (s-fcSEMS 26.6% vs. c-fcSEMS 22.8%). SEMS occlusions also occurred with comparable frequency in both groups (s-fcSEMS 3.8% vs. c-fcSEMS 5.1%). Six patients experienced more than one adverse event: two in the s-fcSEMS group (migration plus bleeding; migration plus occlusion) and four in the c-fcSEMS group (three cases with migration plus bleeding; one with migration plus occlusion).
To probe for differences in the timing of adverse events we compared clinical success, i.e., the absence of adverse events including migration during the first 30 days after SEMS insertion. We observed no significant difference between the s-fcSEMS and c-fcSEMS groups in terms of clinical success over this time frame (Fig. 2).
Fig. 2.
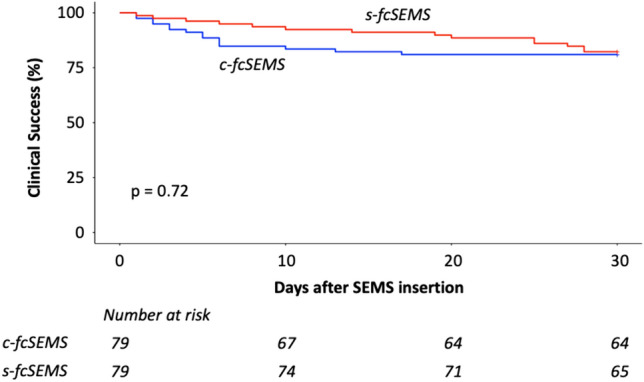
30 days of clinical success; Clinical success was defined as a functional stent in situ without any adverse events (stent migration or any other). c-fcSEMS conventional fully covered self-expanding metal stent, s-fcSEMS segmented self-expanding metals stent, SEMS self-expanding metals stent; Log-rank test was used to test for statistical significance
We performed binary logistic regression analysis across the entire patient cohort, i.e., both groups, to probe for an association between potential risk factors—specifically sex, age, etiology (malignant vs. benign stricture), anatomy (unaltered vs. post-surgical), and SEMS diameter—and migration or any adverse event. However, we did not detect any such association in our data set (Supplemental Table 4 and 5).
Next, we analyzed the subgroup of patients with malignant strictures (Supplemental Table 6). In this subgroup, there was no significant difference between groups in terms of overall adverse event rate (c-fcSEMS 37.1% vs. s-fcSEMS 31.7%). Severe complications occurred numerically more often in the c-fcSEMS group, but in this smaller subset the difference fell short of statistical significance (c-fcSEMS 14.5% vs. s-fcSEMS 5%). The incidence of SEMS occlusion and SEMS migration were seen with approximately the same frequency.
Discussion
s-fcSEMS are an innovative variant of fcSEMS. It is conceivable that the design with multiple small stent segments connected by loops within a full-length silicone cover might allow s-fcSEMS to adapt better to the surrounding anatomy. This might translate into a lower risk of stent migration or injury to surrounding structures. To our knowledge, this is the first report of a direct comparison between c-fcSEMS and s-fcSEMS. Our findings suggest that the segmented design does not provide a benefit in terms of preventing SEMS migration, but s-fcSEMS may be associated with a lower risk of severe complications, i.e., clinically significant bleeding and perforation.
The technical success rate of stent deployment was 100% in both the c-fcSMES and s-fcSEMS groups. This is in keeping with two uncontrolled case series on s-fcSEMS in malignant esophageal stenosis [7, 8]. Likewise, studies on c-fcSEMS usually report technical success rates well above 90% [9]. Thus, initial stent deployment across esophageal stenoses is generally successful with both c-fcSEMS and s-fcSEMS. Based on the data, we have we cannot comment on the clinical success rate of c-fcSEMS versus s-fcSEMS since this is a retrospective analysis and we do not have systematic dysphagia score data before and after SEMS placement. Likewise, we cannot comment on whether clinically more severe dysphagia is associated with a higher rate of adverse events. This interesting point would need to be investigated in a prospective study. In our clinical experience, a c-fcSEMS or s-fcSEMS that is correctly positioned across a stricture will usually result in some relieve of dysphagia. Thus, we consider it likely that technical and clinical success will be correlated at least at early timepoints. However, this assumption will need to be tested in a prospective study where severity of dysphagia can be evaluated.
The most common clinical problem with esophageal stents is migration. A recent metanalysis on SEMS use in malignant stenosis—somewhat surprisingly—reported comparable migration rates for c-fcSEMS (range 6–19%) and partially covered SEMS (6–30%) [9]. A metanalysis of studies on c-fcSEMS use in refractory benign stenosis found a wide range of migration rates of 4–54% (mean 29%) [10]. In our study, we observed similar migration rates for c-fcSEMS (22.8%) and s-fcSEMS (26.6%) that fall well into the range reported in published studies on c-fcSEMS. This is also in line with what has been observed for s-fcSEMS in two published case series by Bi et al. (17%) [7] and Wiese et al. (15%) [8]. Thus, it seems that s-fcSEMS have no advantage over c-fcSEMS in terms of stent migration rate and other approaches are needed to mitigate this problem. Recently, promising results have been reported for fixation of the stent to the esophageal wall by either suturing [11] or a use of specially designed over the scope clips [12].
Stent obstruction due to tissue ingrowth and/or food bolus impaction that necessitated clinical intervention to restore passage also occurred with comparable frequency in c-fcSEMS (5.1%) and s-fcSEMS (3.8%) in our study. Again, these findings are within the range of what has been reported in other studies of c-fcSEMS use in malignant disease (range 0–26%) [9].
Stent-associated bleeding and perforation are less common but far more serious adverse events compared to stent migration and occlusion. In our cohort, such severe complications were observed with significantly lower frequency in the s-fcSEMS group compared to the c-fcSEMS group. When considering bleeding and perforation separately there was a numerical difference between the groups for both types of events, but this fell short of statistical significance.
It is notable that no stent-associated perforation was overserved in the s-fcSEMS group, while we observed 3 perforations among 79 patients in the c-fcSEMS group (3.8%). The latter is within the range of perforation rates reported in earlier studies ranging from 1.9 to 7.3% [3, 13, 14]. If this difference between c-fcSEMS and s-fcSEMS is real and confirmed in prospective studies, it may be because the design of s-fcSEMS enables them to adjust better to the surrounding anatomy making them more atraumatic. This would be clinically highly relevant since perforation is relatively rare, but when it occurs the most catastrophic complication of esophageal stenting. Thus, prospective data on the risk of severe complications ideally from randomized controlled trials are very desirable.
A potential confounder in our study is that we included both benign and malignant stenoses and native as well as surgically altered anatomy in our analysis. Yet, the logistic regression showed no significant impact of these variables to the rates of migration or occurrence of any adverse event. Moreover, if only the subgroup with malignant stenoses (77% of the total cohort) is considered the rate of all adverse events analyzed remained largely unchanged.
In our retrospective analysis, 30 days of clinical success—defined as SEMS in situ without occurrence of migration or any other adverse events—did not differ between the s-fcSEMS and c-fcSEMS groups. Given the retrospective nature of this analysis, we cannot provide reliable data on a long-term follow-up. However, for malignant strictures 30-day mortality following SEMS placement have been reported to be in the range of 17–22% so that 30 days may well be viewed as a meaningful timeframe in this population [15, 16]. Optimal duration of SEMS treatment for benign strictures is not clear, but the ESGE guideline 2021 suggests a duration between 6 and 8 weeks and no longer than 10–12 weeks so that again 30 days can be considered clinically meaningful [2]. According to our data, s-fcSEMS and c-fcSEMS perform similarly in terms of short-term clinical success. Of note, because of the retrospective nature of the study, we cannot comment on relief of dysphagia. This should clearly be included in the definition of clinical success in a prospective study design.
SEMS treatment may affect oncologic outcomes. In the curative setting, SEMS placement prior to surgery has been associated with lower R0 resection rates, more complications, and reduced survival in a multicenter study [3] but not in two other studies [4, 5]. This is a question of clinical importance since SEMS are a fast and reliable way to restore oral intake, improve quality of life, and maintain nutritional status. Moreover, it is mechanistically unclear why SEMS placement would impair oncologic outcomes. One possibility is that the physical pressure exerted by the SEMS on the tumor might somehow promote invasion and/or spread. If that is true, one may speculate that s-fcSEMS are an alternative option worth exploring since they likely exert less pressure on the surrounding tissue and adapt well to the surrounding anatomy. For now, pre-operative stent placement remains controversial and mostly limited to select cases based on multidisciplinary tumor board decisions.
In conclusion, this is the first direct comparison of c-fcSEMS and s-fcSEMS. We found no difference in terms of migration and occlusion rate, but a reduced rate of severe complications, i.e., stent-associated clinically significant bleeding and perforation, associated with s-fcSEMS. This suggests that their design may render them less likely to cause damage to surrounding structures. More data ideally from a prospective randomized trial will be needed to further evaluate this interesting possibility.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Disclosures
Claudius Schlemmer, Torsten Voigtländer, Jan Drews, Carsten Engelke, Jens U. Marquardt, Benjamin Heidrich, Friederike Klein, Heiner Wedemeyer, Martha M. Kirstein, and Thomas von Hahn have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adler DG, Siddiqui AA (2017) Endoscopic management of esophageal strictures. Gastrointest Endosc 86:35–43. 10.1016/j.gie.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Spaander MCW, Van Der Bogt RD, Baron TH, Albers D, Blero D, De Ceglie A, Conio M, Czakó L, Everett S, Garcia-Pagán J-C, Ginès A, Jovani M, Repici A, Rodrigues-Pinto E, Siersema PD, Fuccio L, Van Hooft JE (2021) Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2021. Endoscopy 53:751–762. 10.1055/a-1475-0063 [DOI] [PubMed] [Google Scholar]
- 3.Mariette C, Gronnier C, Duhamel A, Mabrut J-Y, Bail J-P, Carrere N, Lefevre JH, Meunier B, Collet D, Piessen G (2015) Self-expanding covered metallic stent as a bridge to surgery in esophageal cancer: impact on oncologic outcomes. J Am Coll Surg 220:287–296. 10.1016/j.jamcollsurg.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 4.Järvinen T, Ilonen I, Ylikoski E, Nelskylä K, Kauppi J, Salo J, Räsänen J (2017) Preoperative stenting in oesophageal cancer has no effect on survival: a propensity-matched case-control study†. Eur J Cardiothorac Surg 52:385–391. 10.1093/ejcts/ezx097 [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues-Pinto E, Ferreira-Silva J, Sousa-Pinto B, Medas R, Garrido I, Siersema PD, Pereira P, Macedo G (2021) Self-expandable metal stents in esophageal cancer before preoperative neoadjuvant therapy: efficacy, safety, and long-term outcomes. Surg Endosc 35:5130–5139. 10.1007/s00464-020-08002-8 [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen BD, Siersema PD (2018) Esophageal stenting in clinical practice: an overview. Curr Treat Options Gastroenterol 16:260–273. 10.1007/s11938-018-0181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi Y, Ren J, Li J, Yu Z, Han X, Wu G (2019) A novel fully covered self-expandable segmental metallic stents for the treatment of refractory esophageal stenosis. J Thorac Dis 11:1363–1369. 10.21037/jtd.2019.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiese M-S, Dratsch T, Plum PS, Lorenz F, Rieck I, Pinto Dos Santos D, Alakus H, Bludau M, Kleinert R, Goeser T, Bruns CJ, Chon S-H (2021) Palliation of malignant dysphagia with a segmented self-expanding metal stent: a STROBE-compliant article. Medicine 100:e27052. 10.1097/MD.0000000000027052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Wei H, Li Y (2020) Comparison of fully-covered vs partially covered self-expanding metallic stents for palliative treatment of inoperable esophageal malignancy: a systematic review and meta-analysis. BMC Cancer 20:73. 10.1186/s12885-020-6564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuccio L, Hassan C, Frazzoni L, Miglio R, Repici A (2015) Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy 48:141–148. 10.1055/s-0034-1393331 [DOI] [PubMed] [Google Scholar]
- 11.Bick BL, Imperiale TF, Johnson CS, DeWitt JM (2017) Endoscopic suturing of esophageal fully covered self-expanding metal stents reduces rates of stent migration. Gastrointest Endosc 86:1015–1021. 10.1016/j.gie.2017.03.1545 [DOI] [PubMed] [Google Scholar]
- 12.Schiemer M, Bettinger D, Mueller J, Schultheiss M, Schwacha H, Hasselblatt P, Thimme R, Schmidt A, Kuellmer A (2022) Reduction of esophageal stent migration rate with a novel over-the-scope fixation device (with video). Gastrointest Endosc 96:1–8. 10.1016/j.gie.2022.02.001 [DOI] [PubMed] [Google Scholar]
- 13.Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, van Lanschot JJ, Wijrdeman HK, Mulder CJ, Reinders JG, Boot H, Aleman BM, Kuipers EJ, Siersema PD (2004) Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 364:1497–1504. 10.1016/S0140-6736(04)17272-3 [DOI] [PubMed] [Google Scholar]
- 14.Wang MQ, Sze DY, Wang ZP, Wang ZQ, Gao YA, Dake MD (2001) Delayed complications after esophageal stent placement for treatment of malignant esophageal obstructions and esophagorespiratory fistulas. J Vasc Interv Radiol 12:465–474. 10.1016/S1051-0443(07)61886-7 [DOI] [PubMed] [Google Scholar]
- 15.Battersby NJ, Bonney GK, Subar D, Talbot L, Decadt B, Lynch N (2012) Outcomes following oesophageal stent insertion for palliation of malignant strictures: a large single centre series. J Surg Oncol 105:60–65. 10.1002/jso.22059 [DOI] [PubMed] [Google Scholar]
- 16.Eickhoff A, Knoll M, Jakobs R, Weickert U, Hartmann D, Schilling D, Eickhoff JC, Riemann JF (2005) Self-expanding metal stents versus plastic prostheses in the palliation of malignant dysphagia: long-term outcome of 153 consecutive patients. J Clin Gastroenterol 39:877–885. 10.1097/01.mcg.0000180631.61819.4a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


