Abstract
Purpose
To examine intraretinally migrated retinal pigment epithelium cells (iRPECs) in enucleated human eyes with various retinal conditions and corresponding intraretinal hyperreflective bodies (iHRBs) in a large cohort of patients with age-related macular degeneration (AMD) in China.
Design
Population-based study and histomorphometric investigation.
Participants
Participants of the population-based Beijing Eye Study and enucleated human eyes.
Methods
OCT-based and fundus photography-based examination of the macula of the Beijing Eye Study participants and light-microscopical histomorphometry of enucleated human eyes.
Main Outcome Measures
Presence and location of iRPECs and iHRBs.
Results
In the Beijing Eye Study (6551 eyes; 3301 participants), the prevalence of intermediate AMD and late AMD was 331 (5.1%) and 44 (0.6%), respectively. All 42 eyes with intermediate AMD and macular hyperpigmentation had iHRBs at locations corresponding spatially with macular hyperpigmentation on the fundus photographs. Among all eyes with intermediate AMD (n = 331), iHRBs were detected in 262 (79.2%) eyes. The most internal location of the iHRBs was at the ellipsoid zone in 46 (13.9%) eyes, at the external limiting membrane (ELM) in 45 (13.6%) eyes, and in the outer nuclear layer in 145 (43.8%) eyes. Out of the 262 eyes with iHRBs, 186 (71.0%) eyes showed a corresponding defect in the ellipsoid zone, and 128 (48.9%) eyes showed a defect in the ELM. The eyes with an iHRB located beneath the ELM did not show an ELM defect. The iHRBs were associated with a plume-like appearance and with a smoke-like appearance in 20 (7.6%) eyes and 137 (52.3%) eyes, respectively. All iHRBs did not have a shadow on the OCT images. Similar findings were obtained in the eyes with late AMD. Among 237 eyes examined histologically, 21 globes showed iRPECs: 8 eyes in parapapillary α zone/β zone; 5 eyes with myopic patchy atrophies, and 3 eyes with AMD. The iRPECs were spatially associated with an ELM defect and were not surrounded by a basal membrane.
Conclusions
Intraretinal hyperreflective bodies can be found in 3 out of 4 eyes with intermediate AMD, correlate histologically with intraretinally located (migrated) retinal pigment epithelium cells, and correspond spatially with localized defects of the ellipsoid zone and ELM.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Age-related macular degeneration, Drusen, Retinal microglial cells, Retinal photoreceptors, Retinal pigment epithelium
Changes in the retinal pigment epithelium (RPE) belong to the hallmarks of age-related macular degeneration (AMD).1,2 These RPE alterations include an RPE cell loss in association with the development of geographic atrophy in the late stage of AMD and RPE cell proliferation in the subretinal space between the retina and Bruch’s membrane in the case of the neovascular or exudative form of AMD.1, 2, 3, 4, 5, 6 The RPE cell proliferation is characterized by the formation of a subretinal scar in the macular region, with the proliferated RPE cells having contact with, or producing a periodic acid-Schiff (PAS) staining-positive basal membrane. The finding that the proliferating RPE cells produce their basal membrane within the subretinal scar led to the notion that the RPE cell transformation may best be described as a fibrous pseudo-metaplasia, with the RPE cells still keeping some of their basic epidermal characteristics (i.e., the production of their basal membrane).7 Performing a combined histologic and clinical study, Cao et al8 recently reported about the migration/proliferation of single, or few clustered, macular RPE cells into the retina in eyes without choroidal neovascularization. Their research revealed that a plume-like morphology of the intraretinally migrated/proliferated RPE cells increased and eventually regressed during the follow-up, with a complete resolution in about 40% of the eyes. The observation made by Cao et al8 agrees with findings obtained in other investigations in which activated RPE cells and RPE cells in association with AMD-related subretinal fibrosis have been described.9, 10, 11, 12, 13, 14, 15 In a hospital-based retrospective study, Ho et al10 detected in 27 out of 44 (61%) patients with early to intermediate dry AMD intraretinal RPE migration in OCT images, corresponding to RPE pigment clumping on the fundus photographs. The intraretinal RPE cells were detected most often in the outer nuclear layer and less frequently in more anterior retinal layers. The intraretinal RPE migration was observed predominantly above areas of drusen. Applying polarization-sensitive scanning laser ophthalmoscopy, Miura et al14 detected an intraretinal migration of RPE cells in 52 out of 155 (33.5%) eyes with AMD, in particular in those with drusenoid and serous pigment epithelial detachment.
Based on the findings of the preceding investigations, we undertook the present study to examine the occurrence and spatial correlations of intraretinal hyperreflective bodies (iHRBs) and macular hyperpigmentation as a potential surrogate of intraretinally located RPE cells (iRPECs) on OCT images and fundus photographs obtained in a population-based study. An additional goal of our study was to search for iRPECs in human eyes enucleated for various clinical reasons including AMD and correlate these histologic findings with the observations made clinically. Increasing the available information about iHRBs and iRPECs may expand the knowledge about the pathogenesis of AMD in general and the role of iRPECs in particular. It may especially be of interest because macular hyperpigmentation in eyes with intermediate AMD have been recognized as a major risk factor for the progression of the disease.2
Methods
The population-based Beijing Eye Study 2011 was conducted in Beijing in 5 communities in the urban area of the Haidian district and in 3 communities in the village area of Daxing District in the year 2011.16 The Medical Ethics Committee of the Beijing Tongren Hospital approved the study protocol and confirmed its accordance with the Declaration of Helsinki. All study participants gave their written informed consent. Eligibility criteria for inclusion in the investigation were an age of 50+ years and living in the study regions. Out of 4403 eligible people, 3468 (78.8%) individuals (1963 [56.6%] women) participated in the investigation. The study has been described in detail previously.16
The study participants underwent a series of ophthalmological examinations including refractometry and determination of best-correcting refractive error, pneumotonometry, slit-lamp-based examination of the anterior ocular segment, biometry of the right eyes (Lenstar 900 Optical Biometer, Haag-Streit), and photography of the cornea and lens (slit-lamp digital photography, camera type BG-4, Topcon Medical Systems, Inc.), and of the macula and optic disc (fundus camera type CR6-45NM, Canon Inc). We additionally performed spectral-domain OCT (Spectralis, wavelength: 870 nm; Heidelberg Engineering Co) of the macula and optic nerve head. The OCT-based macular imaging included a macular volume scan (25° × 30° field, 31 B-scan lines) and a macular star (6 B-scan lines) centered on the fovea. Each scan line of the macular volume scan and of the macular star was based on 100 averaged scans.
The iHRBs were defined as distinct, bright, single structures with a diameter of smaller than approximately 25 μm and located in the retina between the RPE line and the inner limiting membrane on any of the macular scan lines (Figure 1, Figure 2, Figure 3). In the case of an agglomeration of single iHRBs, the size of the total hyperreflective spot could be markedly larger than 25 μm. The iHRBs had to extend beyond the RPE line. Regions with subretinal or intraretinal exudation were excluded. In addition, we excluded eyes with retinal disease associated with intraretinal or subretinal exudation (e.g., retinal vein occlusion). Hyperreflective appearances located in the inner plexiform layer were interpreted as cross sections of retinal blood vessels if they could be spatially correlated with blood vessels detected on the fundus photographs or on the OCT images. For that purpose, we did not use image alignment software; the alignment was based on the grader’s interpretation of the vessel pattern. We noted the location of the iHRBs in the various retinal layers and assessed whether they were accompanied by a shadow in the sagittal posterior direction. We compared the location of the iHRBs on the OCT images with the location of macular hyperpigmentation on the fundus photographs. In addition, we assessed whether the iHRBs were spatially associated with discontinuities in the ellipsoid zone and in the external limiting membrane (ELM), and whether the iHRBs showed “smoke”-like or “comet-like,” whitish appearances in their vicinity (Fig 2). These appearances had unsharp borders and covered a region with a diameter ranging between 10 μm and 100 μm (Figure 1, Figure 2, Figure 3).
Figure 1.
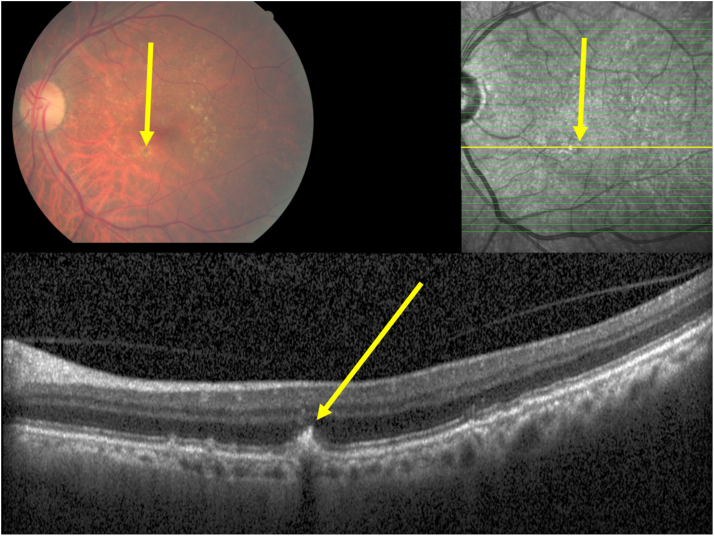
Clinical photograph and optical coherent tomographic images of an eye with an intermediate stage of age-related macular degeneration and macular hyperpigmentation, with the hyperpigmentation on the fundus photograph (yellow arrow) corresponding to a plume-like intraretinal hyperreflective body in the outer nuclear layer (yellow arrow), apparently emerging out of the retinal pigment epithelium line, with a corresponding localized defect in the inner segment/outer segment line and defect in the outer external limiting membrane.
Figure 2.
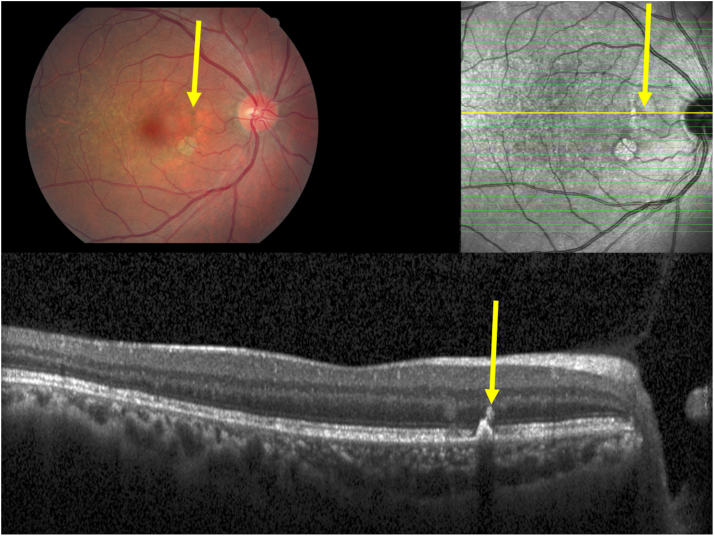
Clinical photograph and optical coherent tomographic images of an eye with an intermediate stage of age-related macular degeneration and macular hyperpigmentation, with the hyperpigmentation on the fundus photograph (yellow arrow) corresponding to a plume-like or smoke-like intraretinal hyperreflective body in the outer nuclear layer (yellow arrow), apparently emerging out of a focal elevation of the retinal pigment epithelium line, with a corresponding localized defect in the inner segment/outer segment line and defect in the outer external limiting membrane.
Figure 3.
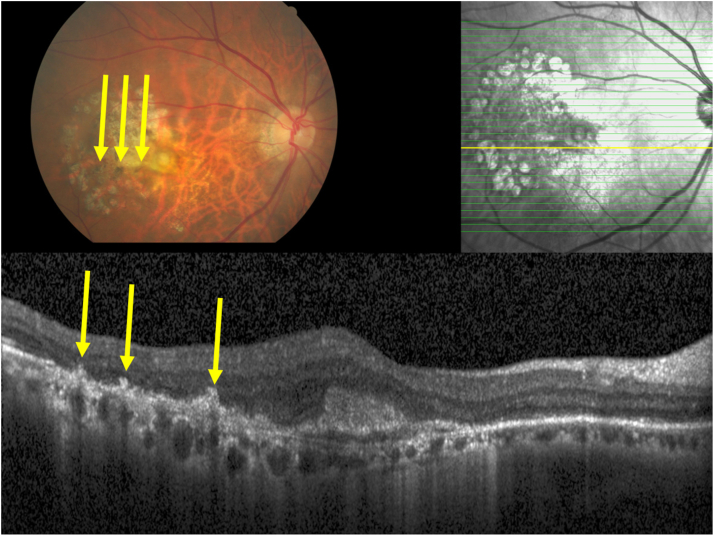
Clinical photograph and optical coherent tomographic images of an eye with late stage of age-related macular degeneration and macular hyperpigmentation, with the hyperpigmentation on the fundus photograph (yellow arrows) corresponding to intraretinal hyperreflective bodies in the outer nuclear layer and outer plexiform layer (yellow arrows), apparently emerging out of focal retinal pigment epithelium elevations, with corresponding localized defects in the inner segment/outer segment line and defects in the outer external limiting membrane.
Using the fundus photographs and OCT images and applying the criteria published by the Beckman Initiative for Macular Research Classification Committee, we defined AMD and differentiated it into an early stage (RPE drusen with a diameter of ≥63 and <125 μm, without pigmentary abnormalities), intermediate AMD (RPE drusen with a diameter of >125 μm or pigmentary abnormalities associated with drusen with a diameter of ≥63 μm), and late AMD, with a neovascular subgroup and a subgroup with geographic atrophy.17 We considered fundus lesions located within 2 average optic disc diameters from the fovea. Specially trained ophthalmologists carried out the basic grading of the fundus images. The fundus photographs and the OCT images of all eyes with pigmentary changes (i.e., eyes with AMD stage 2 with hyperpigmentation and eyes with AMD stage 3 and hyperpigmentation) were reassessed by 3 investigators (S.P.-J., J.B.J., Y.X.W.) who adjudicated in the case of diverging opinions. For this assessment, both the fundus photographs and the OCT images were simultaneously available and were examined in a parallel manner.
In the histologic part of the project, we examined human eyes that had been removed because of diseases like malignant choroidal melanomas and painful secondary angle-closure glaucoma. The Medical Ethics Committee II of the Medical Faculty Mannheim of the Heidelberg University approved the study, confirmed that the study conformed to the regulations formulated in the World Medical Association Declaration of Helsinki, and waived the necessity of informed written consent by the patients because the globes had been enucleated up to 60 years before the start of the present investigations. As also described in detail previously, the eyes had been fixed in a solution consisting of 4% formaldehyde and 1% glutaraldehyde immediately after enucleation, and they had been kept in that solution for 1 week at room temperature.18 After the diameters of the eyes in the horizontal, vertical, and sagittal directions had been determined, a central part with a thickness of approximately 8 mm and running through the center of the cornea and pupil and through the optic nerve head had been excised from the eyes. After dehydration in alcohol and imbedding in paraffin, slides with a thickness of about 5 to 8 μm had been stained by hematoxylin–eosin or by using the PAS method. Although the presence of iRPECs had already been shown in previous studies, our histologic examination was focused on the location of the iRPECs and their spatial association with defects in the ELM and the presence of a PAS-positive basal membrane.8, 9, 10, 11, 12, 13, 14, 15 Other aspects of interest, including migration of iRPECs to specific layers, immunohistochemical analysis, and microglial assessment were not addressed. Using a light microscope, we searched the slides of all eyes for the presence of iRPECs, independent of the underlying clinical disease as the cause for enucleation and independently of the direction of the histologic slide. Retinal pigment epithelium cells at the retinal undersurface in eyes with an artificially detached retina or those with a serous retinal detachment because of a malignant choroidal melanoma were not considered iRPECs. The regions with the uveal melanomas were excluded from the examinations. In the parapapillary region, we differentiated between α zone (characterized by the presence of Bruch’s membrane and the presence of an irregularly structured RPE), β zone (defined by the presence of Bruch’s membrane and absence of RPE), and γ zone (characterized by the absence of Bruch’s membrane).19
The statistical analysis was performed using a commercially available statistical software package (SPSS for Windows, version 27.0, IBM-SPSS). We calculated and expressed the prevalence of the main outcome parameters (i.e., the presence of iHRBs and their spatial correlation with macular hyperpigmentation on the fundus photographs) as the mean and its 95% confidence interval. A 2-sided P value < 0.05 was considered to indicate statistical significance.
Results
Out of 6936 eyes of 3468 individuals examined in the Beijing Eye Study 2011, fundus photographs of sufficient quality to be assessed for the presence of AMD were available for 6891 (99.4%) eyes. Out of these 6891 eyes, 6551 (95.1%) eyes (3301 participants) had serial OCT images of the macula. The mean age of the individuals was 64.3 ± 9.6 years (median: 63.0 years; range: 50–93 years), and the mean axial length was 23.3 ± 1.1 mm (median: 23.1 mm; range: 18.96–30 mm). The group included in the present study as compared with the group of Beijing Eye Study participants not included in the present study were significantly younger (64.3 ± 9.6 years versus 70.6 ± 10.8 years; P < 0.001), although both groups did not differ significantly in axial length (23.3 ± 1.1 versus 23.3 ± 1.3 mm; P = 0.50) and sex (P = 0.25).
Prevalence of early AMD, intermediate AMD, late AMD, and any AMD was 1079 (16.5%), 331 (5.1%), 44 (0.6%), and 1454 (22.2%), respectively. Among the eyes with late AMD (n = 44), 32 (73%) eyes showed choroidal neovascularization, and 13 (30%) eyes had geographic atrophy.
Among the eyes with intermediate AMD, 42 (42 of 331 or 12.7%) eyes showed macular intraretinal hyperpigmentation. The mean age in this group of eyes with intermediate AMD and macular hyperpigmentation was 65.0 ± 9.2 years, and the mean axial length was 22.8 ± 1.3 mm. Among the 44 eyes with late AMD (age: 73.2 ± 10.2 years; mean axial length was 23.5 ± 0.8 mm), 22 (22 of 44 or 50%) eyes showed hyperpigmentation. The mean age in the group of 22 eyes with late AMD and macular hyperpigmentation was 75.2 ± 9.4 years, and the mean axial length was 23.4 ± 0.9 mm. Out of the 22 eyes, 19 (82%) showed also macular hypopigmentation.
All 42 eyes with macular hyperpigmentation of intermediate AMD had iHRBs in their OCT images at locations corresponding spatially with macular hyperpigmentation on the fundus photographs (Figs 1, 2). The most internal location of the iHRBs was at the ellipsoid zone in 4 (9.5%) eyes, between the ellipsoid zone and the ELM in 2 (4.8%) eyes, at the ELM in 5 (11.9%) eyes, in the outer nuclear layer in 21 (50%) eyes, in the outer plexiform layer in 6 eyes (14.3%), and in the inner nuclear layer in 4 (9.5) eyes (Table 1). In 37 (88%) out of the 42 eyes, the iHRBs were spatially correlated with a localized thickening or elevation of the RPE line, 36 (86%) eyes showed a defect in the ellipsoid zone, and 28 (67%) eyes showed a defect in the ELM, with all defects spatially correlated with the iHRBs. The eyes with an iHRB located beneath the ELM did not show an ELM defect. In 10 (24%) of the eyes, the iHRBs were associated with a plume-like or comet-like appearance, and in 23 (55%) eyes, the iHRBs were associated with a smoke-like graying of the area. In all eyes with a plume-like or comet-like appearance, the direction of the comet was almost perpendicularly away from the RPE layer (Figs 1, 2). A regional thinning of the outer nuclear layer in spatial association with the iHRBs was detected in 15 (36%) eyes. All iHRBs did not have a shadow on the OCT images, in contrast to hyperreflective images of the large and medium-sized retinal vessels located in the inner retinal layers, most of which showed a shadow in the direction of the deep retinal layers.
Table 1.
Optical Coherent Tomographic Findings in the Participants of the Beijing Eye Study with Intermediate or Late AMD and Macular Hyperpigmentation
| Intermediate AMD (n = 331) |
Hyperpigmentation in Intermediate AMD (n = 42 out of 331 [12.7%]) |
Late AMD (n = 44) |
Hyperpigmentation in Late AMD, 22 out of 44 (50.0%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| iHRBs presence | – | 262 | 79.2% | 42 | 100% | 29 | 66% | 20 | 91% |
| iHRBs location | At the ellipsoid zone | 46 | 13.9% | 4 | 9.5% | – | – | 1 | 5% |
| Between the ellipsoid zone and external limiting membrane | 3 | 0.9% | 2 | 4.8% | 1 | 2.3% | – | – | |
| External limiting membrane | 45 | 13.6% | 5 | 11.9% | 4 | 9.1% | – | – | |
| Outer nuclear layer | 145 | 43.8% | 21 | 50% | 15 | 34% | 11 | 50% | |
| Outer plexiform layer | 15 | 4.5% | 6 | 14.3% | 5 | 11% | 5 | 23% | |
| Inner nuclear layer | 7 | 2.1% | 4 | 9.5% | 3 | 7% | 3 | 14% | |
| Retinal ganglion cell layer or retinal nerve fiber layer | 2 | 0.6% | – | – | – | – | – | – | |
| iHRBs appearance | Plume-like or comet-like | 20 | 7.6% | 10 | 24% | 17 | 40% | 13 | 59% |
| Smoke-like | 137 | 52.3% | 23 | 55% | 10 | 23% | 7 | 32% | |
| Spatial associations | Corresponding RPE thickening or elevation | 165 | 63.0% | 37 | 88% | – | – | 18 | 82% |
| Corresponding defect in the ellipsoid zone | 186 | 71.0% | 36 | 86% | 29 | 66% | 20 | 91% | |
| Corresponding to a defect in the external limiting membrane | 128 | 48.9% | 28 | 67% | 29 | 66% | 20 | 91% | |
AMD = age-related macular degeneration; iHRBs = intraretinal hyperreflective bodies; RPE = retinal pigment epithelium.
Among all eyes with intermediate AMD (n = 331), iHRBs were detected in 262 (79.2%). The most internal location of the iHRBs was at the ellipsoid zone in 46 eyes (or 13.9% of all 331 eyes with intermediate AMD), between the ellipsoid zone and the ELM in 3 (0.9%) eyes, at the ELM in 45 (13.6%) eyes, in the outer nuclear layer in 145 (43.8%) eyes, in the outer plexiform layer in 15 eyes (4.5%), in the inner nuclear layer in 7 (2.1%) eyes, and in the retinal ganglion cell layer or retinal nerve fiber layer in 2 (0.6%) eyes. In 165 (63%) out of the 262 eyes with iHRBs, the iHRBs were spatially correlated with a localized thickening or elevation of the RPE line, 186 (71.0%) eyes showed a defect in the ellipsoid zone, and 128 (48.9%) eyes showed a defect in the ELM, with all defects spatially correlated with the iHRBs. The eyes with an iHRB located beneath the ELM did not show an ELM defect. The iHRBs were associated with a plume-like or comet-like appearance in 20 (7.6%) eyes, and with a smoke-like graying of the area in 137 (52.3%) eyes. In all eyes with a plume-like or comet-like appearance, the direction of the comet was almost perpendicularly away from the RPE layer. A regional thinning of the outer nuclear layer in spatial association with the iHRBs was detected in 70 (26.7%) eyes. All iHRBs did not have a shadow on the OCT images.
In 20 (91%) out of the 22 eyes with late AMD and macular hyperpigmentation, iHRBs were detected in the OCT images at locations corresponding spatially with hyperpigmented spots in the macular region on the fundus photographs (Fig 3). The most internal location of iHRBs was at the ellipsoid zone in one eye (or 5% of all 22 eyes with late AMD and macular hyperpigmentation), in the outer nuclear layer in 11 (50%) eyes, in the outer plexiform layer in 5 eyes (23%), and in the inner nuclear layer in 3 (14%) eyes (Table 1). In 18 (82%) out of the 22 eyes, the iHRBs were spatially correlated with a localized thickening or elevation of the RPE line, and 20 (91%) eyes showed a defect in the ellipsoid zone and in the ELM, with all defects spatially correlated with the iHRBs. In 13 (59%) of the eyes, the iHRBs were associated with a plume-like or comet-like appearance, and, in 7 (32%) eyes, the iHRBs were associated with a smoke-like graying of the area. As in the eyes with intermediate AMD and macular hyperpigmentation, the direction of the comet was almost perpendicularly away from the RPE layer. A regional thinning of the outer nuclear layer in spatial association with the iHRBs was detected in 9 (41%) eyes. All iHRBs did not have a shadow on the OCT images.
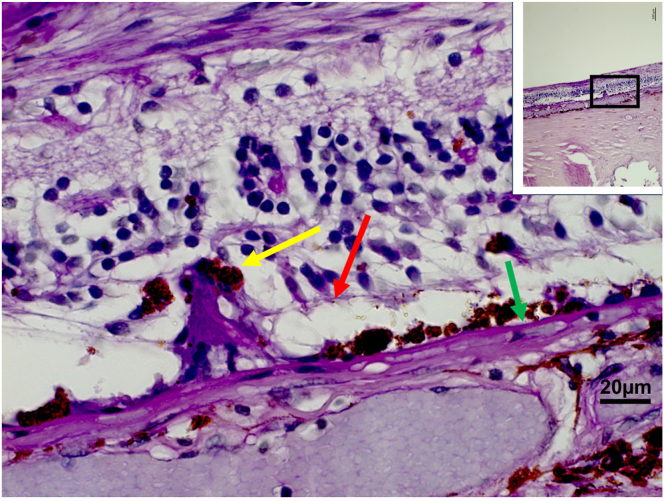
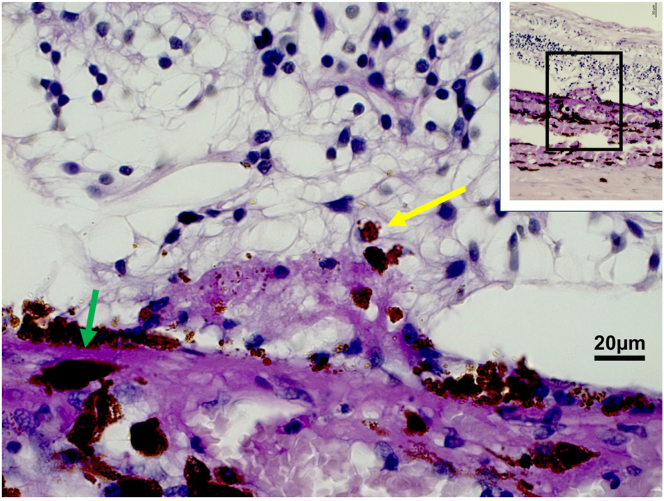
The histologic study included 237 eyes of 237 patients with an age of 61.4 ± 13.9 years and a mean axial length of 25.4 ± 3.0 mm. The study population consisted of 131 eyes with malignant uveal melanoma, 83 globes with end-stage secondary angle-closure glaucoma, 6 eyes with congenital glaucoma, and 18 eyes with other reasons for enucleation such as trauma. Intraretinally migrated RPE cells were detected in 21 globes: in 8 eyes in the region of parapapillary α zone/β zone; in 5 eyes in association with a Bruch’s membrane defect/patchy atrophy with myopic macular degeneration; in 4 eyes with retinitis pigmentosa; in 3 eyes with AMD; and in 1 eye in the region of a presumable retinal laser coagulation spot. In all these eyes, the iRPECs were not surrounded or in contact with a detected PAS-positive membrane, and, in all these eyes, the iRPECs were spatially associated with a defect in the ELM (Figs 4, 5).
Figure 4.
Histo-photograph showing retinal pigment epithelium cells (yellow arrow) proliferating into the retinal outer nuclear layer through a defect in the external limiting membrane (red arrow); green arrow: Bruch’s membrane.
Figure 5.
Histo-photograph showing retinal pigment epithelium cells (yellow arrow) proliferating into the retinal outer nuclear layer; green arrow: Bruch’s membrane.
Discussion
The clinical part of the present study showed the presence of iHRBs in all eyes with intermediate or late AMD with macular hyperpigmentation, with the hyperpigmentation spatially correlating with the iHRBs. The iHRBs extending up to the inner plexiform layer were related to a localized defect in the ellipsoid zone and in the ELM, and all of them did not show a shadow on the OCT images. In addition, the prevalence of iHRBs in all eyes with intermediate AMD, independently of the presence of an ophthalmoscopically detected macular hyperpigmentation, was 262 of 331 or 79.2%. The histologic part of the study demonstrated the intraretinal location of RPE cells in eyes with AMD and other diseases, and all of these iRPECs were spatially associated with defects in the ELM. The findings suggest that RPE cell migration into the retina is a hallmark of intermediate or late AMD, in particular in eyes with macular pigmentations.
The observations made in our study confirm the results of the landmark investigation performed by Cao et al.8 They examined clinical OCT images taken longitudinally of eyes with AMD and assessed histologically and immunohistochemically donor eyes that had undergone an ex vivo OCT examination. The authors found that a plume-like appearance of iHRBs on the clinical OCT images developed and, in a substantial number of eyes, regressed again during follow-up. Immunohistochemically, RPE cells spatially corresponding to iHRBs on the OCT images taken ex vivo showed a loss in their immunoreactivity for retinoid markers and an increase in the immunoreactivity for immune markers. In addition, some RPE cells in the RPE layer demonstrated an aberrant immunoreactivity. The iRPECs with a plume-like morphology contacted retinal capillaries in some eyes. Cao et al8 concluded that iHRBs, as described in the study, were suggestive of a transdifferentiation or an epithelial–mesenchymal transition of the RPE cells, with the gain and loss of function starting with individual RPE cells located in the RPE layer. This process may lead to the migration or proliferation of RPE cells into the retina.8 Following Cao et al,8 we histologically detected pigmented RPE cells in the retina in eyes with various diseases including AMD. Interestingly, these cells were not surrounded by a light-microscopically detected PAS-positive membrane, either because these cells did not have sufficient time to develop a detectable PAS-positive membrane or because they were in the process of undergoing metaplasia. This finding is in contrast to subretinal RPE cell proliferations forming a subretinal scar in eyes with neovascular/exudative myopic macular degeneration and in eyes with neovascular/exudative AMD.7 In the latter diseases, the pigmented RPE cells are in contact with a PAS-positive basal membrane. Because these cells kept a major characteristic of epithelial cells, i.e., the formation of a basal membrane, the finding suggested a fibrous pseudo-metaplasia of the RPE cells. The observations may suggest that an RPE cell proliferation in the subretinal space forming a subretinal scar and undergoing a fibrous pseudo-metaplasia may be differentiated from an intraretinal RPE cell migration/proliferation as in the case of intermediate AMD. One may discuss whether differences in the microenvironment (i.e., the subretinal space with the neovascular choroidal vessels as basis for exudative AMD compared with the intraretinal compartment) may be associated with the difference in the behavior of the displaced RPE cells producing or not producing a detectable PAS-positive basal membrane.
The finding of intraretinally migrating/proliferating RPE cells opens the question of whether these cells are needed for the support of the retinal cells in the process of AMD or whether they are part of a downward development in the course of AMD, eventually leading to macula-related central vision loss. The question arises whether the inward migration/proliferation of RPE cells in eyes with intermediate AMD should therapeutically be addressed. Investigations using RPE cell cultures revealed that some molecules, such as transforming growth factor β and epidermal growth factor, when added to the cell culture medium, increased the migration and proliferation of RPE cells in vitro, whereas the antibodies of the molecules had an inhibitory effect.20 Future studies may explore whether in eyes with intermediate and late AMD an intravitreal application of molecules preventing the RPE cells from migrating/proliferating into the neighboring retinal tissue, and the application of molecules supporting the RPE cells in the RPE layer to prevent them from initiating the process of transdifferentiation and metaplasia, may be useful.21 The finding of iRPECs in eyes with intermediate and late AMD also raises the question of whether in the intermediate and late stage of AMD, the disease affects not only the subretinal compartment and photoreceptor layer but also the middle retinal layers, including the inner nuclear layer.
The observation of a coincidence of iHRBs and spatially correlated defects in the ellipsoid zone and ELM corresponds to the finding obtained in the histologic part of our study in which intraretinal located RPE cells were spatially correlated with defects in the ELM (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5). These observations suggest that the ELM (and the ellipsoid zone) may act as a migration barrier for subretinal (RPE) cells. This notion is supported by the histologic finding that iRPECs could be found also in eyes with disorders other than AMD, such as in the parapapillary α zone and β zone, eyes with myopic macular degeneration with a high myopia-associated macular defect in Bruch’s membrane (patchy atrophy), and an eye with a retinal laser coagulation spot. At all these locations, the ELM showed defects, potentially allowing the RPE cells to migrate inward. Supporting the notion of the ELM as an effective barrier against an intraretinal cell migration are observations that, in eyes with malignant choroidal melanoma, an intact ELM was associated with an absence of tumor cells in the retina.
Intraretinal hyperreflective bodies as detected on OCT images may be differentiated into those occurring in relationship with migrated or proliferated RPE cells and into those found in association with a subretinal and intraretinal exudation (e.g., the so-called hard exudates). The differentiation may be supported by clinical ophthalmoscopy, with hard exudates (as cause for hyperreflective bodies) having a more whitish appearance upon ophthalmoscopy. In addition, iHRBs in association with iRPECs did not cast a posterior shadow on the OCT images in the direction of the choroid, in contrast to some exudation-related iHRBs showing such a shadow (Fig 6).
Figure 6.
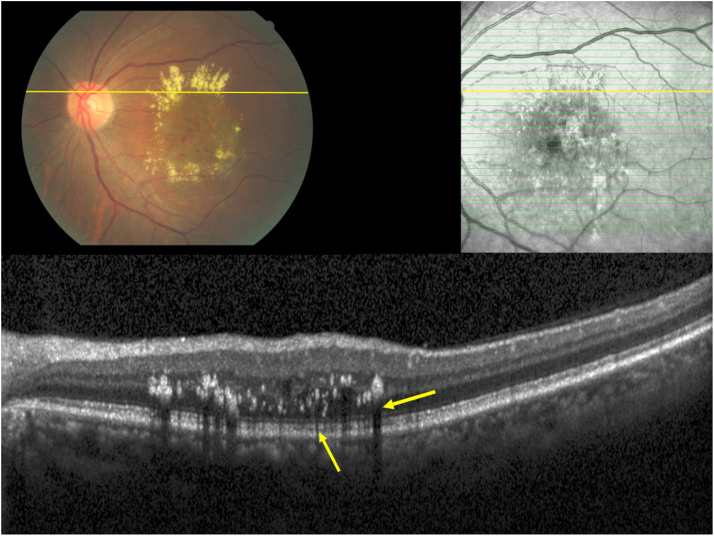
Clinical photograph and optical coherent tomographic images of an eye with exudative macular degeneration and hard exudates on the clinical photograph, corresponding to intraretinal hyperreflective bodies in the outer nuclear layer, outer plexiform layer, and inner nuclear layer (yellow arrows) with corresponding posterior shadows on the OCT images.
Clinical implications of our study are that macular hyperpigmentation in eyes with AMD are usually related to the iHRBs. The high prevalence of iHRBs in eyes with intermediate AMD may imply that iHRBs should be actively searched for in routinely taken OCT images of the macula in patients with AMD. It holds true in particular because the risk of progression from intermediate AMD to late AMD has been shown to be associated with the distribution and extent of iHRBs, especially in the outer retinal layer.22,23
When the findings obtained in our study are discussed, its limitations should be considered. First, the number of patients with intermediate and late AMD with macular hyperpigmentation included in the study was relatively small for a statistical analysis. They were derived, however, from a relatively large number of patients with intermediate AMD (n = 331) and were recruited and examined in a population-based study. The latter is combined with the advantage of a reduced risk of a referral bias. In addition, intraretinal RPE migration leading to macular hyperpigmentation may differ in prevalence between ethnicities. Furthermore, in all eyes with macular hyperpigmentation and intermediate AMD and in 19 out of 21 eyes with late AMD, the macular hyperpigmentation could be correlated with iHRBs. The potential limitation of our study with a small number of eyes with macular hyperpigmentation was additionally addressed by including all eyes with intermediate AMD in the analysis. It showed that the prevalence of iHRBs was relatively high, with 262 of 331 (or 79.2%). Second, the use of a 31 B-scan volume scan over a 25° × 30° field was not very dense; hence, some pigmentary changes visible on the fundus photographs might have been missed in the OCT images between OCT line scans. Third, iHRBs could have been due to intraretinal hard exudates due to an intraretinal or subretinal exudation or due to iRPECs; hence, their differentiation might have been difficult. The group of eyes with intermediate AMD, however, did not have an intraretinal or subretinal exudation due to the diagnostic criteria of intermediate AMD (as compared with late AMD) and due to the exclusion of eyes with a retinal disease associated with an exudation (e.g., retinal vein occlusions). In the case of late AMD, it might not always have been possible to clearly differentiate between iHRBs due to exudation or due to iRPECs. Fourth, because the histologic slides did not stem from the same individuals examined clinically, they did not allow a direct clinical–histologic correlation. Knowledge about the histology of iRPECs, in addition to the OCT histology-based findings of the Beijing Eye Study, may however have increased the information obtained. Fifth, the Beijing Eye Study included Han Chinese; hence, the results of the clinical part of our study may not directly be transferred to individuals of other ethnicities. Sixth, in the histologic part of our investigation, we did not perform immunohistochemical examinations due to the retrospective character of the histologic part of the study; hence, the melanin-loaded intraretinal cells could not unequivocally be classified as RPE cells. Seventh, because we did not perform immunohistochemistry, we could not identify amelanotic RPE cells, which, because of their metaplasia, might have lost their melanin load and their melanin-producing ability. Neither could we determine, whether the iRPECs were migrating, proliferating, or both. By the same token, we could not search for retinal microglial cells which have also been discussed to be the cause of iHRBs. Because in our clinical-epidemiological study, the iHRBs spatially correlated with macular hyperpigmentation as detected on fundus photographs, it might have been unlikely that the iHRBs represented activated retinal microglial cells. Eighth, the histologic section of some eyes did not run through the fovea, because the location of the malignant melanoma determined the meridional orientation of the section. In addition, serial sections were not available, and the underlying reasons leading to the enucleation of the globes might have influenced the results.
In conclusion, this combined clinical-epidemiological and histologic investigation revealed that macular hyperpigmentation in eyes with intermediate and late AMD are usually associated with iHRBs, which, according to their shape and according to histologic studies, represent migrating/proliferating RPE cells. Clinically and histologically, the iHRBs/intraretinal RPE cells are associated with defects in the ELM and ellipsoid zone.
Manuscript no. XOPS-D-24-00161R1.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s):
S.P.-J.: European patent EP 3 271 392, JP 2021-119187, and US 2021 0340237 A1: “Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia”; Patent application: European patent office number: 23170806.6: Jonas JB, Panda-Jonas S, Jonas RA, Jonas SB. Epidermal Growth Factor Inhibition in the Prophylactic or Therapeutic Treatment of Unwanted Proliferation, Migration or Metaplasia of the Retinal Pigment Epithelium.
R.A.J.: European patent EP 3 271 392, JP 2021-119187, and US 2021 0340237 A1: “Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia”; Patent application: European patent office number: 23170806.6: Jonas JB, Panda-Jonas S, Jonas RA, Jonas SB. Epidermal Growth Factor Inhibition in the Prophylactic or Therapeutic Treatment of Unwanted Proliferation, Migration or Metaplasia of the Retinal Pigment Epithelium.
J.B.J.: European patent EP 3 271 392, JP 2021-119187, and US 2021 0340237 A1: “Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia”; Patent application: European patent office number: 23170806.6: Jonas JB, Panda-Jonas S, Jonas RA, Jonas SB. Epidermal Growth Factor Inhibition in the Prophylactic or Therapeutic Treatment of Unwanted Proliferation, Migration or Metaplasia of the Retinal Pigment Epithelium.
The other authors have no proprietary or commercial interest in any materials discussed in this article.
Financial Support: Research Development Fund of Beijing Municipal Health Commission (2019-4); National Natural Science Foundation of China (82271086). The funder of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
HUMAN SUBJECTS: Human subjects were included in this study. The Medical Ethics Committee of the Beijing Tongren Hospital approved the study protocol and confirmed its accordance with the Declaration of Helsinki. All study participants gave their written informed consent.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Panda-Jonas, R.A. Jonas, Wang, J.B. Jonas
Data collection: Panda-Jonas, Wang, J.B. Jonas
Analysis and interpretation: Panda-Jonas, R.A. Jonas, Xu, Wang, J.B. Jonas
Obtained funding: Wang
Overall responsibility: Panda-Jonas, R.A. Jonas, Xu, Wang, J.B. Jonas
References
- 1.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Guymer R.H., Campbell T.G. Age-related macular degeneration. Lancet. 2023;401:1459–1472. doi: 10.1016/S0140-6736(22)02609-5. [DOI] [PubMed] [Google Scholar]
- 3.Fleckenstein M., Mitchell P., Freund K.B., et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–390. doi: 10.1016/j.ophtha.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Sadda S.R., Guymer R., Holz F.G., et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of Atrophy Report 3. Ophthalmology. 2018;125:537–548. doi: 10.1016/j.ophtha.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guymer R.H., Rosenfeld P.J., Curcio C.A., et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology. 2020;127:394–409. doi: 10.1016/j.ophtha.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spaide R.F., Jaffe G.J., Sarraf D., et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas S.B., Panda-Jonas S., Jonas J.B., Jonas R.A. Histology of neovascular myopic macular degeneration. Sci Rep. 2021;11 doi: 10.1038/s41598-021-01500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao D., Leong B., Messinger J.D., et al. Hyperreflective foci, optical coherence tomography progression indicators in age-related macular degeneration, include transdifferentiated retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2021;62:34. doi: 10.1167/iovs.62.10.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacks D.N., Johnson M.W. Transretinal pigment migration: an optical coherence tomographic study. Arch Ophthalmol. 2004;122:406–408. doi: 10.1001/archopht.122.3.406. [DOI] [PubMed] [Google Scholar]
- 10.Ho J., Witkin A.J., Liu J., et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118:687–693. doi: 10.1016/j.ophtha.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanzottera E.C., Messinger J.D., Ach T., et al. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:3269–3278. doi: 10.1167/iovs.15-16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaratnasingam C., Messinger J.D., Sloan K.R., et al. Histologic and optical coherence tomographic correlates in drusenoid pigment epithelium detachment in age-related macular degeneration. Ophthalmology. 2017;124:644–656. doi: 10.1016/j.ophtha.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curcio C.A., Zanzottera E.C., Ach T., et al. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:BIO211–BIO226. doi: 10.1167/iovs.17-21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura M., Makita S., Sugiyama S., et al. Evaluation of intraretinal migration of retinal pigment epithelial cells in age-related macular degeneration using polarimetric imaging. Sci Rep. 2017;7:3150. doi: 10.1038/s41598-017-03529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Cao D., Messinger J.D., et al. Histology and clinical imaging lifecycle of black pigment in fibrosis secondary to neovascular age-related macular degeneration. Exp Eye Res. 2022;214 doi: 10.1016/j.exer.2021.108882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Y.N., Wang Y.X., Xu L., et al. Fundus tessellation: prevalence and associated factors: The Beijing Eye Study 2011. Ophthalmology. 2015;122:1873–1880. doi: 10.1016/j.ophtha.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Ferris F.L., III, Wilkinson C.P., Bird A., et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panda-Jonas S., Auffarth G.U., Jonas J.B., Jonas R.A. Elongation of the retina and ciliary body in dependence of the sagittal eye diameter. Invest Ophthalmol Vis Sci. 2022;63:18. doi: 10.1167/iovs.63.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonas J.B., Jonas S.B., Jonas R.A., et al. Parapapillary atrophy: histological gamma zone and delta zone. PLOS ONE. 2012;7 doi: 10.1371/journal.pone.0047237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern J., Temple S. Retinal pigment epithelial cell proliferation. Exp Biol Med (Maywood) 2015;240:1079–1086. doi: 10.1177/1535370215587530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikbov M.M., Khalimov T.A., Panda-Jonas S., Jonas J.B. Intravitreal application of epidermal growth factor in non-exudative age-related macular degeneration. Br J Ophthalmol. 2022;106:1762–1766. doi: 10.1136/bjophthalmol-2021-319582. [DOI] [PubMed] [Google Scholar]
- 22.Verma A., Nittala M.G., Corradetti G., et al. Longitudinal evaluation of the distribution of intraretinal hyper-reflective foci in eyes with intermediate age-related macular degeneration. Curr Eye Res. 2024:1–7. doi: 10.1080/02713683.2024.2343334. [DOI] [PubMed] [Google Scholar]
- 23.Verma A., Corradetti G., He Y., et al. Relationship between the distribution of intra-retinal hyper-reflective foci and the progression of intermediate age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2023;261:3437–3447. doi: 10.1007/s00417-023-06180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]