Abstract
Background
Two programs to reduce expenditures for common gastrointestinal drugs were introduced simultaneously by British Columbia (BC) Pharmacare in 1995. Reference-based pricing restricted reimbursement for all histamine-2 receptor antagonists (H2RAs) to the cost of the least expensive H2RA available, generic cimetidine. Special authority restricted reimbursement for proton pump inhibitors (PPIs) to patients who met certain eligibility criteria. We evaluated the effect of reference-based pricing for H2RAs and special authority for PPIs on dispensing and reimbursement for senior citizen beneficiaries of BC Pharmacare.
Methods
Itemized monthly claims data for upper gastrointestinal drugs were obtained from BC Pharmacare for all beneficiaries 65 years of age or older. Periods before and after implementation of reference-based pricing and special authority were compared with respect to defined daily doses dispensed per 100 000 beneficiaries, BC Pharmacare reimbursement per 100 000 beneficiaries, BC Pharmacare reimbursement per defined daily dose and beneficiary contributions per defined daily dose. We used regression models to project forward trends in expenditures observed before implementation of the new policies and hence to estimate accrued cost savings.
Results
Before reference-based pricing and special authority, the numbers of defined daily doses that were dispensed and total BC Pharmacare reimbursements for H2RAs appeared to be declining gradually, whereas those for PPIs were rising. With reference-based pricing, the monthly defined daily dose of cimetidine dispensed increased more than 4-fold, to 116 257 per 100 000 beneficiaries, while those of other restricted H2RAs decreased by more than half, to 50 927 per 100 000 beneficiaries. Special authority immediately reduced the dispensed volumes of PPIs by one-fourth, but growth in volume then appeared to resume at its previous rate. The estimated annualized cost savings achieved by reference-based pricing and special authority were $1.8 million to $3.2 million for H2RAs (depending on the estimation method used) and $5.5 million for PPIs. However, beneficiary contributions for H2RAs increased from negligible amounts to approximately 16% of total drug expenditures.
Interpretation
Reference-based pricing and special authority appear to have been successful in altering prescribing habits and reducing provincial expenditures for upper gastrointestinal drugs, but they have increased the financial burden on senior citizen beneficiaries.
Rising expenditures for prescription medications have strained government-subsidized drug programs worldwide and have prompted the introduction of policies to control expenditures for high-cost drug classes. One such approach adopted both in Canada and abroad is reference-based pricing, which limits reimbursement of a group of drugs with similar therapeutic effect but different active ingredients to the “reference price,” a weighted average price of the lowest cost drug(s) within the group. Drug plan beneficiaries can use one of the higher-cost drugs if they pay the difference between the drug's retail price and the reference price. Variants of reference-based pricing have been applied in many jurisdictions, but individual policies differ in terms of the drug groups targeted, the setting of the reference price and the mechanisms for exemption,1,2 all of which limit the generalizability of policy effects across jurisdictions. Generalizability is also limited by differences in drug prices and prescribing patterns, both of which might affect potential savings due to reference-based pricing. In general, savings related to reference-based pricing could be offset in several ways: large numbers of patients could be approved for exemption; manufacturers could increase the prices of reference drugs; or physicians could switch patients to other, more costly drug classes. Moreover, restrictions on government reimbursement could also shift costs to nonexempt beneficiaries, who would then pay the price difference to maintain their access to a particular drug. Another policy to reduce drug expenditures involves restricting reimbursement for specific drugs to patients who meet certain eligibility criteria. This policy is known in British Columbia (BC) as “special authority.”
The simultaneous introduction of 2 policies by BC Pharmacare in 1995 provided an opportunity to study these and other effects in a Canadian setting. The policies of reference-based pricing of histamine-2 receptor antagonists (H2RAs) and special authority for proton pump inhibitors (PPIs) were intended to control expenditures for 2 high-cost drug classes. A preliminary analysis of claims data suggested that reference-based pricing had significantly reduced BC Pharmacare expenditures on H2RAs in the year after its introduction.3 However, no comprehensive or long-term analysis of either of the 2 policies has since been reported.
Methods
Before the introduction of reference-based pricing, BC Pharmacare limited reimbursement of each individual H2RA to the cost of its lowest-cost formulation, usually a generic equivalent. This “low-cost alternative” policy was announced in March 1994 and implemented in April 1994. Reference-based pricing was announced in early September 1995 and introduced on Oct. 1, 1995. This policy further limited reimbursement for all H2RAs to the cost of generic cimetidine, the lowest-cost H2RA then available (hereafter, generic cimetidine is referred to as the reference standard, and all other H2RAs are referred to as restricted H2RAs). Beneficiaries who required a restricted H2RA for medical reasons, including those who were receiving warfarin, phenytoin or theophylline and who satisfied specific criteria, could be exempted from reference-based pricing upon petition by the physician. Alternatively, nonexempt patients could choose to pay the cost difference out of pocket. Low doses of restricted H2RAs (ranitidine at less than 300 mg/day, famotidine at less than 40 mg/day or nizatidine at less than 300 mg/day) were not subject to reference-based pricing. Until Dec. 1, 1995, patients receiving their first refill prescription could be given a fully reimbursed 2-week supply of a restricted H2RA upon petition to BC Pharmacare.
The special authority policy restricted full reimbursement for PPIs to patients who satisfied specific clinical criteria. Approved indications for a PPI included treatment of esophagitis unresponsive to H2RA, eradication of Helicobacter pylori, and management of rare conditions such as Barrett's esophagus, Zollinger–Ellison syndrome and connective tissue diseases. The announcement and introduction of special authority for PPIs coincided with the introduction of reference-based pricing for H2RAs. Special authority for omeprazole was announced in September 1995 and introduced in October 1995, with initial prescription renewals exempted until December 1995. The policy was extended to lansoprazole in May 1996 and to pantoprazole in November 1997, when these products became available to BC Pharmacare beneficiaries. All prescriptions for PPIs issued by gastroenterologists were automatically exempted from the special authority policy. BC Pharmacare has not restricted reimbursement of prescriptions for other upper gastrointestinal drugs, including sucralfate, prokinetic agents (e.g., cisapride and domperidone) and misoprostol.
Aggregate monthly claims data for H2RAs, PPIs, sucralfate, prokinetic agents and misoprostol were provided by BC Pharmacare for the period January 1993 to May 1999, inclusive, to cover periods before and after introduction of the reference-based pricing and special authority policies. We analyzed claims data only for senior citizens (65 years of age or older), as they represented the largest beneficiary group and had the highest per capita consumption of gastrointestinal drugs. Data included the numbers of prescriptions and unit doses dispensed, the costs reimbursed by BC Pharmacare and the payments made by beneficiaries.
Prescribing volumes were converted to defined daily doses to better reflect the intensity of pharmaceutical use. The defined daily dose represents the assumed mean maintenance dose per day for a drug when used for its main indication in adults; it is assigned to each chemical substance (defined as a fifth-level anatomical therapeutic chemical class) by the World Health Organization Collaborating Centre for Drug Statistics Methodology.4 To control for growth in the number of beneficiaries, all data were converted to rates per 100 000 senior citizens, on the basis of annual population data obtained from Statistics Canada.5 All senior citizens enrolled in British Columbia's provincial health insurance plan are eligible for BC Pharmacare benefits.
For each gastrointestinal drug and drug class, the following values were calculated for each month: (1) numbers of defined daily doses dispensed per 100 000 senior citizens, (2) total BC Pharmacare expenditures per 100 000 senior citizens, (3) total out-of-pocket expenditures per 100 000 senior citizens and (4) mean BC Pharmacare reimbursement per defined daily dose, defined as (2) divided by (1).
Because the reference-based pricing and special authority policies were announced months before they were universally enforced, it was anticipated that utilization immediately before implementation might reflect anticipatory prescribing or “stockpiling.” The data were therefore segregated into 5 discrete periods for analysis: January 1993 to August 1994, a historical comparator period; September 1994 to August 1995, the 12-month baseline period immediately before the policy announcement; September to December 1995, a transition period between announcement of the new policies and universal enforcement; January to December 1996, the 12-month period immediately after enforcement began; and January 1997 to May 1999, a follow-up period.
For both H2RAs and PPIs, expenditure trends observed in the first 3 periods (up to the time when the policies were universally enforced) were estimated with regression models and then projected forward to predict the expenditures that would likely have accrued in the absence of the policies. Because the expenditures for H2RAs were already declining (at a decreasing rate) before implementation of reference-based pricing, we elected to fit expenditures to the log of a time trend for the primary analysis. Estimates of the savings attributable to the introduction of reference-based pricing of H2RAs were particularly sensitive to the choice of period for comparison. Because H2RA expenditures appeared to stabilize during the baseline period (immediately before announcement of the policy), a sensitivity analysis was undertaken in which baseline expenditures were assumed to represent the expenditures had reference-based pricing not been introduced. Trends in PPI expenditures were fit with a standard linear trend. An indicator variable equal to 1 for observations from March and April 1994 was included to control for fluctuations in expenditures related to introduction of the “low-cost alternative” policy. An indicator variable equal to 1 for observations during the interval between the announcement of the new policies and universal enforcement was constructed to directly estimate savings over this transition period. The models were then used to extrapolate pre-policy expenditures to the 12 months immediately after universal enforcement and the later follow-up period, with total BC Pharmacare savings estimated as the cumulative difference between observed and expected monthly expenditures. To reflect sampling error in these estimates, confidence intervals were constructed using 1000 bootstrap replications of the difference between predicted and actual expenditures.
Results
Prescription volumes for gastrointestinal drugs for each period are summarized in Table 1. The 12-month period from September 1994 to August 1995 was considered the baseline against which we evaluated the impact of the reference-based pricing and special authority policies, because it immediately preceded the announcement and implementation of these policies.
Table 1
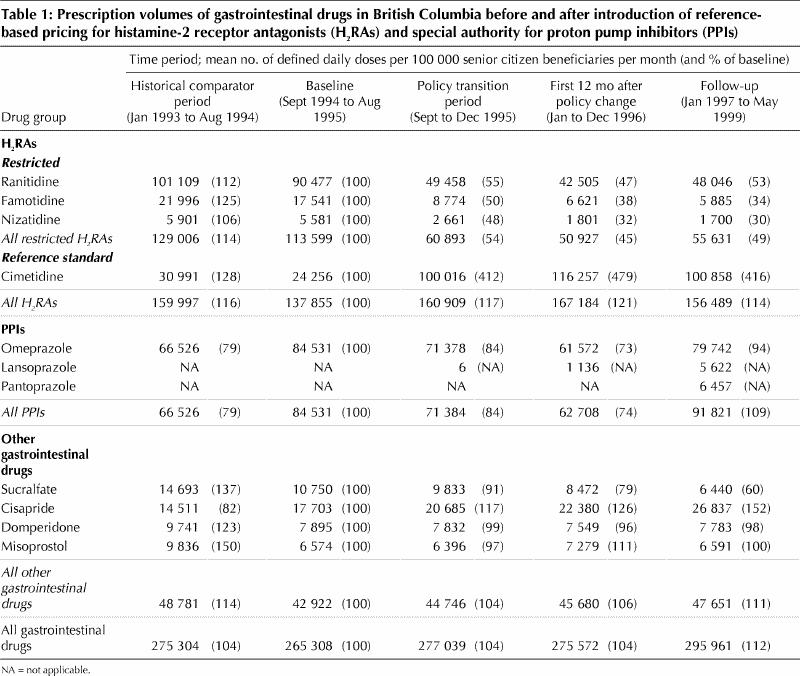
During the baseline period, the mean monthly number of defined daily doses of H2RAs prescribed was 137 855 per 100 000 senior citizen beneficiaries, of which 24 256 (18%) were for the reference standard, cimetidine (Table 1). From the baseline period to the 12-month period immediately after implementation (January to December 1996), there was only a modest 21% increase in the mean monthly number of defined daily doses of H2RAs dispensed, but there were pronounced changes in the mix of individual H2RAs. Mean monthly cimetidine prescriptions rose by 379% to 116 257 defined daily doses per 100 000 senior citizen beneficiaries, accounting for 70% of all H2RAs, whereas prescribing of restricted H2RAs fell by 55%. From the 12-month period after implementation (January to December 1996) to the follow-up period (January 1997 to May 1999), the mean number of defined daily doses of restricted H2RAs rose but those for cimetidine and H2RAs in aggregate declined.
During the baseline period, the mean monthly number of defined daily doses of PPIs prescribed was 84 531 per 100 000 senior citizen beneficiaries (Table 1). This number fell by 26%, to 62 708 per 100 000 beneficiaries, in the 12-month period after implementation, but climbed to 9% over baseline (91 821 per 100 000 beneficiaries) in the follow-up period. The same interval saw only a gradual increase in the use of other gastrointestinal drugs, from a monthly mean of 42 922 defined daily doses per 100 000 beneficiaries in the baseline period to 47 651 in the follow-up period, an increase of 11%. This increase was due entirely to increases in the use of cisapride.
Changes in BC Pharmacare expenditures are summarized in Table 2. A 300% increase in monthly expenditures for cimetidine was offset by lower expenditures for restricted H2RAs. Thus, overall monthly expenditures for H2RA fell to 58% of baseline in the 12-month period after implementation of reference-based pricing and remained low in the follow-up period. In contrast, monthly BC Pharmacare expenditures for PPIs fell to 74% of baseline in the 12 months after implementation of the special authority policy but rose to 107% of baseline in the subsequent follow-up period. Monthly expenditures for cisapride increased progressively, reaching 143% of the baseline value in the follow-up period. Reimbursement for other gastrointestinal drugs stayed relatively constant or declined.
Table 2
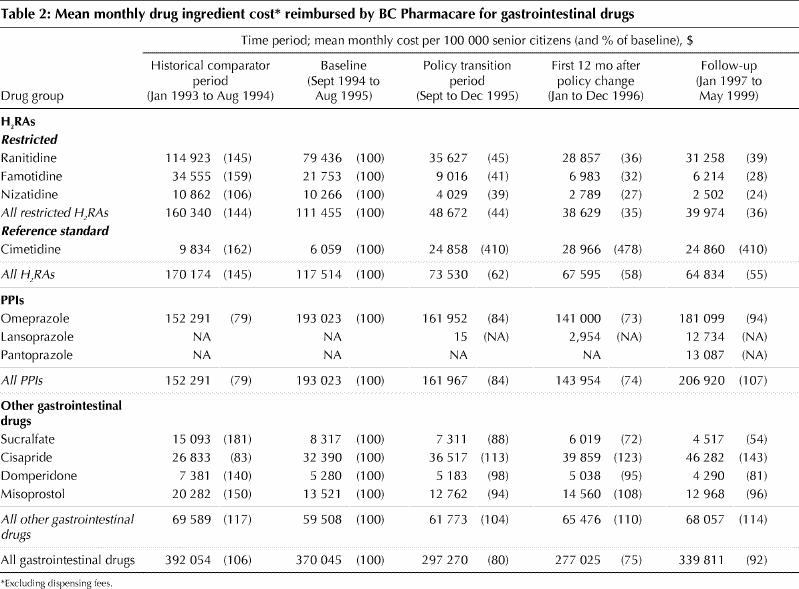
The projections of trends in expenditure from before to after the change in policy for H2RAs (Fig. 1) and PPIs (Fig. 2) revealed that the savings achieved by the 2 policies differed. For H2RAs, the total estimated savings for the period January 1996 to May 1999, inclusive (i.e., after the policy change), were $6.0 million (95% confidence interval [CI] $4.5 million to $7.6 million) or $1.8 million per year. For PPIs, the estimated savings over the same period were $18.8 million (95% CI $16.1 million to $21.5 million) or $5.5 million per year. The estimated total savings over the 4-month transition period (September to December 1995) were $0.7 million (95% CI $0.3 million to $1.2 million) for H2RAs and $1.0 million (95% CI $0.4 million to $1.7 million) for PPIs. The secondary analysis of H2RA expenditure trends, which assumed that expenditures after the change to reference-based pricing would have remained constant at the rates observed in the baseline period, almost doubled the estimates of H2RA savings for the transition period and the periods following to $12.1 million (95% CI $10.9 million to $13.2 million) or approximately $3.2 million annually.
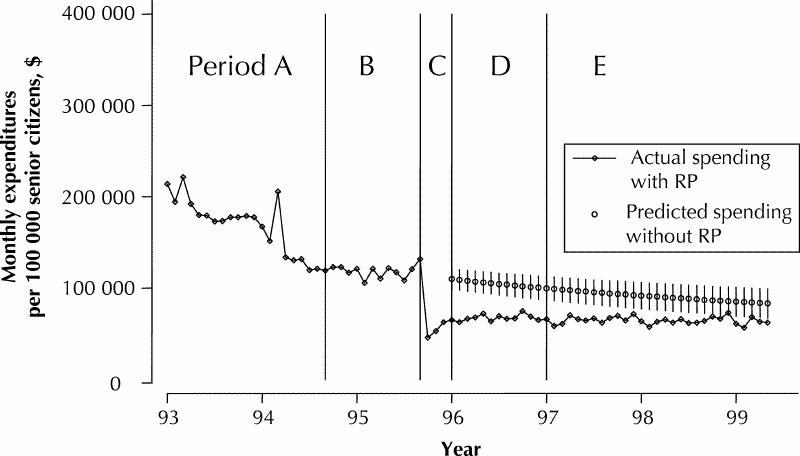
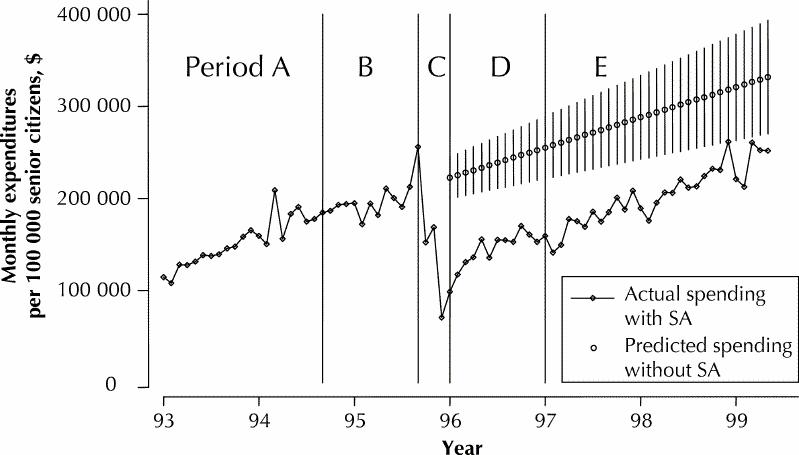
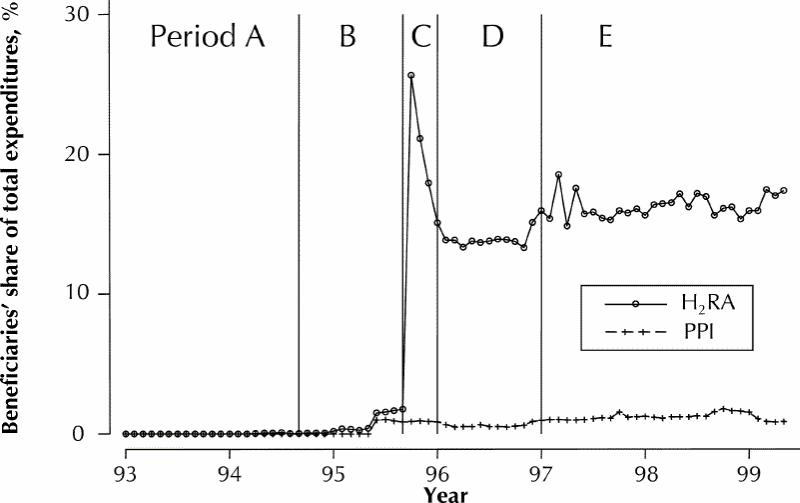
The total out-of-pocket expenditures incurred by senior citizen beneficiaries for H2RAs and PPIs were negligible before the introduction of the reference-based pricing and special authority policies (Fig. 3). However, after the new policies were introduced, expenditures by beneficiaries reached approximately 16% of total expenditures on H2RAs in the follow-up period (January 1997 to May 1999) or $0.03 per defined daily dose. Expenditures for PPIs in the follow-up period rose to just over 1% of total costs or $0.08 per defined daily dose.
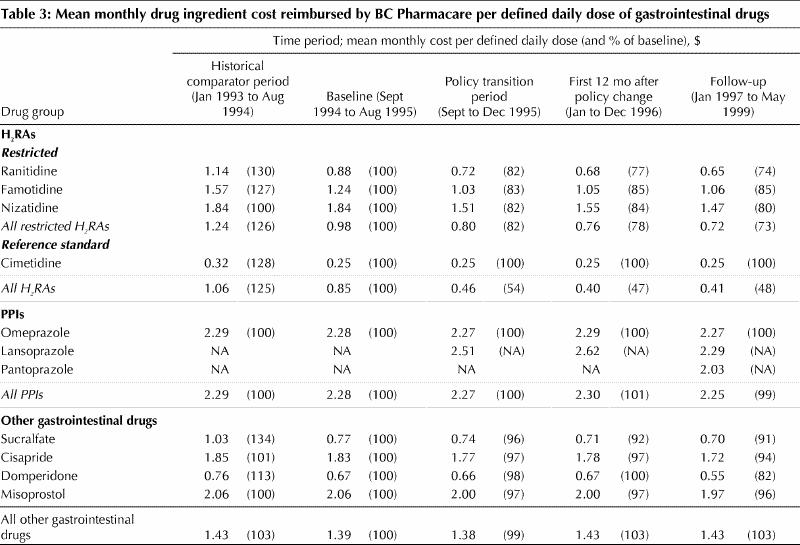
For all periods analyzed, the mean reimbursement by BC Pharmacare per defined daily dose of cimetidine (the reference standard) was unchanged at $0.25 (Table 3). The mean reimbursement per defined daily dose of restricted H2RAs fell from $0.98 to $0.76 between the baseline period and the 12-month period after the policy went into effect but remained above the reference price because some restricted H2RAs were still reimbursed at full cost for beneficiaries who had been granted exemption from the policy. In the same interval, reimbursement by BC Pharmacare per defined daily dose of all H2RAs fell by more than half, from $0.85 to $0.40, which reflected both the shift to greater use of the lower-cost generic cimetidine and the declining reimbursement per defined daily dose of restricted H2RAs. In contrast, reimbursement by BC Pharmacare per defined daily dose of PPIs and other gastrointestinal drugs such as cisapride did not change appreciably.
Table 3
Interpretation
Expenditures by Canadian provincial drug plans for upper gastrointestinal drugs are substantial. In 1993, omeprazole and ranitidine were BC Pharmacare's third and fourth most costly drugs, accounting for more than $20 million in expenditures.6 Our results confirm that the introduction of reference-based pricing for H2RAs by the BC Ministry of Health increased the use of generic cimetidine, a less costly alternative to other H2RAs. Reference-based pricing was also associated with lower mean costs per defined daily dose of all H2RAs and hence lower overall expenditures for these drugs by BC Pharmacare. At the same time, the special authority policy for PPIs was associated with a sustained reduction in the volume of PPIs dispensed to seniors each month but did not appear to reduce the rate of growth of prescriptions for these drugs. Nevertheless, during the first 41 months after introduction of these policies, the annual savings to BC Pharmacare were estimated at $1.8 million to $3.2 million for H2RAs (depending on the method of estimation used) and $5.5 million for PPIs. These values represent approximately 3.1% to 4.1% of the total drug ingredient costs (excluding dispensing fees) paid by BC Pharmacare for senior citizen beneficiaries in 1997.6 Our analysis underestimated savings to the entire BC Pharmacare program because only senior citizens were included; other beneficiaries, such as recipients of social assistance, were excluded.
H2RAs and PPIs both suppress secretion of gastric acid, but they do so by different mechanisms. They are indicated for the prevention and treatment of common acid-related upper gastrointestinal disorders, including peptic ulcer disease, gastroesophageal reflux disease (GERD) and the gastropathy associated with nonsteroidal anti-inflammatory drugs. Because GERD alone affects up to 20% of the adult population,7 a large number of BC Pharmacare beneficiaries are potential candidates for acid-suppressive therapies. Although the BC Therapeutics Initiative program has recommended H2RAs (specifically cimetidine) as first-line therapy for GERD,8 the optimal strategies for prescribing H2RAs and PPIs for patients with GERD remain somewhat controversial.9,10 PPIs are well recognized as producing greater acid suppression and better clinical efficacy than H2RAs and are generally well tolerated.11,12 However, H2RAs are effective in many patients and are generally less costly. Within the H2RA class, there is also little evidence of clinically important differences in effectiveness.11,12,13 Thus, BC Pharmacare preferred that patients who require less intense acid suppression be converted from a PPI to a H2RA and, among H2RAs, to the least costly alternative. Although prokinetic drugs can also be used to treat upper gastrointestinal disorders, they are generally less effective than acid suppressors and one (cisapride) was withdrawn from the Canadian market in 2000 because of its risk profile.
In this analysis, mean BC Pharmacare expenditures for PPIs fell immediately after implementation of the special authority policy but subsequently rose to exceed the levels observed before implementation of that policy. Our projections from the trends observed for the period January 1993 to August 1995 (before the change in policy) revealed that the observed and expected trends in PPI expenditures after implementation of the special authority policy were roughly parallel. Thus, although the new policy did not appear to alter the underlying growth in PPI use, it shifted the curve downward, and savings continued to accrue despite rising total monthly expenditures. Analyses not adjusted for underlying trends in utilization could have underestimated these savings significantly. The reasons for the observed growth in PPI use are unclear and are beyond the scope of this analysis. Increased demand might have reflected genuine need, with better clinical recognition of disorders that warrant PPI, or product promotion might have been more effective. However, the rate of expansion also suggests that BC Pharmacare should consider reviewing the special authority criteria and the mechanisms for granting approval.
The introduction of reference-based pricing increased out-of-pocket expenditures by senior citizen beneficiaries using restricted drugs. After implementation of the policy, these contributions were estimated to represent approximately 16% of total expenditures on H2RAs ($0.03 per defined daily dose) and 1% of total expenditures on PPIs ($0.08 per defined daily dose), providing a significant source of savings to BC Pharmacare. These proportions were remarkably stable throughout the follow-up periods. The social and ethical implications of shifting such costs to a potentially vulnerable patient population are open to debate. However, further analysis of cost shifting to this and other beneficiary groups is required.
This study could not evaluate the impact of the reference-based pricing and special authority policies on health expenditures other than those for prescription drugs. Because the H2RAs are generally less effective than PPIs at reducing gastric acid secretion, it is possible that cost savings achieved by a reduction in the use of PPIs occurred at the expense of related health outcomes. Furthermore, reimbursement for cisapride increased by 43% from the baseline period (September 1994 to August 1995) to the follow-up period (January 1997 to May 1999), but this compound has since been removed from the marketplace because of rare but serious adverse effects. Unfortunately, we had access only to aggregate-level data for this analysis. However, patient-level database linkage could determine whether the reference-based pricing and special authority policies have influenced rates of physician consultation to review treatment options, management of patients intolerant of drug switches, time consumed in pharmacist consultation, or use of over-the-counter and alternative remedies.
Many jurisdictions worldwide have introduced cost-containment programs that limit access to high-volume drugs such as H2RAs and PPIs in government-funded prescription drug programs, but they have met with mixed success.1,2,3,14,15,16,17 This analysis suggests that the combination of reference-based pricing for H2RAs and special authority for PPIs reduced government pharmacy expenditures to some extent. Further analyses of these trends and reviews of other endpoints such as health outcomes, patient satisfaction and utilization of nonpharmaceutical resources would shed light on other indirect effects of reference-based pricing.
Acknowledgments
We thank Sean Burnett and Robert Hart at BC Pharmacare for assistance with data retrieval. Financial support for the research was provided by the Health Transitions Fund of Health Canada. Dr. Marshall holds a Clinician Scientist Award from the Canadian Institutes for Health Research (CIHR). Dr. Grootendorst acknowledges career support from the Rx&D Health Research Foundation and CIHR. Dr. Holbrook is the recipient of a Health Career Investigator Award sponsored by the CIHR, Social Sciences and Humanities Research Council and National Health Research and Development Program.
Footnotes
This article has been peer reviewed.
Contributors: John K. Marshall, Paul V. Grootendorst, Bernie J. O'Brien, Lisa R. Dolovich, Anne M. Holbrook, and Adrian R. Levy were responsible for the conception and design of the study, for analyzing and interpreting the data, and for critically revising the manuscript. Dr. Grootendorst acquired the data, and Dr. Marshall drafted the manuscript. All authors approved the manuscript for publication.
Competing interests: None declared.
Correspondence to: Dr. John K. Marshall, Division of Gastroenterology (4W8), McMaster University Medical Centre, 1200 Main St. W, Hamilton ON L8N 3Z5; fax 905 521-4958; marshllj@mcmaster.ca
References
- 1.Grootendorst P, Holbrook A. Evaluating the impact of reference-based pricing. CMAJ 1999;161:273-4. Available: www.cma.ca/cmaj/vol-161/issue-3/0273.htm [PMC free article] [PubMed]
- 2.Dickson M, Redwood H. Pharmaceutical reference prices: How do they work in practice? Pharmacoeconomics 1998;14:471-9. [DOI] [PubMed]
- 3.Narine L, Senathirajah M, Smith T. Evaluating reference-based pricing: initial findings and prospects. CMAJ 1999;161:286-8. Available: www.cma.ca/cmaj/vol-161/issue-3/0286.htm [PMC free article] [PubMed]
- 4.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment. Oslo: World Health Organization; 2000.
- 5.Table 051-0001: Estimates of population, by age group and sex, Canada, provinces and territories, annual (persons unless otherwise noted). In: Statistics Canada's socio-economic database (CANSIM II). Ottawa: Statistics Canada; 2002. Data available through: cansim2.statcan.ca/cii/cii_1_e.htm (accessed 2002 Apr 17).
- 6.British Columbia Ministry of Health Services. Pharmacare trends 2000. Victoria: The Ministry; 2001.
- 7.Nandurkar S, Talley NJ. Epidemiology and natural history of reflux disease. Baillieres Best Pract Res Clin Gastroenterol 2000;14:743-57. [DOI] [PubMed]
- 8.Therapeutics Initiative. Review and update. Ther Lett 1995;9:1-2. Available: www.ti.ubc.ca/pages/letter9.html (accessed 2002 Apr 19).
- 9.Beck IT, Champion MC, Lemire S, Thomson AB, Anvari M, Armstrong D, et al. The second Canadian consensus conference of the management of patients with gastroesophageal reflux disease. Can J Gastroenterol 1997;11:7B-20B. [PubMed]
- 10.Thomson AB, Chiba N, Armstrong D, Tougas G, Hunt RH. The Second Canadian Gastroesophageal Reflux Disease Consensus: moving forward to new concepts. Can J Gastroenterol 1998;12:551-6. [DOI] [PubMed]
- 11.Veldhuyzen van Zanten SJO, Flook N, Chiba N, Armstrong D, Barkun A, Bradette M, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. CMAJ 2000;162(12 Suppl):S3-S23. Available: www.cma.ca/cmaj/vol-162/issue-12/pdf/dyspepsia.pdf [PMC free article] [PubMed]
- 12.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997;112:1798-810. [DOI] [PubMed]
- 13.Feldman M, Burton ME. Histamine receptor antagonists, standard therapy for acid-peptic diseases. N Engl J Med 1990;323:1672-80,1749-55. [DOI] [PubMed]
- 14.Bursey F, Crowley M, Janes C, Turner CJ. Cost analysis of a provincial drug program to guide the treatment of upper gastrointestinal disorders. CMAJ 2000;162:817-23. Available: www.cma.ca/cmaj/vol-162/issue-6/0817.htm [PMC free article] [PubMed]
- 15.McManus P, Marley J, Birkett DJ, Lindner J. Compliance with restrictions on the subsidized use of proton pump inhibitors in Australia. Br J Clin Pharmacol 1998; 46:409-11. [DOI] [PMC free article] [PubMed]
- 16.Brufsky JW, Ross-Degnan D, Calabrese D, Gao X, Soumerai SB. Shifting physician prescribing to a preferred histamine-2-receptor antagonist: effects of a multifactorial intervention in a mixed-model health maintenance organization. Med Care 1998;36:321-32. [DOI] [PubMed]
- 17.McBride JE, Pater JL, Dorland JL, Lam YM. Extent and variation of omeprazole prescribing in an elderly population of Ontario. Ann Pharmacother 1997;31:411-6. [DOI] [PubMed]


