Abstract
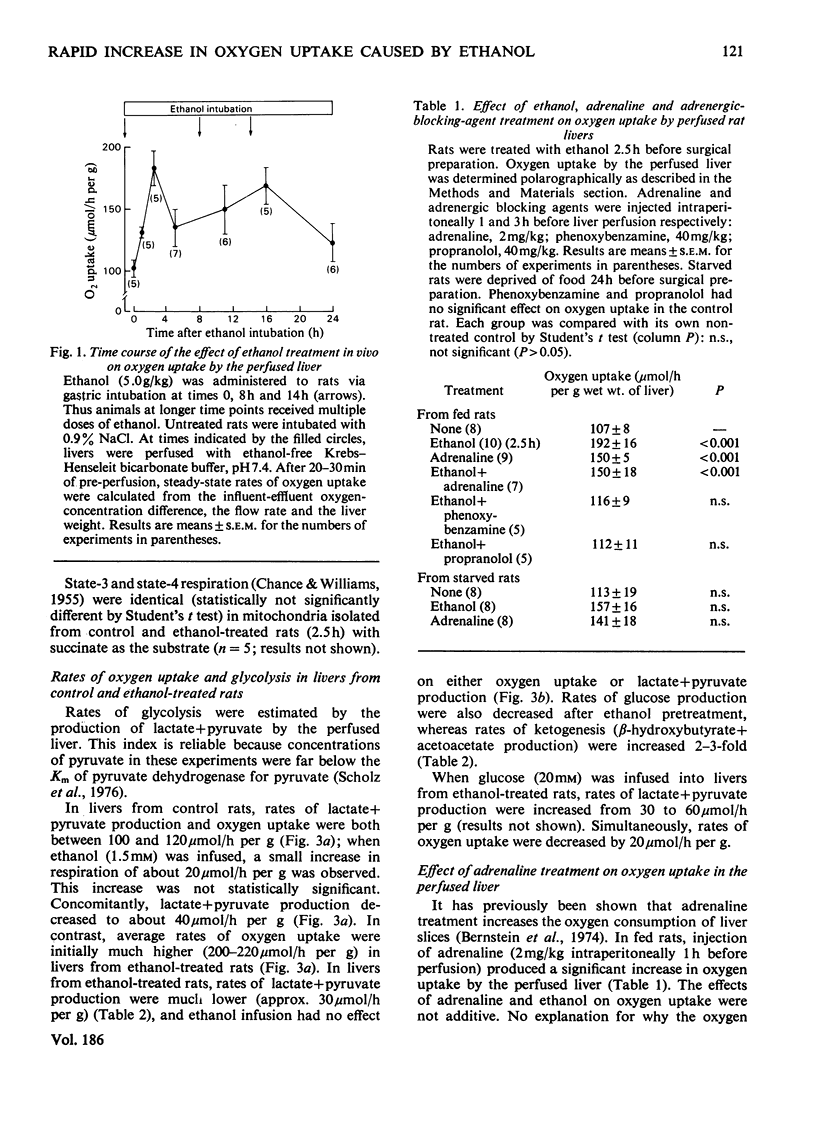
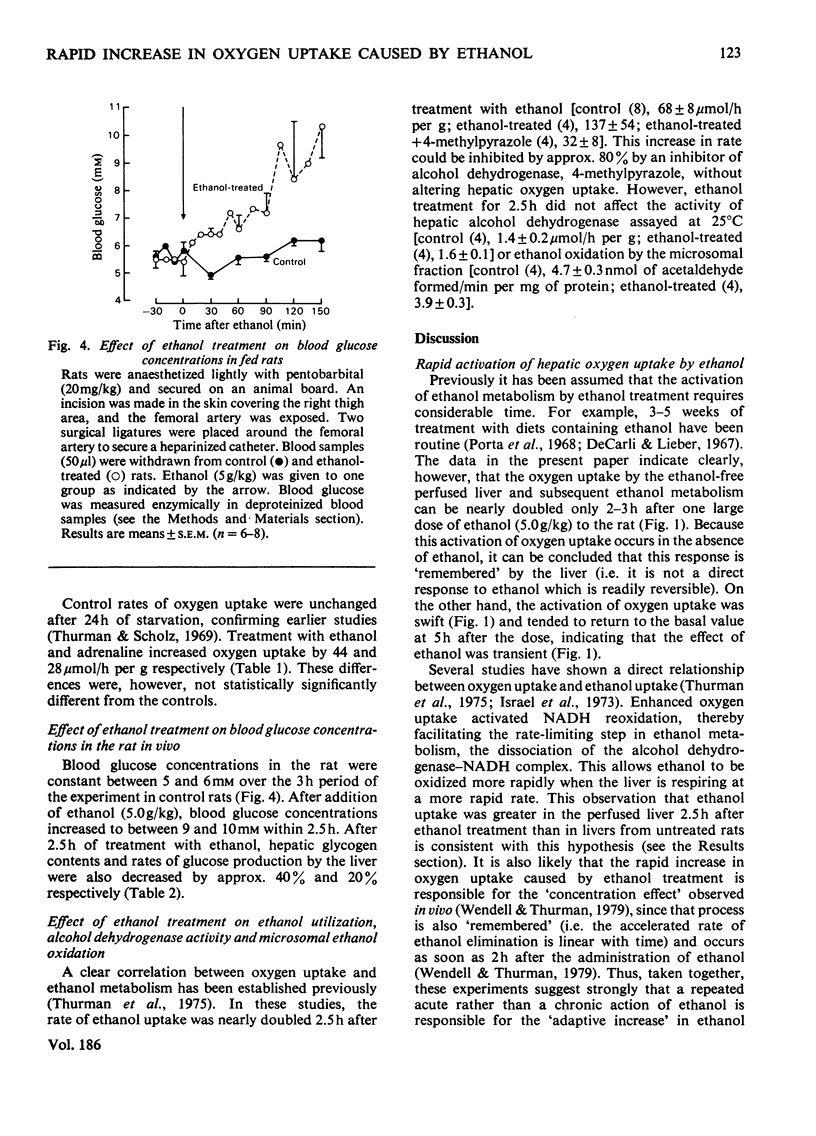
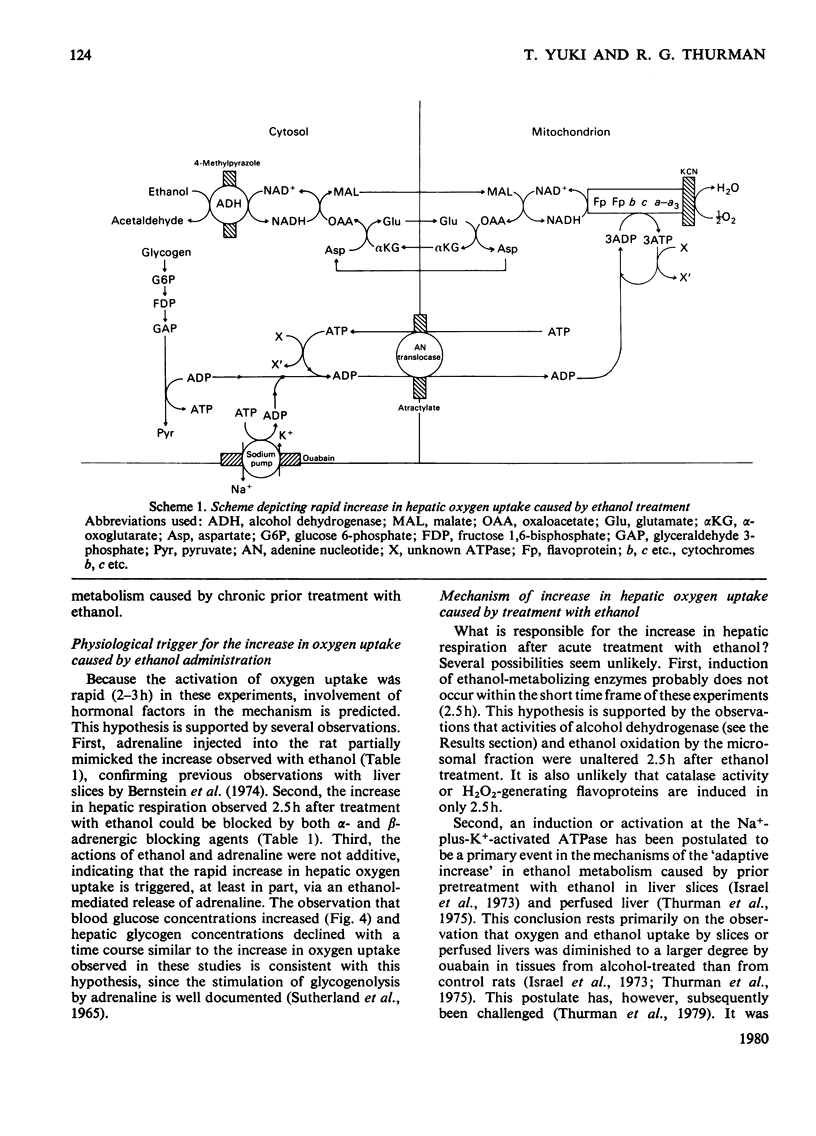
Gastric intubation of female Sprague-Dawley rats with 5 g of ethanol/kg body wt. nearly doubled oxygen uptake by the isolated perfused rat liver maximally after only 2.5 h of treatment (Swift Increase in Alcohol Metabolism). Inhibition of enhanced oxygen uptake by KCN (2mM) and 4-methylpyrazole (0.8 mM) suggested the involvement of the mitochondrial respiratory chain and alcohol dehydrogenase in this phenomenon. Glycolysis was depressed after ethanol treatment. Diminished ATP generation via glycolysis accounts for a portion (23-50%) of the increased oxygen uptake, assuming that other rates of biosynthesis remain constant. Injection of adrenaline (2 mg/kg) 1 h before perfusion mimicked partially the action of ethanol on hepatic oxygen uptake. The increases produced by ethanol and adrenaline were not additive, suggesting that adrenaline is involved in the action of ethanol. Moreover, the increase in hepatic oxygen uptake produced by 2.5 h of ethanol treatment could be blocked by either alpha-(phenoxybenzamine; 40 mg/kg) or beta-(propranolol; 40 mg/kg) adrenergic blocking agents. Blood glucose increased after ethanol treatment, supporting the involvement of glycogenolytic hormones in this effect. These data indicate that at least part of the stimulated oxygen uptake after treatment with ethanol is a result of lower rates of glycolytic ATP generation resulting from hormone (e.g. adrenaline etc.) action. The ADP not phosphorylated in the cytosol enters the mitochondria, where it stimulates oxygen uptake.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams M. A., Cooper C. Quantitative analysis of metabolism of hepatic triglyceride in ethanol-treated rats. Biochem J. 1976 Apr 15;156(1):33–46. doi: 10.1042/bj1560033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J., Videla L., Israel Y. Hormonal influences in the development of the hypermetabolic state of the liver produced by chronic administration of ethanol. J Pharmacol Exp Ther. 1975 Mar;192(3):583–591. [PubMed] [Google Scholar]
- Bernstein J., Videla L., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Changes related to energetic parameters of the cell. Biochem J. 1973 Jun;134(2):515–521. doi: 10.1042/bj1340515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- FONG J., SCHAFFER F. L., KIRK P. L. The ultramicrodetermination of glycogen in liver; a comparison of the anthrone and reducing-sugar methods. Arch Biochem Biophys. 1953 Aug;45(2):319–326. doi: 10.1016/s0003-9861(53)80009-3. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A., Remmer H., Estabrook R. W. Cytochrome P-450 of liver microsomes--one pigment or many. Biochem Biophys Res Commun. 1968 Mar 27;30(6):607–612. doi: 10.1016/0006-291x(68)90555-x. [DOI] [PubMed] [Google Scholar]
- Israel Y., Videla L., Macdonald A., Bernstein J. Metabolic alterations produced in the liver by chronic ethanol administration. Comparison between the effects produced by ethanol and by thyroid hormones. Biochem J. 1973 Jun;134(2):523–529. doi: 10.1042/bj1340523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970 May 25;245(10):2505–2512. [PubMed] [Google Scholar]
- Mendelson J. H., Mello N. K. Experimental analysis of drinking behavior of chronic alcoholics. Ann N Y Acad Sci. 1966 Sep 23;133(3):828–845. doi: 10.1111/j.1749-6632.1966.tb50930.x. [DOI] [PubMed] [Google Scholar]
- Porta E. A., Gomez-Dumm C. L. A new experimental approach in the study of chronic alcoholism. I. Effects of high alcohol intake in rats fed a commercial laboratory diet. Lab Invest. 1968 Apr;18(4):352–364. [PubMed] [Google Scholar]
- Rawat A. K., Lundquist F. Influence of thyroxine on the metabolism of ethanol and glycerol in rat liver slices. Eur J Biochem. 1968 Jun;5(1):13–17. doi: 10.1111/j.1432-1033.1968.tb00329.x. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., OYE I., BUTCHER R. W. THE ACTION OF EPINEPHRINE AND THE ROLE OF THE ADENYL CYCLASE SYSTEM IN HORMONE ACTION. Recent Prog Horm Res. 1965;21:623–646. [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., McKenna W. R., McCaffrey T. B. Pathways responsible for the adaptive increase in ethanol utilization following chronic treatment with ethanol: inhibitor studies with the hemoglobin-free perfused rat liver. Mol Pharmacol. 1976 Jan;12(1):156–166. [PubMed] [Google Scholar]
- Thurman R. G., Scholz R. Ethanol metabolism in perfused rat liver at low ethanol concentrations: involvement of alcohol dehydrogenase in the adaptive increase in ethanol metabolism due to chronic pretreatment with ethanol. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1443–1446. [PubMed] [Google Scholar]
- Thurman R. G., Scholz R. Interaction of glycolysis and respiration in perfused rat liver. Changes in oxygen uptake following the addition of ethanol. Eur J Biochem. 1977 May 2;75(1):13–21. doi: 10.1111/j.1432-1033.1977.tb11499.x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Scholz R. Mixed function oxidation in perfused rat liver. The effect of aminopyrine on oxygen uptake. Eur J Biochem. 1969 Oct;10(3):459–467. doi: 10.1111/j.1432-1033.1969.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Yuki T., Bleyman M. A., Wendell G. The adaptive increase in ethanol metabolism due to pretreatment with ethanol: a rapid phenomenon. Drug Alcohol Depend. 1979 Jan-Mar;4(1-2):119–129. doi: 10.1016/0376-8716(79)90052-8. [DOI] [PubMed] [Google Scholar]
- Tobon F., Mezey E. Effect of ethanol administration on hepatic ethanol and drug-metabolizing enzymes and on rates of ethanol degradation. J Lab Clin Med. 1971 Jan;77(1):110–121. [PubMed] [Google Scholar]
- Wendell G. D., Thurman R. G. Effect of ethanol concentration on rates of ethanol elimination in normal and alcohol-treated rats in vivo. Biochem Pharmacol. 1979;28(2):273–279. doi: 10.1016/0006-2952(79)90515-x. [DOI] [PubMed] [Google Scholar]


