Abstract
Background
Locally advanced melanoma has a variable prognosis. Currently, there are no reliable criteria to stratify the risk of disease relapse and identify those patients who will benefit the most from adjuvant therapies. Circulating tumor DNA (ctDNA) is an emerging biomarker measuring the presence of tumor-derived DNA in blood.
Patients and methods
We used a bespoke, tumor-informed assay (RaDaR®, NeoGenomics, Inc.) to detect ctDNA in 276 prospectively collected plasma samples from 66 melanoma patients receiving definitive treatment. Collection time points included landmark (after completion of local treatment) and every 3-6 months for up to 2 years.
Results
ctDNA was detected in at least one plasma sample in 19 patients (29%), including 6/65 (9%) at landmark (post-surgical sample). Positive ctDNA at landmark was associated with shorter overall survival (OS; median OS 22.7 months versus not reached, log-rank P value = 0.01) and a trend towards a shorter relapse-free survival (RFS; median RFS 15.7 months versus not reached, log-rank P value = 0.07). In 10 patients, ctDNA detection preceded disease relapse by a median of 128 days (range 8-406 days).
Conclusions
Our data indicate that ctDNA detection after surgery can identify patients with worse prognosis, and serial ctDNA measurements may enable earlier identification of disease recurrence.
Key words: melanoma, liquid biopsy, circulating tumor DNA, molecular residual disease, biomarker
Highlights
-
•
Post-surgery positive ctDNA in high-risk melanoma is associated with worse OS (HR 6.04) and systemic RFS (HR 3.77).
-
•
ctDNA detection before surgery is associated with shorter systemic RFS (HR 9.72) in high-risk melanoma patients.
-
•
Twenty-six patients (26%) of ctDNA-positive patients were BRAF/NRAS wild type, indicating a broader applicability of bespoke ctDNA technology.
-
•
ctDNA detection precedes radiological relapse by a median of 4 months, supporting its use to identify disease recurrence.
Introduction
Locally advanced melanoma is a highly heterogeneous disease with varying risk of relapse (e.g. 39% among patients with stage IIIA, compared to ∼70% of patients with stage IIIC disease1, 2, 3). Adjuvant treatment with immune checkpoint inhibitors (ICI), or for patients whose tumors harbor BRAF mutations with BRAF/MEK-targeted agents, is proven to prolong relapse-free-survival (RFS) in stage III and resected stage IV disease.4, 5, 6, 7 Pembrolizumab and nivolumab [anti-programmed cell death protein 1 (PD-1) ICI] have been demonstrated to increase RFS in stage IIB and IIC disease.8, 9, 10 However, pathology-based staging systems are limited in optimally defining the risk of recurrence, as relapses are observed even in the earliest disease stages. Conversely, a significant proportion of patients treated with adjuvant treatment are either overtreated (would be cured with surgery alone) or experience relapse despite systemic therapy. Reliable biomarkers to define the risk of recurrence and identify patients who would truly benefit from adjuvant treatment are urgently needed to improve patient outcomes.
Circulating tumor DNA (ctDNA) is a minimally invasive approach for the detection of tumor-related genetic material in the peripheral blood. Widely available for clinical use in the advanced setting, ctDNA testing to guide treatment and follow-up decisions is currently under evaluation in localized disease across a broad range of tumor types.11, 12, 13, 14, 15, 16 In melanoma, ctDNA dynamics demonstrated the potential to reflect disease status and to predict treatment outcomes ahead of conventional imaging.17 De Simoni et al. recently reviewed the use of ctDNA in non-metastatic melanoma.18 One of the main limitations in most available melanoma studies is the low throughput via the use of droplet PCR (dPCR) methodology. This technique detects the presence of ctDNA through the identification of a restricted number of molecular alterations, such as BRAF and NRAS mutations. Importantly, >20% of cutaneous melanoma cases do not harbor a known driver genomic alteration19; therefore, their ctDNA would be undetectable by dPCR. The proportion is even larger when considering rare melanoma subtypes such as mucosal or acral melanomas.20 Tumor heterogeneity and clonal evolution might render single-gene mutations found in primary melanomas not very informative when tracking these in metastatic lesions.21,22 The ability to investigate the genomic profile beyond a single driver mutation is a key element to expand the number of patients deriving benefit from the use of ctDNA to guide their clinical management.
Bespoke tumor-informed ctDNA panels are highly sensitive assays to detect ctDNA with a potential to improve risk stratification in the curative setting. This method consists of sequencing tumor tissue sample and selecting relevant mutations for a custom-built ctDNA assay. For patients with metastatic disease receiving anti-PD-1 treatment, a decrease in bespoke ctDNA levels has demonstrated an association with treatment response.23 In the locally advanced setting, molecular residual disease (MRD) assessment through bespoke ctDNA panels yields better prognostication in multiple cancers.24, 25, 26 Within this context, tumor-informed bespoke ctDNA panels may improve upfront risk stratification and early detection of disease relapse in patients with melanoma, a hypothesis also supported by the results of two small recently published studies.27,28
We hypothesize that a bespoke tumor-informed ctDNA assay can distinguish patients with MRD and predict relapse in a cohort of patients with locally advanced melanoma, helping to inform adjuvant treatment decisions.
Patients and methods
Study design and population
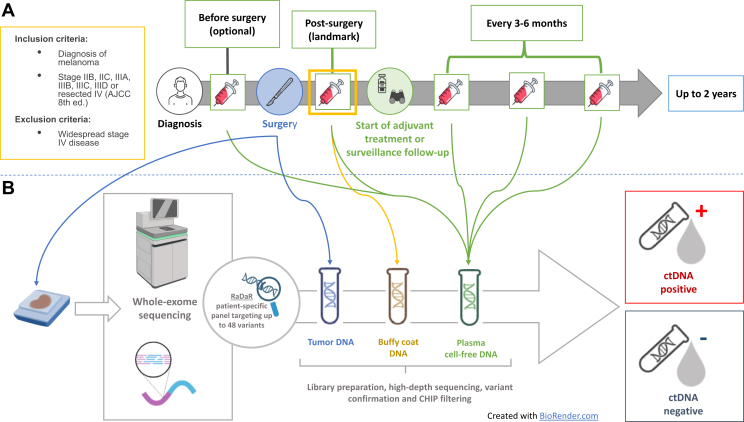
Serial ctDNA Monitoring as a Predictive Biomarker in Advanced NeoplAsms (SAMBA) is a prospective, Princess Margaret Cancer Centre–led, investigator-initiated study (NCT03702309)29 approved by the Institutional Research Ethics Board (#19-5694). In this study, longitudinal blood samples were prospectively collected from high-risk melanoma patients (stage IIB-resected stage IV, according to the American Joint Committee on Cancer 8th edition) during standard-of-care surveillance follow-up or adjuvant treatment (targeted agents or immunotherapy). Collection time points included landmark (after completion of local treatment), and every 3-6 months, approximately at the time of radiological assessment for up to 2 years. Additional blood samples were collected before local treatment and at the time of disease progression, when feasible (study schema is summarized in Figure 1). Up to 30 ml of peripheral blood were prospectively collected in Streck Cell-Free DNA (La Vista, NE) tubes at each time point. Samples were subsequently analyzed using the RaDaR® assay® (NeoGenomics, Inc., Cambridge UK and Durham, NC). All the patients enrolled signed informed consent for participation in the study. The results were not used to guide clinical decisions.
Figure 1.
The Serial ctDNA Monitoring as a Predictive Biomarker in Advanced NeoplAsms (SAMBA) study schema. (A) Patients with high-risk resected stage IIB-IV resected melanoma undergoing adjuvant treatment or surveillance were eligible for the study. Blood samples were collected at landmark (within 3 months from the completion of local treatment) and then every 3-6 months around the time of radiological restaging for up to 2 years. Additional plasma samples were collected before surgery and at progression, when feasible. (B) Archival tumor tissue was collected from all the patients and analyzed with whole-exome sequencing to identify somatic mutations and design patient-specific ctDNA panel. Longitudinal plasma samples were analyzed with the RaDaR assay. ctDNA, circulating tumor DNA.
Bespoke ctDNA analysis with RaDaR assay
RaDaR® is a highly sensitive next-generation sequencing (NGS) assay for the detection of ctDNA in patients’ blood using a tumor-informed approach. Tumor-specific variants were identified through whole-exome sequencing (WES) of DNA extracted from a formalin-fixed paraffin-embedded tumor tissue sample. Custom software was then used to prioritize tumor variants and design a patient-specific primer panel to interrogate up to 48 amplicons each capturing at least one such somatic variant. Variant selection process used a proprietary algorithm to identify the variant set most appropriate for the sensitive detection of plasma ctDNA in each specific patient and to ensure that the selected primer pairs were well suited for multiplex PCR. Variant selection was independent from their actionability.
To define the detectability of ctDNA in specific samples, the statistical significance of the observed mutant counts for each of the variants was determined using a statistical framework incorporating the entire set of patient-specific variants. Samples were classified as ‘ctDNA positive’ when their cumulative statistical score was above a pre-set threshold defined during the assay’s analytical development.
Procedures for WES, panel design, cell-free DNA extraction, processing of sequencing data, and determination of residual or recurrent disease were carried out as previously described in detail.24,30,31 WES details are reported in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2024.103978.
Statistical analysis
Descriptive statistics were used to summarize the clinical characteristics of the study’s population. Differences by ctDNA status were analyzed using the Wilcoxon rank-sum, chi-square, and Fisher’s exact tests as appropriate. Overall survival (OS) and RFS were compared among patients with positive versus undetectable ctDNA status at landmark. OS was defined as time from the date of surgery to the date of death; otherwise, it was censored at the last follow-up. RFS was defined as the time from the date of surgery to the date of first recurrence or death, otherwise censored at the last follow-up. Median follow-up time was determined from median time to censoring using the reverse Kaplan–Meier estimator. Kaplan–Meier curves, log-rank tests, and univariable Cox proportional hazards regression models were used to compare survival across groups. Time-dependent sensitivity and specificity were assessed for predicting OS and RFS. Cumulative sensitivity and dynamic specificity were computed using the Kaplan–Meier estimator for predicting OS and RFS events by 6, 12, 18, and 24 months after surgery.32 All statistical analyses were carried out in R (version 4.2.1), using two-sided tests with a significance alpha level of <0.05.
Results
Patient characteristics
Between July 2018 and April 2022, 66 patients were enrolled in SAMBA, and 276 longitudinal samples were collected for analysis. All the participants were identified and enrolled at the Princess Margaret Cancer Centre in Toronto, Canada. Patient characteristics are summarized in Table 1. The median age was 65 years (range 27-87 years), and 71% were male. Most of the patients had stage III disease (46, 70%), followed by resected stage IV (13, 20%) and stage II disease (7, 10%). Cutaneous melanoma was the most common subtype (51, 77%), followed by melanoma of unknown primary (6, 9%), mucosal (5, 8%), and acral melanoma (4, 6%). BRAF and NRAS mutations were observed in 28 (42%) and 16 (24%) cases, respectively. The median follow-up time was 39 months [95% confidence interval (CI) 27-87 months]. Seven patients (11%) received neoadjuvant systemic treatment (three immunotherapy, three targeted therapy, and one participating in a clinical trial). All patients (100%) had surgical resection, followed by post-operative radiation in 15 cases (23%). Most patients received adjuvant anti-PD-1 ICI (41, 62%), while 14 (21%) did not receive any systemic treatment. Of the remaining patients, eight (12%) received adjuvant targeted therapy and three (5%) were enrolled in a clinical trial randomizing to immunotherapy or placebo. Twenty-two patients (33%) experienced disease progression during follow-up; the median follow-up time was 39 months (range 6-58 months). The estimated 2-year OS was 84% (95% CI 75% to 94%), and 2-year RFS was 68% (95% CI 58% to 81%).
Table 1.
Clinical characteristics of the study population
| Total N of patients | 66 |
| Age, median (range), years | 65 (27-87) |
| Follow-up, median (95% CI), months | 39 (27-87) |
| Gender, n (%) | |
| Female | 19 (29%) |
| Male | 47 (71%) |
| Stage, n (%) | |
| II | 7 (11%) |
| III | 46 (70%) |
| IV | 13 (20%) |
| Melanoma subtype, n (%) | |
| Cutaneous | 51 (77%) |
| Mucosal | 5 (8%) |
| Acral | 4 (6%) |
| Unknown primary | 6 (9%) |
| Primary site, n (%) | |
| Trunk | 17 (26%) |
| Extremities | 24 (36%) |
| Head and neck | 17 (26%) |
| Lymph nodes (unknown primary) | 5 (8%) |
| Genital | 1 (1.5%) |
| GI | 1 (1.5%) |
| Other | 1 (1.5%) |
| BRAF, n (%) | |
| Mutated | 28 (42%) |
| Wild-type | 38 (58%) |
| NRAS, n (%) | |
| Mutated | 16 (24%) |
| Wild-type | 50 (76%) |
| Neoadjuvant systemic therapy, n (%) | |
| None | 59 (89%) |
| Immunotherapy | 3 (5%) |
| Targeted therapy | 3 (5%) |
| Unknown (clinical trial) | 1 (1.5%) |
| Adjuvant systemic therapy, n (%) | |
| None | 14 (21%) |
| Immunotherapy | 41 (62%) |
| Targeted therapy | 8 (12%) |
| Unknown (clinical trial) | 3 (5%) |
| Adjuvant radiation, n (%) | |
| Yes | 15 (23%) |
| No | 51 (77%) |
| Progressive disease, n (%) | |
| Yes | 22 (33%) |
| No | 44 (67%) |
CI, confidence interval; GI, gastrointestinal.
ctDNA detectability in pre- and post-surgery plasma samples
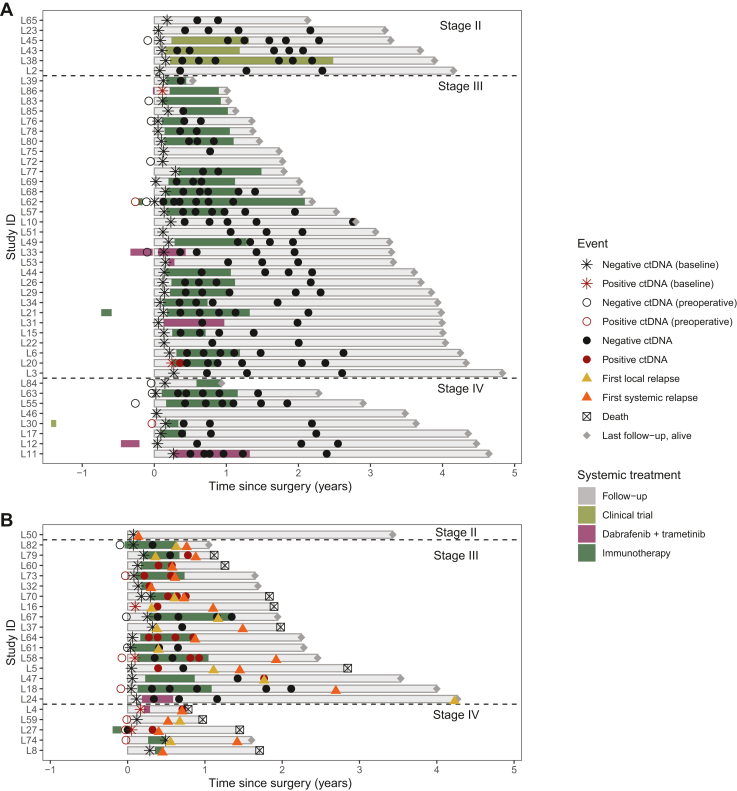
A total of 276 plasma samples from 66 patients were analyzed. The median number of plasma collections per patient was 4 (range 1-9). Of those, 35 (13%) samples from 19 patients (29%) had ctDNA detected by the RaDaR assay. A total of 9/19 (47%), 5/19 (26%), and 5/19 (26%) patients with at least one collection with detected ctDNA were positive for BRAF mutation, NRAS mutation, or both BRAF/NRAS wild-type, respectively. Of the 19 patients with at least one plasma collection positive for ctDNA, 14 (74%) had stage IIIB-IIID disease and 5 (26%) had resected stage IV disease. None of the 12 patients with stage IIB-IIIA melanoma had ctDNA detected at any time point (Figure 2). The most frequent melanoma subtype in the population with at least one collection positive for ctDNA was cutaneous melanoma (15 patients, 79%) followed by unknown primary (3 patients, 16%) and mucosal melanoma (1 patient, 5%) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103978).
Figure 2.
Swimmer plot showing the change in ctDNA detectability during adjuvant treatment and survival follow-up (N = 66 patients). Each line represents a specific patient. The landmark (time 0) corresponds to the time of definitive surgery. Patients are divided according to disease stage. The length of each line corresponds to the duration of survival from the time of surgery to death or last follow-up. ctDNA+ and ctDNA− collections are indicated with red and black circles, respectively. The type and duration of systemic treatment are represented by lines of different colors. Patients who did not experience progressive disease (A) and who did experience progressive disease (B) are represented separately. ctDNA, circulating tumor DNA.
Plasma samples collected before definitive surgery were available for 19/66 patients (29%). Eight of them (42%) were positive for ctDNA with a median estimated variant allele fraction (eVAF) of 0.0337% (range 0.00127%-2.52%) (Figure 2). Landmark plasma samples, collected within 3 months from completion of local treatment and before the start of systemic therapy or surveillance follow-up, were available for 65/66 subjects (99%). Of these six (9%) had ctDNA detected by RaDaR, with a median eVAF of 0.0437% (Figure 3A). At least one additional longitudinal plasma collection was carried out for 57 patients (86%), undergoing adjuvant systemic treatment (44/57, 77%) or surveillance follow-up (13/57, 23%). The median number of post-operative plasma samples collected per patient was 4 (range 1-8).
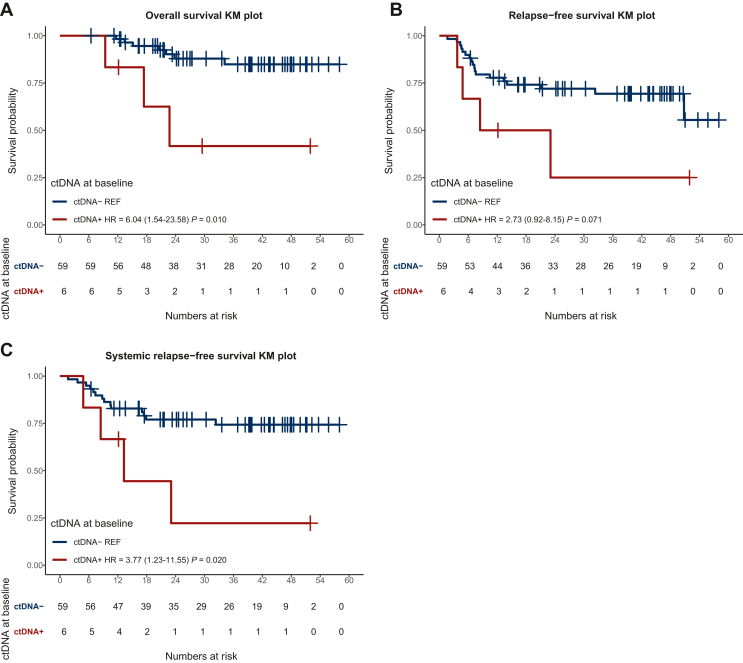
Figure 3.
Overall(OS) and relapse-free survival (RFS) in patients with ctDNA+ versus ctDNA− at the first collection post-surgery (landmark). We observed a significant prolongation of median OS in patients with ctDNA− in this population (A). No significant difference was observed in terms of RFS for the overall population based on post-op ctDNA detectability when considering both local and systemic recurrence (B). However, patients with a positive landmark collection had a significantly shorter systemic RFS (C). ctDNA, circulating tumor DNA; HR, hazard ratio; KM, Kaplan-Meier curves.
Of the 22 patients who experienced disease relapse, 18/22 (82%) had distant metastases and 4/22 (18%) had only local recurrence. Twelve of the 18 patients with distant relapse (67%) had at least one plasma sample collected in the 6 months before the recurrence. Of these, 8/12 (67%) had ctDNA detected by RaDaR. Two of the four patients with ctDNA not detected in the 6 months before radiological progression developed disease recurrence only to the brain, while the other two had metastatic disease to the lung and the liver, and to the liver and the lymph nodes, respectively.
Three of the four patients who presented with only local disease recurrence had available plasma samples collected within 6 months before the date of relapse. Only one patient of the three patients had detectable ctDNA at the time of the local recurrence (1/3, 33%).
Post-operative ctDNA to identify patients with higher risk of relapse and detect minimal residual disease
Patients with detected ctDNA at their first post-operative collection (landmark) had a significantly shorter OS when compared to subjects with undetected ctDNA after surgery [median 22.7 months versus median not reached, hazard ratio (HR) 6.04 (1.54-23.58) P = 0.01] (Figure 3A). RFS was not significantly different between those with detected versus undetected ctDNA after surgery [median 15.7 months versus not reached, HR 2.73 (0.92-8.15) P = 0.07] (Figure 3B). A significant difference in terms of RFS between patients with positive and undetectable ctDNA at landmark was, however, observed if considering only patients with systemic relapse [median 13.3 months versus not reached, HR 3.77 (1.23-11.55), P = 0.02] (Figure 3C). All the six patients with positive ctDNA at landmark were diagnosed with stage III or IV disease. Two of them received adjuvant immunotherapy (patients 20 and 58), one received adjuvant targeted therapy (patient 4), one had neoadjuvant immunotherapy (patient 27), one had neoadjuvant targeted therapy and adjuvant immunotherapy (patient 86), and one received no systemic treatment (patient 16) (Figure 2). Both patients who received adjuvant immunotherapy only cleared ctDNA at subsequent collections. In one of them (patient 58), ctDNA became detectable again at 9 months from landmark and the patient developed subsequent disease recurrence. The other patient (patient 20) remained ctDNA negative and is free of disease recurrence 51 months after surgery. The patient who received both neoadjuvant targeted therapy and adjuvant immunotherapy (patient 86) had no ctDNA collections available apart from landmark sample; he remained free of disease relapse at his last follow-up, 12 months after surgery. The patient who received adjuvant targeted therapy (patient 4) had only two ctDNA collections available: one at landmark and one at 6 months which coincided with the time of disease relapse; both collections were ctDNA positive. Similarly, the patient who received no systemic treatment (patient 16) had only two ctDNA collections available, one at landmark and one at the time of progression (3 months after landmark), both ctDNA positive (timeline of ctDNA collections and clinical characteristics of patients 4, 16, and 58 who developed disease progression are described in detail in the section ‘Dynamic changes in ctDNA for the early identification of disease recurrence’). No statistically significant difference in RFS was found between patients with positive and negative landmark collection in the group who received adjuvant systemic therapy (median 23.1 months versus 50.8 months, P = 0.664) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103978). Characteristics of patients with ctDNA positivity at any time point and at landmark are summarized in Supplementary Tables S3 and S4, available at https://doi.org/10.1016/j.esmoop.2024.103978, respectively.
The cumulative sensitivity and dynamic specificity of ctDNA detected by RaDaR at landmark in predicting RFS are reported in Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103978. At 6, 12, 18, and 24 months, the sensitivity/specificity/ positive predictive value/negative predictive value were as follows: 6 months (25%, 92.9%, 33.3%, 89.8%), 12 months (18.7%, 93.6%, 49.1%, 77.7%), 18 months (16.5%, 94.7%, 55.1%, 74.3%), and 24 months (20.7%, 97.1%, 76.9%, 72.1%).
Preoperative ctDNA to identify patients with higher risk of systemic relapse
Among patients with preoperative ctDNA collection, no deaths were observed in n = 11 patients with negative preoperative ctDNA (median follow-up 23.3 months), and two deaths were observed among n = 8 patients with positive preoperative ctDNA (median follow-up 27.9 months). No significant difference was observed in terms of OS (not reached versus not reached, P = 0.13) or RFS [15.2 versus not reached, HR 3.15 (0.78-12.70), P = 0.11] between patients with positive versus non-detectable ctDNA in the preoperative collection (Supplementary Figure S2A and B, available at https://doi.org/10.1016/j.esmoop.2024.103978). However, patients with positive preoperative ctDNA had a significantly shorter systemic RFS [HR 9.72 (1.17-80.98), P = 0.036] (Supplementary Figure S2C, available at https://doi.org/10.1016/j.esmoop.2024.103978).
Dynamic changes in ctDNA for the early identification of disease recurrence
All patients who developed detectable ctDNA in blood samples collected after landmark experienced disease progression (Figure 2). Ten subjects had ctDNA detected before the evidence of radiological progression (Figures 2 and 4). In these patients, ctDNA detection preceded disease relapse by a median of 128 days (range 8-406 days). The ctDNA changes over time and the clinical history of these 10 patients are described in detail below. A summary of these results is provided in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.103978.
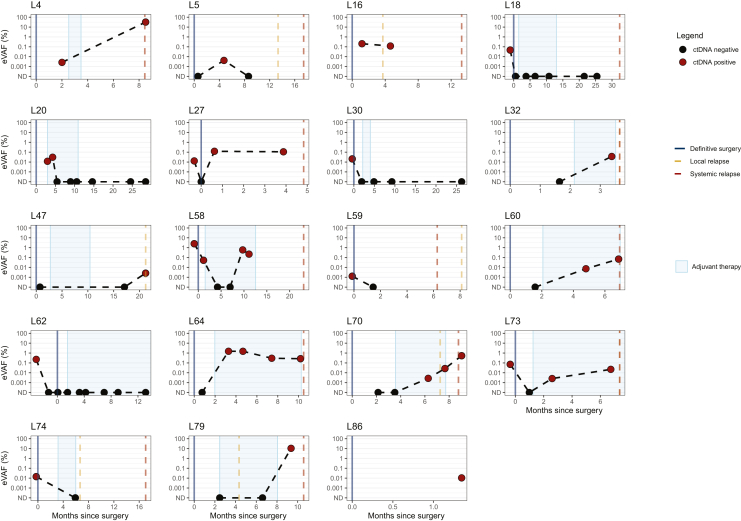
Figure 4.
Longitudinal plots showing ctDNA changes over time in 19 patients with at least one positive ctDNA collection. The red dots indicate positive for ctDNA collections while the black dots indicate negative for ctDNA collections. The time of definitive surgery is indicated by a blue line while the time of local and systemic progression corresponds to the orange and red dashed lines, respectively. ctDNA, circulating tumor DNA; eVAF, estimated variant allele fraction.
Patient 4: initially diagnosed with stage IVb BRAF-mutant cutaneous melanoma with a biopsy-proven metastatic nodule to the lung treated with definitive radiation. The patient was started on adjuvant treatment with targeted therapy. Landmark collection was ctDNA positive with a 0.0026% eVAF. Six months later, the patient was diagnosed with metastatic disease to the brain. ctDNA analysis done at the time of progression confirmed persistence of ctDNA positivity with an increased eVAF of 0.0321%.
Patient 16: diagnosed with stage IIIC BRAF-mutant cutaneous melanoma who underwent surveillance, with ctDNA positivity at landmark (eVAF of 0.0205%). At 3 months from surgery, local progression to lymph nodes was detected on imaging. ctDNA was positive at progression (eVAF of 0.12%).
Patient 27: diagnosed with BRAF and NRAS wild-type stage IV cutaneous melanoma (recurrent in-transit, unresectable lesion on the thigh). The patient started on neoadjuvant anti-PD-1 ICI. The preoperative plasma sample collected after three cycles of immunotherapy was positive for ctDNA (eVAF 1.29E−02 %eVAF). At the time of the surgical resection ctDNA was not detectable; however, it rapidly increased in the following weeks up to %eVAF of 1.23E-01 at the landmark collection. The patient was diagnosed with systemic progressive disease 4 months after surgery.
Patient 32: diagnosed with stage IIIC BRAF-mutated cutaneous melanoma. After surgery, the patient started adjuvant anti-PD-1 ICI. ctDNA was not detected at landmark collection. At 3 months, ctDNA became detectable (%eVAF 3.66E−02), and radiological restaging confirmed progressive disease a few days later.
Patient 58: diagnosed with stage IIIB, NRAS-mutant cutaneous melanoma. Both preoperative and landmark ctDNA were positive (%eVAF of 2.52 and 0.053, respectively). The patient received adjuvant anti-PD-1 ICI with cleared ctDNA at 3 and 6 months, following initiation of ICI. At 9 months from landmark, ctDNA was detected again (0.59 %eVAF). At 11 months from surgery, a new liver lesion was detected on imaging. The biopsy was negative for cancer and the patient continued immunotherapy with shrinkage of the liver lesion and a slight decrease in ctDNA levels (%eVAF 0.22). About 23 months after surgery, the patient had clear radiological disease progression.
Patient 60: diagnosed with stage IIIB, CDKN2A-mutant, cutaneous melanoma, received adjuvant anti-PD-1 ICI. At landmark, ctDNA was undetectable. Radiological progressive disease to liver and bone occurred 7 months from surgery. Analysis of a plasma sample collected 2 months before progression demonstrated ctDNA positivity (0.0073 %eVAF). ctDNA at the time of radiological progression was positive, with an eVAF of 0.0694%.
Patient 64: diagnosed with stage IIIC, BRAF-mutant, cutaneous melanoma, received adjuvant anti-PD-1 ICI. ctDNA was undetectable in landmark plasma sample. All four additional plasma collections (at 3, 5, 7, and 10 months) were ctDNA positive (%eVAF 1.5, 1.5, 0.2, and 0.2). Radiological evidence of disease progression occurred 10 months after surgery.
Patient 70: diagnosed with stage IIIC, NRAS-mutant, cutaneous melanoma; received adjuvant anti-PD-1 ICI. At landmark and at 3 months, ctDNA was undetectable. However, ctDNA became positive with eVAF 0.00271% at 6 months after surgery and continued to rise at 9 and 12 months. This patient had evidence of local and systemic progression 1 and 2 months after ctDNA was detected, respectively.
Patient 73: diagnosed with stage IIIB, NRAS-mutated melanoma of unknown primary. The preoperative collection was positive for ctDNA (eVAF 7.15E−02%), while the landmark collection ctDNA was undetectable. Following surgery, adjuvant anti-PD-1 ICI was initiated. At 3 months, ctDNA was detected (%eVAF 2.60E−03). The patient was diagnosed with radiological PD to the lymph nodes almost 5 months later.
Patient 79: diagnosed with stage IIIC, NRAS-mutant cutaneous melanoma, received adjuvant anti-PD-1 ICI. At landmark, ctDNA was negative, and the patient had local progression 5 months after surgery and systemic progression to the bone and lung at 11 months. ctDNA was detected about 2 months before systemic progression (%eVAF 10.5).
Seven of the 22 patients who experienced disease recurrence (32%) had undetectable ctDNA in all available plasma samples profiled, including four subjects with systemic progression. Two of them (patient 8 and patient 50) had plasma collected only at landmark; however, they both relapsed within 3 months from their landmark collection. The other two (patient 37 and patient 82) had radiological disease recurrence 1 and 6 months after the landmark, respectively.
Discussion
In our study, ctDNA was detected in 29% of patients undergoing definitive treatment for locally advanced melanoma, with 9% exhibiting ctDNA at landmark. The presence of ctDNA at this juncture was associated with inferior OS and systemic RFS. These observations align with existing studies on ctDNA in melanoma,17 reinforcing the adverse prognostic implications of ctDNA detectability post-definitive treatment. Noteworthy is the heightened impact of ctDNA positivity in patients who did not receive adjuvant treatment, suggesting that systemic treatments may rescue a subset of high-risk individuals prone to relapse. However, the limited sample size raises caution against definitive conclusions.
In a subset of patients with available preoperative plasma collections, we observed an association between detectability of ctDNA before surgery and shorter systemic RFS. Our results are consistent with prior literature data. Lee et al. reported a correlation between the presence of positive preoperative ctDNA and worse clinical outcomes in patients undergoing complete lymph nodal dissection, independently of disease substage.33
Our methodology involved the application of RaDaR, a tailored NGS technique, eliminating the necessity for specific driver mutations. This stands in contrast to most ctDNA studies in melanoma,17,34, 35, 36, 37, 38 utilizing droplet PCR (dPCR) targeting distinct mutations. Indeed, 26% of patients with detectable ctDNA in our study were BRAF and NRAS wild-type, underscoring the broader applicability of this technology, especially in non-cutaneous melanoma, which frequently does not harbor driver alterations.19
Although the sensitivity of ctDNA assay at landmark was ∼20%, it had a high clinical specificity at ∼94%. These figures align with most ctDNA studies in melanoma focusing on MRD.34,35,37,39,40 For instance, Long et al., using an alternative tumor-informed ctDNA assay detecting up to 200 variants (Invitae Personalized Cancer Monitoring) in locally advanced melanoma patients, reported a sensitivity of 23% for relapse detection at 24 months, with a specificity of 88%.34 However, a study by Eroglu et al. using a set of up to 16 variants (Signatera) reported a higher sensitivity of 83%.27 This study included 69 melanoma patients; however, only 30 of them received local treatment while the others had unresectable disease. Despite the encouraging results, there were only six relapses in this study (in contrast with 18 relapses in our study). As these are very small numbers, new recurrences may significantly affect the results, and therefore these data should be carefully followed up. In a study recently published by Weber et al., the RaDaR assay was used to detect minimal residual disease in patients with high-risk melanoma receiving adjuvant treatment with pembrolizumab alone or in combination with a personalized RNA-based vaccine.40 The authors observed a trend for shorter recurrence-free survival in patients with detectable ctDNA at landmark, in line with our results.
Compared to other published studies,27,28 our population included a greater proportion of patients with rare melanoma subtypes. Only 1/5 mucosal melanoma patients and 0/4 acral melanoma patients had at least one collection positive for ctDNA (see Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103978). Despite the small number of patients, this observation might suggest that ctDNA has a lower sensitivity in rare melanoma subtypes. Interestingly, the mucosal melanoma patient who had detectable ctDNA in his preoperative collection (patient 59, see Figure 4) is the only one in this group who experienced disease relapse.
Our findings diverge from the application of RaDaR in detecting MRD in other cancers such as locally advanced head and neck squamous cell carcinoma,24 breast,41 and non-small-cell lung cancer,25 where sensitivity rates reached up to 100%. In a systematic review by Mittal et al. investigating MRD utility by ctDNA following a definitive treatment across multiple tumor types and varying ctDNA techniques, the aggregated sensitivity for ctDNA at landmark was 58.3%.42 The reasons for the perceived decreased sensitivity of ctDNA in melanoma MRD detection remain elusive. We speculate that even in locally advanced disease, melanoma sheds limited ctDNA, possibly due to its skin/lymph node location, falling below the detection threshold of current methods. Favoring that hypothesis, Al-Mondhiry et al., in a small study of stage III melanoma with satellite/in-transit or nodal metastases, found that only 4 (28%) of 14 patients had ctDNA detected before surgery.43 Of note, all cases with ctDNA positivity had a nodal burden of >1.5 cm, suggesting that a minimal burden of disease is required for ctDNA to be detected in nodal-only disease. Conversely, in our cohort and in the study by Brunsgaard et al.,28 42% and 46% of patients had ctDNA detected before surgery, suggesting that lymph nodes and satellites may function as ‘sanctuary’ sites.
Importantly, 10 patients exhibited ctDNA detection preceding conventional imaging, with a median lead time of 128 days. In a small study published by Brunsgaard et al., bespoke ctDNA detection with the Signatera assay preceded disease relapse in 3/6 patients.28 To our knowledge SAMBA is the largest study demonstrating the possibility to use bespoke ctDNA for the early detection of disease relapse in high-risk melanoma patients. The ability to intercept disease progression before radiological PD offers a window for altering treatment, and potentially leads to better oncology outcomes.
Our study has several limitations. Firstly, it was conducted in parallel with standard-of-care management, with minimal procedure burden to patients. Therefore, blood draws for ctDNA acquisition were only typically carried out every 3-4 months, which may limit the ability of ctDNA to intercept disease recurrence even sooner than what we encountered. Furthermore, the adjuvant treatment regimen decision was based on patients’ preference and physicians’ recommendation. The lack of randomization could confound the accuracy analysis of RaDaR. Secondly, the median number of plasma samples analyzed per patient was 4. This was largely due to the coronavirus disease 2019 (COVID-19) outbreak when blood sample collections were temporarily interrupted for several months to prioritize patients’ safety and reduce the risk of contagion. It is known that longitudinal sampling increases the likelihood of MRD detection by ctDNA,42 which may also partially explain the overall low detection rate observed.42 Lastly, less than half of our patients had pre-definitive treatment samples collected, impacting the interpretation of results.
Despite these constraints, our study underscores the potential of ctDNA as a predictive tool and advocates for further clinical trials exploring its role in guiding treatment decisions. Our findings have led to the development of the investigator-initiated CLEAR-Me trial, an interception study to detect and clear MRD in patients with high-risk melanoma (NCT06319196). CLEAR-Me is a phase II trial in which high-risk melanoma patients are pre-screened for detectable ctDNA following definitive surgery and, if ctDNA positive, are then randomized (2 : 1) to receive adjuvant treatment with an anti-PD-1 agent alone or in combination with an anti-lymphocyte activating 3. If, as demonstrated by the SAMBA study, ctDNA analysis can identify patients with poorer survival, a multiagent adjuvant treatment might improve patients’ outcomes as compared to current standard of care. This interventional study is only an example of the potential applications of ctDNA for the implementation of novel therapeutic strategies aimed at improving the care of cancer patients.
Acknowledgements
We thank all the patients who participated in the SAMBA study and their families.
Funding
This study was supported by the LIBERATE study (NCT03702309), which is an institutional liquid biopsy program at the University Health Network supported by the BMO Financial Group Chair in Precision Cancer Genomics (Chair held by Dr Lillian Siu). The following grants and awards have also contributed to supporting this study: Douglas Wright Melanoma Award—University of Toronto (SG), David Cornfield Melanoma Fund Award—University of Toronto (SG), ASCO Merit Award (SG), Cancer Genomic Program Grant—Princess Margaret Cancer Center (DVA) Division of Medical Oncology and Hematology Junior Faculty Award—Princess Margaret Cancer Center (AS), Catalyst and Invest in Research Princess Margaret grants—Princess Margaret Cancer Foundation (AS).
Disclosure
DVA: honoraria from Libbs, Novartis Brazil, Pfizer Brazil; advisory board for MSD Brazil. GJ: NeoGenomics employee and shareholder. CP, RV, and PR: NeoGenomics employees. DPA: fellowship funding from Roche and Merck in 2022; honoraria and sat on advisory boards for Novartis and Merck. LLS: consultant for (advisory board): Merck, Pfizer, AstraZeneca, Roche, GlaxoSmithKline, Voronoi, Arvinas, Tessa, Navire, Relay, Rubius, Daiichi Sanyko, Coherus, Marengo, InteRNA, Tubulis, LTZ Therapeutics, NGM Biotherapeutics; stock ownership: Agios (spouse); leadership position: Treadwell Therapeutics (spouse); grant/research support from (clinical trials): Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, GlaxoSmithKline, Roche/Genentech, AstraZeneca, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Shattucks, BionTech, 23Me, EMD Serono. AS: consultant for (advisory board): Merck, Bristol-Myers Squibb, Oncorus, Janssen, Medison & Immunocore; grant/research support from (clinical trials): Novartis, Bristol-Myers Squibb, Symphogen AstraZeneca/Medimmune, Merck, Bayer, Surface Oncology, Northern Biologics, Janssen Oncology/Johnson & Johnson, Roche, Regeneron, Alkermes, Array Biopharma/Pfizer, GSK, NuBiyota, Oncorus, Treadwell, Amgen. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Mohr P., Kiecker F., Soriano V., et al. Adjuvant therapy versus watch-and-wait post surgery for stage III melanoma: a multicountry retrospective chart review. Melanoma Manage. 2019;6:MMT33. doi: 10.2217/mmt-2019-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbe C., Keim U., Suciu S., et al. Prognosis of patients with stage III melanoma according to American Joint Committee on Cancer Version 8: a reassessment on the basis of 3 independent stage III melanoma cohorts. J Clin Oncol. 2020;38:2543–2551. doi: 10.1200/JCO.19.03034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershenwald J.E., Scolyer R.A., Hess K.R., et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J., Mandala M., Del Vecchio M., et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont A.M.M., Blank C.U., Mandala M., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 6.Grossmann K.F., Patel S.P., Sondak V.K., et al. Final analysis of overall survival (OS) and relapse-free-survival (RFS) in the intergroup S1404 phase III randomized trial comparing either high-dose interferon (HDI) or ipilimumab to pembrolizumab in patients with high-risk resected melanoma. J Clin Oncol. 2021;39:9501. 9501. [Google Scholar]
- 7.Long G.V., Hauschild A., Santinami M., et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 8.Luke J.J., Rutkowski P., Queirolo P., et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718–1729. doi: 10.1016/S0140-6736(22)00562-1. [DOI] [PubMed] [Google Scholar]
- 9.Long G.V., Del Vecchio M., Weber J., et al. Adjuvant therapy with nivolumab versus placebo in patients with resected stage IIB/C melanoma (CheckMate 76K) SKIN J Cutaneous Med. 2023;7(2) [Google Scholar]
- 10.Kirkwood J.M., Del Vecchio M., Weber J., et al. Adjuvant nivolumab in resected stage IIB/C melanoma: primary results from the randomized, phase 3 CheckMate 76K trial. Nat Med. 2023;29:2835–2843. doi: 10.1038/s41591-023-02583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tie J., Cohen J.D., Lahouel K., et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386:2261–2272. doi: 10.1056/NEJMoa2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles T., Assaf Z.J., Davarpanah N., et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595:432–437. doi: 10.1038/s41586-021-03642-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee B., Lipton L., Cohen J., et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol. 2019;30:1472–1478. doi: 10.1093/annonc/mdz200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honore N., van Marcke C., Galot R., et al. Tumor-agnostic plasma assay for circulating tumor DNA detects minimal residual disease and predicts outcome in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol. 2023;34:1175–1186. doi: 10.1016/j.annonc.2023.09.3102. [DOI] [PubMed] [Google Scholar]
- 15.Ng H.Y., Ko J.M.Y., Lam K.O., et al. Circulating tumor DNA dynamics as prognostic markers in locally advanced and metastatic esophageal squamous cell carcinoma. JAMA Surg. 2023;158:1141–1150. doi: 10.1001/jamasurg.2023.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vellanki P.J., Ghosh S., Pathak A., et al. Regulatory implications of ctDNA in immuno-oncology for solid tumors. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandini S., Zanna I., De Angelis S.P., et al. Circulating tumour DNA and melanoma survival: a systematic literature review and meta-analysis. Crit Rev Oncol Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103187. [DOI] [PubMed] [Google Scholar]
- 18.De Simoni E., Spagnolo F., Gandini S., et al. Circulating tumor DNA-based assessment of molecular residual disease in non-metastatic melanoma. Cancer Treat Rev. 2024;129 doi: 10.1016/j.ctrv.2024.102788. [DOI] [PubMed] [Google Scholar]
- 19.Hayward N.K., Wilmott J.S., Waddell N., et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 20.Wang M., Banik I., Shain A.H., Yeh I., Bastian B.C. Integrated genomic analyses of acral and mucosal melanomas nominate novel driver genes. Genome Med. 2022;14:65. doi: 10.1186/s13073-022-01068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi H., Hugo W., Kong X., et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannan B., Perego M., Somasundaram R., Herlyn M. Heterogeneity in melanoma. Cancer Treat Res. 2016;167:1–15. doi: 10.1007/978-3-319-22539-5_1. [DOI] [PubMed] [Google Scholar]
- 23.Bratman S.V., Yang S.Y.C., Iafolla M.A.J., et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1:873–881. doi: 10.1038/s43018-020-0096-5. [DOI] [PubMed] [Google Scholar]
- 24.Flach S., Howarth K., Hackinger S., et al. Liquid BIOpsy for MiNimal RESidual DiSease detection in head and neck squamous cell carcinoma (LIONESS) – a personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br J Cancer. 2022;126:1186–1195. doi: 10.1038/s41416-022-01716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale D., Heider K., Ruiz-Valdepenas A., et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. 2022;33:500–510. doi: 10.1016/j.annonc.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahin I.H., Lin Y., Yothers G., et al. Minimal residual disease-directed adjuvant therapy for patients with early-stage colon cancer: CIRCULATE-US. Oncology (Williston Park) 2022;36:604–608. doi: 10.46883/2022.25920976. [DOI] [PubMed] [Google Scholar]
- 27.Eroglu Z., Krinshpun S., Kalashnikova E., et al. Circulating tumor DNA-based molecular residual disease detection for treatment monitoring in advanced melanoma patients. Cancer. 2023;129:1723–1734. doi: 10.1002/cncr.34716. [DOI] [PubMed] [Google Scholar]
- 28.Brunsgaard E.K., Bowles T.L., Asare E.A., et al. Feasibility of personalized circulating tumor DNA detection in stage II and III melanoma. Melanoma Res. 2023;33:184–191. doi: 10.1097/CMR.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genta S., Araujo D.V., Keshavarzi S., et al. Leveraging personalized circulating tumor DNA (ctDNA) for detection and monitoring of molecular residual disease in high-risk melanoma. J Clin Oncol. 2022;40:9579. 9579. [Google Scholar]
- 30.Lipsyc-Sharf M., de Bruin E.C., Santos K., et al. Circulating tumor DNA and late recurrence in high-risk hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer. J Clin Oncol. 2022;40:2408–2419. doi: 10.1200/JCO.22.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dijk N., Gil-Jimenez A., Silina K., et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26:1839–1844. doi: 10.1038/s41591-020-1085-z. [DOI] [PubMed] [Google Scholar]
- 32.Heagerty P.J., Lumley T., Pepe M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.H., Saw R.P., Thompson J.F., et al. Pre-operative ctDNA predicts survival in high-risk stage III cutaneous melanoma patients. Ann Oncol. 2019;30:815–822. doi: 10.1093/annonc/mdz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long G.V., Desai K., Tang T., et al. Association of pre-treatment ctDNA with disease recurrence and clinical and translational factors in patients with stage IIIB-D/IV melanoma treated with adjuvant immunotherapy (CheckMate 915) Ann Oncol. 2022;33(7):S904. [Google Scholar]
- 35.Lee R.J., Gremel G., Marshall A., et al. Circulating tumor DNA predicts survival in patients with resected high-risk stage II/III melanoma. Ann Oncol. 2018;29:490–496. doi: 10.1093/annonc/mdx717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsavela G., Lee J., Calapre L., et al. Circulating tumor DNA predicts outcome from first-, but not second-line treatment and Identifies melanoma patients who may benefit from combination immunotherapy. Clin Cancer Res. 2020;26:5926–5933. doi: 10.1158/1078-0432.CCR-20-2251. [DOI] [PubMed] [Google Scholar]
- 37.Khattak A., Weber J.S., Sullivan R.J., et al. Minimal residual disease by circulating tumor DNA as a biomarker of recurrence free survival in resected high-risk melanoma patients treated with mRNA-4157/V940, a personalized cancer vaccine, and pembrolizumab. J Clin Oncol. 2023;41:LBA9515. [Google Scholar]
- 38.Syeda M.M., Wiggins J.M., Corless B.C., et al. Circulating tumour DNA in patients with advanced melanoma treated with dabrafenib or dabrafenib plus trametinib: a clinical validation study. Lancet Oncol. 2021;22:370–380. doi: 10.1016/S1470-2045(20)30726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiggins J.M., Wiggins J.M., Alegun J., et al. Association of circulating tumor DNA kinetics with disease recurrence in patients with stage IIIB/C/IV melanoma treated with adjuvant immunotherapy in Checkmate 238. J Clin Oncol. 2023;41:9577. [Google Scholar]
- 40.Weber J.S., Carlino M.S., Khattak A., et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet. 2024;403:632–644. doi: 10.1016/S0140-6736(23)02268-7. [DOI] [PubMed] [Google Scholar]
- 41.Cutts R.J., Coakley M., Garcia-Murillas I., et al. Abstract 536: Molecular residual disease detection in early-stage breast cancer with a personalized sequencing approach. Cancer Res. 2021;81(supp 13):536. [Google Scholar]
- 42.Mittal A., Molto Valiente C., Tamimi F., et al. Utility of ctDNA in predicting relapse in solid tumors after curative therapy: a meta-analysis. JNCI Cancer Spectr. 2023;7:pkad040. doi: 10.1093/jncics/pkad040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Mondhiry J., Stahl C., Venna S.S., Jang S. Detection of ctDNA in patients with stage III cutaneous melanoma with satellite/in-transit or nodal metastases. J Clin Oncol. 2023;41 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.