Abstract
Adoptive T-cell immunotherapy holds great promise for the treatment of viral complications in immunocompromised patients resistant to standard anti-viral strategies. We present a retrospective analysis of 78 patients from 19 hospitals across Australia and New Zealand, treated over the last 15 years with “off-the-shelf” allogeneic T cells directed to a combination of Epstein–Barr virus (EBV), cytomegalovirus (CMV), BK polyomavirus (BKV), John Cunningham virus (JCV) and/or adenovirus (AdV) under the Australian Therapeutic Goods Administration’s Special Access Scheme. Most patients had severe post-transplant viral complications, including drug-resistant end-organ CMV disease, BKV-associated haemorrhagic cystitis and EBV-driven post-transplant lymphoproliferative disorder. Adoptive immunotherapy is well tolerated with few adverse effects. Importantly, 46/71 (65%) patients show definitive clinical improvement including reduction in viral load, clinical symptoms and complete resolution of end-organ disease. In addition, seven high-risk patients remain disease free. Based on this long-term encouraging clinical experience, we propose that a dedicated nationally funded centre for anti-viral cellular therapies should be considered to provide T cell therapies for critically ill patients for compassionate use.
Subject terms: Immunotherapy, Viral infection, T cells, Outcomes research
Adoptive T-cell immunotherapy offers promise to patients who are resistant to standard anti-viral strategies. Here the authors describe clinical observations in patients with viral complications treated with adoptive immunotherapy over the last 15 years.
Introduction
Opportunistic infections remain a significant cause of morbidity and mortality in immunocompromised patients, despite the ongoing development of new therapies1. Much of this disease burden is seen in solid organ transplant (SOT) and haematopoietic stem cell transplant (HSCT) recipients. Infectious complications of bacterial, fungal or viral origin occur in the majority of these transplant recipients who, due to underlying drug-induced immunosuppression, graft-versus-host disease or inborn errors of immunity (IEIs), are unable to generate efficient adaptive cellular immune responses associated with pathogen control2,3. Current standard-of-care typically relies upon effective antimicrobial therapies, including antibiotics, antifungals and anti-viral therapies. Some of these therapies are provided pre-emptively or prophylactically, particularly in settings of latent viral infection, to prevent disease following reactivation. Although reduction in immunosuppression is also used as a clinical management strategy to control persistent infection, this may increase the risk of graft rejection following SOT or graft-versus-host disease following HSCT. Despite these effective measures, infections are still associated with 17% of all SOT recipient deaths, 24% of deaths following allogeneic HSCT and 15% of deaths following autologous HSCT4,5. The risk of infection-related morbidity and mortality is further increased in patients who receive a T cell-depleted graft in an allogeneic haploidentical HSCT6.
In Australia, Lindsay et al. 7 reported that between 2013 and 2018, 409 allogeneic HSCT recipients, including 45 children, had infection-related mortality, equating to 34% of all-cause mortality during that period. In children, 61% of the infection-related deaths were caused by a viral infection or virus-related cancer, whereas in adults, this rate was 25%. In SOT recipients, up to 20–30% of infection-related mortality is associated with a viral infection4. Prevalent among these viral causes of mortality are the reactivation of latent viruses normally associated with lifelong asymptomatic infection, including human cytomegalovirus (CMV)8, Epstein–Barr virus (EBV)9, BK polyomavirus (BKV) and John Cunningham virus (JCV)10,11, and infection with respiratory viruses including adenoviruses (AdV)12. Anti-viral drug therapies are either not available for these viruses or become ineffective due to drug resistance, particularly with CMV infections. Diseases caused by latent viral infections are also prevalent in patients with inborn errors of immunity (IEI) and in other actively immunosuppressed patients, such as those with severe autoimmune disease.
The establishment of effective adaptive immunity is a key mechanism for the ongoing control of all viral infections. Clearance of virus-infected cells is primarily mediated by virus-specific T cells (CD8+ and CD4+ T cells) that directly recognise and kill infected cells via the specific interaction of their unique T cell receptor with viral peptides presented by human leucocyte antigens (HLA) on the surface of infected cells. In addition, CD4+ T cells can also contribute to viral clearance in additional ways, i.e. cytokine production, help to CD8+ T cells, and recruitment of other immune cells. These observations have led to the development of virus-specific adoptive T cell therapy involving in vitro expansion of virus-specific T cells either from the patient, a HSCT donor, or more recently from healthy third-party blood donors who are suitably HLA matched to the patient (generally, this involves matching through two or more HLA alleles)13–17. In addition, immunomagnetic cell sorting of interferon-gamma-secreting virus-specific T cells and HLA-peptide multimer-based enrichment of antigen-specific T cells have also been used for adoptive immunotherapy18,19.
Here we describe our experience over the past 15 years providing autologous and allogeneic virus-specific T cells for compassionate use under the Therapeutic Goods Administration (TGA) Special Access Scheme (SAS). These T cells have been used for the treatment of disease related to five different viruses and end-organ diseases of the lung, gut, liver, kidney, eyes or brain. These T cell therapies have also been used in patients with virus-associated cancers or autoimmune disorders. Retrospective analysis of clinical data from these patients demonstrate that these virus-specific cellular therapies are safe, have minimal side effects, and can be successfully used to treat complex diseases in seriously ill patients who have exhausted standard clinical interventions.
Results
Patient characteristics
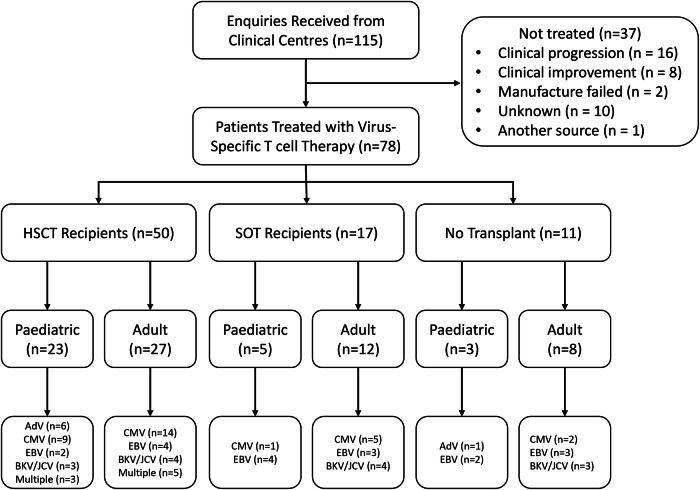
From 2008 to the end of 2023, QIMR Berghofer Medical Research Institute received 115 requests to provide T cells for compassionate use, with a significant increase in requests over the past 2 years (Figs. 1 and 2A). These requests have come from clinical centres treating adult and paediatric patients (Fig. 2B), predominantly in Queensland (QLD) and Victoria (VIC) (Fig. 2C). More than half (54%) of these requests have been for the treatment of patients with underlying haematological malignancies (Fig. 2D), with viral complications following allogeneic HSCT (Fig. 2E). Of the HSCT recipients, 15% had an underlying IEI that would have increased their susceptibility to opportunistic infections; the majority of these patients were paediatric. Paediatric patients with an IEI are often treated using T cell-depleted haploidentical HSCT, which renders them at increased susceptibility to post-transplant infectious complications. Requests have also been made for access to treatment for SOT recipients (n = 25) who are heavily immunosuppressed to prevent organ rejection and are unresponsive to standard-of-care treatment options, including anti-viral therapies and/or reduction of immunosuppression. We have also provided T cell therapies for patients with EBV-associated head and neck cancers (n = 2) and autoimmune diseases (n = 6) that have been linked to EBV or following organ transplant to treat disease (Fig. 1).
Fig. 1.
Flow diagram showing participant allocation, follow-up, and analysis.
Fig. 2. Characteristics of SAS patients.
A The number of SAS requests we received between 2008 and 2023. B The proportions of adult and paediatric patients in this cohort. C The geographic location of origin of the patients. D Underlying diseases in the patients. E The types of organ transplants within the patient cohort.
Most patients had complications associated with a single virus (Fig. 3A), the most prevalent of which was CMV. However, several were experiencing post-transplant complications with multiple viruses. The most prevalent clinical indication associated with SAS requests was persistent viral reactivation (Fig. 3B). Patients also presented with a variety of end-organ diseases in the lung, gut, liver, kidney, eyes or brain. BKV-specific T cell requests were for nephropathy (including nephritis) in renal transplant patients and haemorrhagic cystitis in HSCT patients, while JCV-specific T cell requests were for the treatment of progressive multifocal leukoencephalopathy (Fig. 3B). The majority of EBV-associated lymphomas were post-transplant; however, 10 requests were received for non-transplant patients.
Fig. 3. Viral disease characteristics.
A The viral causes of disease in the patient cohort. B The types of viral disease detected in patients.
T cell Therapy Manufacturing and Characterisation
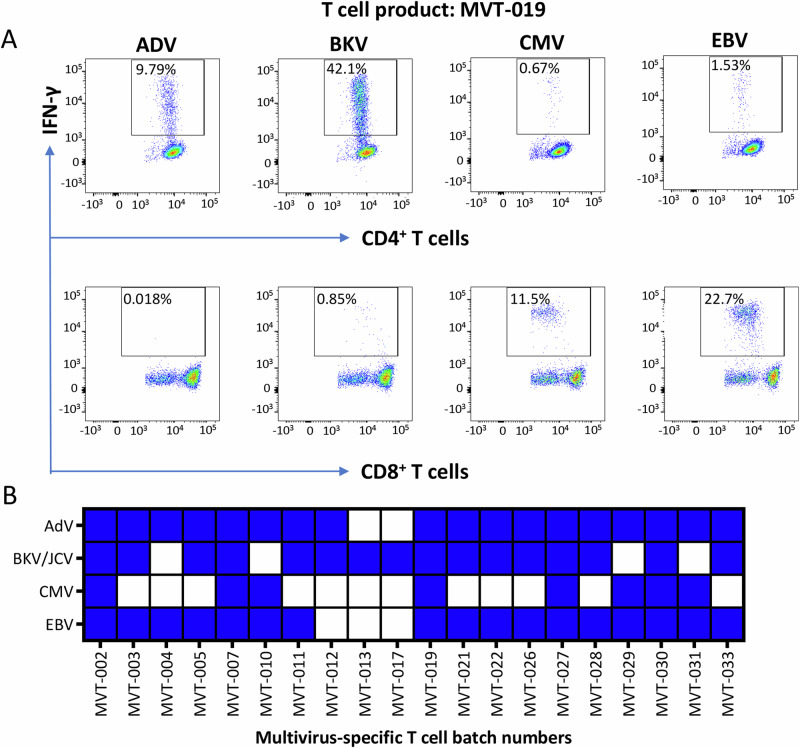
The initial approach undertaken to deliver T cells for compassionate use was through the generation of either autologous or allogeneic single-use T cell products. Between 2008 and 2019, 18 patients received T cells containing single-virus-specific CD8+ T cells, CD4+ T cells or both (Table 1). In 2019, a bank of allogeneic multi-virus-specific T cells for a phase I open-label clinical trial was manufactured (Fig. 4). These T cell products were designed to provide CD4+ and CD8+ T cell specificity for multiple viruses (AdV, BKV/JCV, CMV and EBV) in a single batch (Table 2, Supplementary Data 1). Twenty batches of allogenic multi-virus-specific T cells have been manufactured from a single blood collection (350–400 mL) from 20 healthy volunteers, producing an average of 115 vials (range 23–150 vials) of T cell therapy product (4 × 107 T cells/vial) (Fig. 4). The antigen specificity of each batch of T cells has been assessed using intracellular cytokine assays (Supplementary Fig. 1). Products were then selected for use based on HLA matching between patient and product, and the presence of the appropriate virus-specific T cells. Representative data from one of the multi-virus-specific T cell products with reactivity against AdV, BKV/JCV, CMV and EBV are shown in Fig. 5A. Of the 20 T cell products, five showed reactivity against all four viruses, 11 against three viruses, while the remaining four products showed reactivity against one or two viruses (Fig. 5B, Supplementary Fig. 2). Antigen-specific T cells in all products demonstrated a consistent functional profile, characterised by the correlative production of IFN-γ with TNF, IL-2 and CD107a (Supplementary Fig. 3). None of these products showed alloreactivity against HLA-mismatched targets.
Table 1.
Single-use T cell therapies generated for compassionate use
| Therapy | Product Code | CD8+ T cell specificity | CD4+ T cell specificity |
|---|---|---|---|
| Autologous | GBL-028 | CMV | - |
| CMV-006 | CMV | - | |
| CMV-SAS-001 | CMV | - | |
| HLN-039 | EBV | - | |
| HLN-040 | EBV | - | |
| MUS-003 | EBV | - | |
| SAS-002b | CMV | - | |
| SOT-013 | CMV | - | |
| SOT-025 | BKV | BKV | |
| Allogeneic | SAS-003 | CMV | CMV |
| SAS-005b | CMV | CMV | |
| SOT-024 | CMV | - | |
| SOT-027 | CMV | CMV | |
| SOT-028 | - | BKV | |
| SOT-029 | BKV | BKV | |
| SOT-030 | AdV | AdV | |
| GBL-029 | CMV | - | |
| GBL-030 | - | AdV |
Fig. 4. Schematic of the process used for the manufacture and administration of T cell therapy.
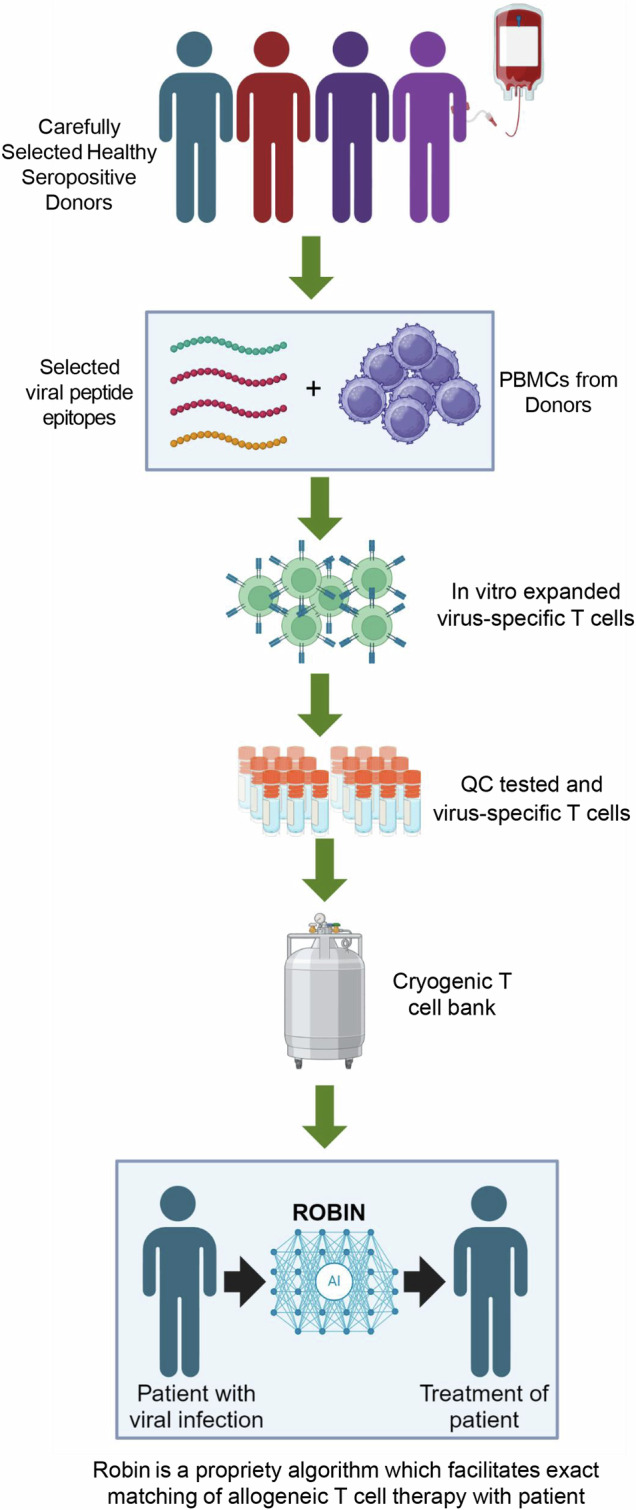
Peripheral blood mononuclear cells (PBMC) isolated from the peripheral blood of healthy volunteers were stimulated with selected viral peptide epitopes and then cultured for 14 to 17 days in the presence of IL-2. Subsequently, virus-specific T cells were assessed for antigen-specific reactivity and stored as a cryogenic bank. Virus-specific T cells were matched with appropriate patients based on HLA class I and class II alleles as outlined in the material and methods section. (created with BioRender.com under a paid subscription).
Table 2.
Banked allogeneic multi-virus-specific T cells
| Product Code | Number of Patients Treated | CD8+ T cell specificity | CD4+ T cell specificity |
|---|---|---|---|
| MVT-002 | 5 | AdV, CMV, EBV | AdV, BKV/JCV, EBV |
| MVT-003 | 5 | EBV | AdV, BKV/JCV, EBV |
| MVT-004 | 4 | EBV | AdV |
| MVT-005 | 2 | AdV, BKV/JCV, EBV | AdV, BKV/JCV, EBV |
| MVT-007 | 1 | CMV, EBV | AdV, BKV/JCV |
| MVT-010 | 3 | CMV, EBV | AdV, CMV |
| MVT-011 | 2 | EBV | AdV, BKV/JCV, EBV |
| MVT-012 | 1 | - | AdV, BKV/JCV |
| MVT-013 | 4 | BKV/JCV | BKV/JCV |
| MVT-017 | 1 | BKV/JCV | BKV/JCV |
| MVT-019 | 4 | CMV, EBV | AdV, BKV/JCV, CMV, EBV |
| MVT-021 | 1 | BKV/JCV, EBV | AdV, BKV/JCV, EBV |
| MVT-022 | 1 | BKV/JCV, EBV | AdV, BKV/JCV, EBV |
| MVT-026 | 1 | EBV | AdV, BKV/JCV, EBV |
| MVT-027 | 8 | CMV, EBV | AdV, BKV/JCV |
| MVT-028 | 4 | BKV/JCV, EBV | AdV, EBV |
| MVT-029 | 6 | CMV, EBV | AdV, CMV, EBV |
| MVT-030 | 6 | CMV, EBV | AdV, BKV/JCV, CMV, EBV |
| MVT-031 | 1 | CMV, EBV | AdV, BKV/JCV |
| MVT-033 | 0 | EBV, BKV | AdV |
Fig. 5. Multi-virus-specific T cell immunotherapy.
A Representative flow cytometry analysis showing multiple virus T cell specificities in a single T cell product. B Virus specificities of batches of T cell product within the multi-virus-specific T cell bank.
Retrospective analysis of clinical outcomes following adoptive T cell therapy
From the 115 requests received, 78 patients have been treated with T cell therapy (Supplementary Data 2). We have previously reported on the clinical outcomes of some of these patients20–24. The remaining patients (n = 37) did not receive therapy due to either progressive disease/death (n = 16), failure in manufacturing process of autologous T cell therapy (n = 2), access to T cell therapy from another source (n = 2) or clinical improvement prior to treatment (n = 8) (Figs. 1 and 6A). We were unable to identify the precise reason for not accessing T cell therapy for 10 patients. Response to T cell therapy was defined by resolution of persistent viraemia as assessed by monitoring of plasma viral DNA load, and improvement in clinical symptoms and signs from different disease manifestations assessed according to standard clinical practice. Of the 71 patients who had active disease at the time of T cell infusion, 46 (65%) showed clinical improvement, based on viral load reduction or disease improvement (Fig. 6B, Supplementary Data 2). Representative analyses of viral loads pre and post T cell therapy from two of these patients, with CMV viraemia and AdV viraemia, are shown in Figs. 6C and 6D respectively. Table 3 summarises retrospective clinical responses of adult and paediatric patients following adoptive T cell therapy stratified by viral infection and transplant status. It should be noted that many patients remained on standard-of-care treatment during their course of T cell therapy, e.g. ganciclovir or foscarnet to treat CMV. In addition, some patients showed evidence of a virological response to T cell therapy without an improvement in end-organ disease. Seven patients who were treated prophylactically due to a high risk of viral disease (due to either recurrent viral reactivation/disease, donor/recipient serostatus and underlying immunosuppression/immunodeficiency) also remained disease free following T cell therapy. We were unable to obtain clinical outcome data for one patient, while the remaining 23 patients who had late stage progressive disease and/or high levels of viral load showed no evidence of response to treatment. These results show that the compassionate use of virus-specific T cell therapy can provide therapeutic benefit, especially for patients with viral infections post-transplant, who have failed to respond to first-line therapy or are unable to tolerate standard therapies.
Fig. 6. Clinical outcome following adoptive T cell therapy.
A The proportion of patients who were treated with the supplied T cell therapy. B The proportion of patients who displayed clinical improvement. C Representative analysis of viraemia relative to the administration of T cells for a patient with CMV complications. D Representative analysis of viraemia relative to the administration of T cells for a patient with AdV complications.
Table 3.
Summary of clinical responses to adoptive T cell therapy
| Total | Virological/Disease Response | Remained Virus/ Disease Free | No Response | Unknown | |
|---|---|---|---|---|---|
| HSCT | |||||
| Paediatric | |||||
| Adenovirus | 6 | 5 | - | 1 | - |
| Cytomegalovirus | 9 | 6 | 1 | 2 | - |
| Epstein–Barr virus | 2 | 2 | - | - | - |
| Polyomavirus (BKV/JCV) | 3 | 1 | - | 2 | - |
| Multiple Viruses | 3 | 2 | 1 | - | - |
| Adult | |||||
| Cytomegalovirus | 14 | 6 | 2 | 6 | - |
| Epstein–Barr virus | 4 | - | 1 | 3 | - |
| Polyomavirus (BKV/JCV) | 4 | 4 | - | - | - |
| Multiple Viruses | 5 | 4 | - | 1 | - |
| SOT | |||||
| Paediatric | |||||
| Cytomegalovirus | 1 | 1 | - | - | - |
| Epstein–Barr virus | 4 | 3 | 1 | - | - |
| Adult | |||||
| Cytomegalovirus | 5 | 4 | - | - | 1 |
| Epstein–Barr virus | 3 | 2 | - | 1 | - |
| Polyomavirus (BKV/JCV) | 4 | 3 | - | 1 | - |
| No Transplant | |||||
| Paediatric | |||||
| Adenovirus | 1 | - | - | 1 | - |
| Epstein–Barr virus | 2 | - | 1 | 1 | - |
| Adult | |||||
| Cytomegalovirus | 2 | - | - | 2 | - |
| Epstein–Barr virus | 3 | 1 | - | 2 | - |
| Polyomavirus (BKV/JCV) | 3 | 2 | - | 1 | - |
| Total | 78 | 46 | 7* | 24 | 1 |
*These patients were at high risk of either recurrent viral reactivation or end organ disease.
Discussion
The increase in the number of solid organ and bone marrow transplants over the last three decades has had a significant impact on the lives of many people worldwide, who otherwise would have succumbed to their disease. Despite the remarkable success of transplanting tissue between genetically distinct individuals, the need to suppress or deplete the recipient’s immune system to prevent graft rejection leaves them highly susceptible to a variety of opportunistic infections1,3. While the development of anti-viral drugs, particularly against CMV, has had a dramatic impact on mortality associated with viral disease following transplantation, many patients still succumb to these opportunistic infections or develop toxicities from anti-viral drugs. While rituximab and cidofovir are used for the treatment of EBV and AdV respectively, concerns on their long-term use include B cell suppression, hypogammaglobulinemia, renal toxicity, and increased susceptibility to infections25–27. Consequently, there remains a lack of effective treatments for EBV, AdV and BKV/JCV. Virus-specific adoptive T cell immunotherapy has provided a last line of treatment for many patients without any remaining viable options. Although generally unproven in terms of effectiveness in randomised clinical studies (with the exception of some recently published studies with encouraging results)28–30, evidence over the last three decades has demonstrated real-world effectiveness of this approach31. Our own observations over the last 15 years provides additional support for the use of virus-specific T cell therapy to reduce the morbidity and mortality associated with viral infections in immunocompromised patients32.
The first evidence of virus-specific adoptive T cell therapy in transplant patients was provided in 1992 by Riddell and colleagues, who demonstrated that CMV-specific T cells could be generated from HSCT donors and administered to transplant recipients33. This was closely followed by work from Rooney and colleagues, who demonstrated the use of EBV-specific T cell therapy in HSCT recipients34. Due to the presence of genetic modification that allows long-term tracking, these EBV-specific T cells have since been shown to survive for more than two decades in the recipients35,36. Since these early observations, multiple studies have reported the use of donor-derived virus-specific T cell therapy in more than 150 HSCT recipients12,37–39.
One limitation of the donor-derived approach is the need for the HSCT donor to have immunological memory to the virus in question. This approach is also not applicable to SOT patients when donor T cells are not available. We and others have shown that autologous T cell therapy can be generated from both SOT and HSCT recipients, demonstrating a good safety profile and clinical efficacy31. We used this autologous approach in several patients treated compassionately in the current report. However, this approach is not feasible for all patients, predominantly due to difficulties in manufacturing cells from heavily immunocompromised patients. To overcome this limitation, Dorothy Crawford’s group pioneered the use of healthy donor blood to generate a bank of allogeneic EBV-specific T cells40,41. In this setting, donors were selected to provide broad HLA coverage, allowing matching between the T cell donor and the patient with viral disease. Based on this approach, the allogeneic EBV-specific T cell therapy tabelecleucel (Ebvallo) was recently approved to treat EBV-associated PTLD in Europe and has also been granted priority review by the FDA42,43. It is unclear if and when this will be available for patients in other regions of the world. This allogeneic approach has been extended to CMV and other virus-associated diseases in adult and paediatric settings, although most have not moved beyond early phase clinical studies44–46. We have also treated a number of patients included in the current report using allogeneic T cell therapy specific for a single virus.
Despite emerging data showing potential for allogeneic virus-specific T cell banks, it is challenging to generate a bank with broad HLA coverage that can target all known transplant-associated infectious complications, particularly those that are rare. To overcome this, Leen et al. pioneered the development of T cell therapy products containing multiple virus specificities47. This has now been extended by other groups to products including up to eight pathogens, including fungi48–51. Most patients in the current report were treated using T cells generated using a multi-virus-targeted approach. While EBV and/or CMV-specific T cells were typically dominant in these products, the majority also contained BKV/JCV- and AdV-specific CD4+ T cells. Importantly, we didn’t see any obvious evidence of reduced efficacy against these viruses, indicating that the use of a multi-virus-targeted approach does not impact the potency of virus-specific T cell therapy. It is important to emphasise that this is only an observational retrospective analysis and not a formal clinical trial.
Based on this retrospective analysis, we can draw two key conclusions. First, adoptive T cell therapy (either autologous or allogeneic) was generally safe, with no reported serious adverse reactions. Importantly, there was no evidence of precipitation of graft rejection or graft-versus-host disease. Second, virus-specific T cell therapy seems to be more effective in patients who had less disease burden and were treated early rather than at a late stage of clinical symptoms, evidenced by 23 patients who had late-stage disease and did not respond to adoptive T cell therapy. Overall, the compassionate use of virus-specific T cell therapies provides an opportunity to deliver therapies to severely immunocompromised patients who otherwise have limited options.
Methods
Regulatory and ethical approvals
Compassionate access to autologous or allogeneic virus-specific T cell therapies was provided through the Therapeutic Goods Administration (TGA), Special Access Scheme (SAS). Requests for access to T cell therapy were made to QIMR Berghofer Medical Research Institute by clinical centres across Australia (Supplementary Table 1). Each request was reviewed by a panel of three independent clinical experts and based on their advice regarding the appropriateness of T cell therapy for the case, the supply was approved by QIMR Berghofer. An SAS Category A notification form or SAS Category B application was also submitted to the TGA by the treating physician and informed consent was obtained from the patient or their parent/guardian. For each custom-manufactured batch of allogeneic T cell therapy from a donor selected by the requesting clinician, donor informed consent was also obtained. For SAS Category B applications, approval was obtained from the TGA prior to T cell manufacture and/or treatment. Access to T cell therapies was provided according to the TGA guidelines and did not require ethics committee approval. Sex- or gender-based analyses was not carried out as part of the study. Patients were treated based upon clinical need.
To generate a bank of “off-the-shelf” allogeneic virus-specific T cell therapies, healthy blood donors were recruited through the Australian Bone Marrow Donor Registry. Written informed consent was provided prior to participation. Approval for the cell therapy manufacturing study was obtained from the Australian Red Cross Lifeblood Human Research Ethics Committee and the QIMR Berghofer Medical Research Institute Human Research Ethics Committee. Additional products were generated from blood donated by healthy adults participating in a clinical trial (ACTRN12620000141943). These products were generated from healthy donors and added to our bank if excess material was available and the donor provided extended consent for use of their donated material to treat future patients. Ethics approval for the clinical trial was obtained through Children’s Health Queensland Human Research Ethics Committee and the QIMR Berghofer Medical Research Institute Human Research Ethics Committee. The use of human study participants was conducted in accordance with the criteria set by the Declaration of Helsinki.
Manufacture of Virus-Specific T cells
For autologous T cell therapy manufacture, peripheral blood mononuclear cells (PBMC) were isolated from patients then stimulated with clinical-grade custom HLA class I and class II-restricted peptide epitopes from CMV, BKV, JCV or AdV. Autologous EBV-specific T cells were manufactured using the AdE1-LMpoly vector as previously described52. For allogeneic virus-specific T cell therapy manufacture, PBMC were isolated from healthy donor blood then cryopreserved prior to use. PBMC were thawed and stimulated with HLA class I and class II-restricted peptide epitopes from CMV, BKV/JCV, EBV and AdV (Fig. 4). Peptide sequence, antigen source and HLA-restriction/s are provided in Supplementary Data 1. Synthetic peptides used in this study were custom manufactured by JPT Peptide Technologies, Berlin, Germany. The first seven batches were generated using the BKV pool without the JCV peptides. The remaining batches were generated using pools that encompass both BKV- and JCV-encoded peptides. T cells were then cultured in RPMI 1640 medium supplemented with 5% human AB serum and recombinant interleukin 2 (IL-2; 200 IU/mL; Miltenyi Biotec, Bergisch Gladbach, Germany, catalogue number: 170-076-14) for 14 to 17 days. T cell products were cryopreserved in Albumex 4 (CSL Behring, Melbourne, Australia, catalogue number: 34500165) containing 10% dimethyl sulfoxide (WAK-Chemie Medical, Steinbach, Germany, catalogue number: WAK-DMSO-10) (Fig. 4). Antigen specificity and HLA restriction of T cell therapy batches were determined using intracellular cytokine assays as outlined below. T cell release criteria included >70% viability post-thaw, >70% CD3+ T cells post-thaw, negative for microbial growth, negative for mycoplasma and <3 EU/mL endotoxin. HLA restriction was considered positive if the response to a HLA-restricted peptide epitope was greater than the mean of the no-peptide control plus three standard deviations.
Intracellular cytokine assays
Intracellular cytokine assays were used to define the specificity and HLA restriction of T cells generated for adoptive T cell therapy, in a two-step process. To define virus-specific reactivity, T cell therapy products were stimulated with pools of virus-specific peptides, then CD4+ T cells and CD8+ T cells were assessed as previously described32 for the expression of interferon gamma (IFN-γ), tumour necrosis factor (TNF), IL-2 and/or the mobilisation of CD107a, a surrogate for T cell-mediated cytotoxicity. Antibodies used in the study are provided in Supplementary Table 2. Products containing virus-specific CD4+ T cells and/or CD8+ T cells were then assessed for reactivity to individual HLA-matched peptide epitopes using an IFN-γ intracellular cytokine assay32. Flow cytometric acquisition was performed using a BD LSR Fortessa cell analyser with FACSDiva software (BD Biosciences, Melbourne, Australia). T cell reactivity to a virus-specific peptide pool or HLA-restricted peptide epitope was considered positive if the response was greater than the mean plus three standard deviations of the response of T cells cultured in the absence of stimulus. Post-acquisition analysis was also performed using FlowJo software (FlowJo, Ashland, Oregon, USA). The gating strategy used to defined virus-specific T cell reactivity is provided in Supplementary Fig. 1.
Assessment of T cell alloreactivity
Potential alloreactivity of T cells manufactured for allogeneic use was assessed using a panel of K562 cell lines, each transfected with a single HLA class I allele. Allogeneic T cell therapy products were incubated with each K562-HLA class I cell line at a responder-to-stimulator ratio of 10:1 and assessed for intracellular IFN-γ production. Products were considered alloreactive against an HLA class I allele if >1% of lymphocytes produced IFN-γ following subtraction of the background response generated against untransfected K56253.
Selection and administration of banked allogeneic T cells
To select matching T cell products from the bank of allogeneic virus-specific T cells for compassionate use in patients, an in-house computer-based algorithm was used. This algorithm prioritised the number of HLA-matched virus-specific responses, followed by the frequency of the virus-specific T cell response and subsequently global HLA matching to the recipient. Where possible, allogeneic products were matched to the recipient by a minimum of two HLA alleles. Between four and twelve vials of T cells were provided to the site for each patient; in most cases, six vials were provided. For each infusion, one vial of T cell product was thawed and diluted to 20 mL in clinical-grade normal saline, then intravenously infused over 5–10 minutes. The dose provided to adult patients was 4 × 107 cells/infusion, and the dose for paediatric patients was 2 × 107 cells/m2 body surface area. Infusions were generally administered fortnightly, or weekly at the discretion of the clinical team.
Safety and clinical assessments
All patients were monitored for any evidence of adverse reactions for up to 4 hours following each infusion of T cell therapy. In addition, as part of standard clinical care, viral load measurements, end-organ disease assessments and/or clinical symptoms were recorded. These records were used in the current retrospective analysis post T cell therapy, to assess clinical outcomes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We would like to thank Prof. David Whiteman and Prof. Grant Ramm for coordinating review of special access requests from clinical centres. We would also like to express gratitude to all anonymous clinical reviewers who reviewed requests for access to T cell therapies.
Author contributions
R.K., M.A.N. and CS conceived the study plan, contributed to data analysis and drafting of the original manuscript. G.R.A., A.P., L.B., S.R., S.B., J. R. L.L.T., P.C., M.S., L.L., S.S. contributed to T cell therapy manufacturing, functional analysis of T cell therapies and/or data analysis. N.H., J.S., R.P., C.L., D.S., M.T., W.Y.N., K., S., A.M., D.D., T.S., P.M., M.W., C.F., A.G., D.K., A.B., K.C., M.D., Z.H.Y., S.J.H., A.K., S.T., I.R., R.M.K., S.D., D.R., B.W., K.M., A.N., B.G., S.I., X.B., K.M., C.T., D.H., D.H., R.C., T.C., S.S.W., L.C., J.F., A.I., D.P., J.C., P.S., S.K.T., S.H., E.S., G.J., M.N., S.R., P.H., D.C., S.C., R.F., N.I., P.M. were responsible for T cell infusion, clinical management of the patients, follow up data collection and analysis. H.R., K.K.M., M.V., and M.A.N. coordinated the clinical follow-up, T cell therapy access, T cell therapy manufacturing coordination, drafting of the clinical protocol and/or T cell therapy batch record drafting. All authors contributed to drafting and reviewing of the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
We have provided a detailed list of the peptides used for manufacturing T cell therapies in Supplementary Data 1 and clinical history of patients in Supplementary Data 2. Any additional data that support the findings of this study are included in the supplementary information or available from the authors, as are the unique reagents used in this manuscript. The raw numbers for charts and graphs are available in the source data file wherever possible. Source data are provided with this paper.
Competing interests
R.K. and C.S. are listed as inventors on international patent applications describing virus-specific T cell therapy. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54595-2.
References
- 1.Kotton, C. N., Huprikar, S. & Kumar, D. Transplant infectious diseases: a review of the scientific registry of transplant recipients published data. Am. J. Transpl.17, 1439–1446 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Timsit, J. F. et al. Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients. Intensive Care Med45, 573–591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Delden, C. et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the swiss transplant cohort study. Clin. Infect. Dis.71, e159–e169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soborg, A. et al. Trends in underlying causes of death in solid organ transplant recipients between 2010 and 2020: Using the CLASS method for determining specific causes of death. PLoS One17, e0263210 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Styczynski, J. et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transpl.55, 126–136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquirol, A. et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transpl.56, 2432–2444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsay, J. et al. Infection-related mortality in adults and children undergoing allogeneic hematopoietic cell transplantation: an australian registry report. Transpl. Cell Ther.27, 798 e791–798 e710 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Stewart, A. G. & Kotton, C. N. What’s new: updates on cytomegalovirus in solid organ transplantation. Transplantation108, 884–897 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Yamada, M., L’Huillier, A. G. & Green, M. A focused review of epstein-barr virus infections and ptld in pediatric transplant recipients: guidance from the ipta and ecil guidelines. J. Pediatr. Infect. Dis. Soc.13, S31–S38 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Abudayyeh, A. et al. Poor immune reconstitution is associated with symptomatic BK polyomavirus viruria in allogeneic stem cell transplant recipients. Transpl. Infect. Dis.1910.1111/tid.12632 (2017). [DOI] [PubMed]
- 11.Cortese, I., Reich, D. S. & Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol.17, 37–51 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feghoul, L. et al. Adenovirus infection and disease in paediatric haematopoietic stem cell transplant patients: clues for antiviral pre-emptive treatment. Clin. Microbiol Infect.21, 701–709 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer, T. et al. Posoleucel, an allogeneic, off-the-shelf multivirus-specific t-cell therapy, for the treatment of refractory viral infections in the post-hct setting. Clin. Cancer Res29, 324–330 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzannou, I. et al. Off-the-shelf virus-specific t cells to treat bk virus, human herpesvirus 6, cytomegalovirus, epstein-barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol.35, 3547–3557 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson, A. et al. Third-party bk virus-specific cytotoxic t lymphocyte therapy for hemorrhagic cystitis following allotransplantation. J. Clin. Oncol.39, 2710–2719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galletta, T. J. et al. Third-Party and Patient-Specific Donor-Derived Virus-Specific T Cells Demonstrate Similar Efficacy and Safety for Management of Viral Infections after Hematopoietic Stem Cell Transplantation in Children and Young Adults. Transpl. Cell Ther.29, 305–310 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Keller, M. D. et al. Antiviral cellular therapy for enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation (ACES): a two-arm, open label phase II interventional trial of pediatric patients with risk factor assessment. Nat. Commun.15, 3258 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moosmann, A. et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood115, 2960–2970 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Cobbold, M. et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J. Exp. Med202, 379–386 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, G. R. et al. Successful immunotherapy of HCMV disease using virus-specific T cells expanded from an allogeneic stem cell transplant recipient. Am. J. Transpl.10, 173–179 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Holmes-Liew, C. L. et al. Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin. Transl. Immunol.4, e35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierucci, P. et al. Novel autologous T-cell therapy for drug-resistant cytomegalovirus disease after lung transplantation. J. Heart Lung Transpl.35, 685–687 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Jahan, S. et al. T-cell adoptive immunotherapy for BK nephropathy in renal transplantation. Transpl. Infect. Dis.22, e13399 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pender, M. P. et al. Epstein-Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Mult. Scler.20, 1541–1544 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petropoulou, A. D. et al. Increased infection rate after preemptive rituximab treatment for Epstein-Barr virus reactivation after allogeneic hematopoietic stem-cell transplantation. Transplantation94, 879–883 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Luterbacher, F. et al. Case report: persistent hypogammaglobulinemia more than 10 years after rituximab Given Post-HSCT. Front Immunol.12, 773853 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vora, S. B., Brothers, A. W. & Englund, J. A. Renal toxicity in pediatric patients receiving cidofovir for the treatment of adenovirus infection. J. Pediatr. Infect. Dis. Soc.6, 399–402 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulou, A. et al. SARS-CoV-2-specific T cell therapy for severe COVID-19: a randomized phase 1/2 trial. Nat. Med29, 2019–2029 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Chandraker, A. et al. Posoleucel in kidney transplant recipients with bk viremia: multicenter, randomized, double-blind, placebo-controlled phase 2 trial. J. Am. Soc. Nephrol.35, 618–629 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreras, C. et al. Results of phase 2 randomized multi-center study to evaluate the safety and efficacy of infusion of memory T cells as adoptive therapy in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia and/or lymphopenia (RELEASE NCT04578210). Cytotherapy26, 25–35 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Khanna, R. & Smith, C. Cellular immune therapy for viral infections in transplant patients. Indian J. Med. Res.138, 796–807 (2013). [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, C. et al. Autologous adoptive t-cell therapy for recurrent or drug-resistant cytomegalovirus complications in solid organ transplant recipients: a single-arm open-label phase i clinical trial. Clin. Infect. Dis.68, 632–640 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Riddell, S. R. et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T-cell clones. Science257, 238–241 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Rooney, C. M. et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet345, 9–13 (1995). [DOI] [PubMed] [Google Scholar]
- 35.Heslop, H. E. et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med.2, 551–555 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Rooney, C. M. et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood92, 1549–1555 (1998). [PubMed] [Google Scholar]
- 37.Savoldo, B. et al. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am. J. Transpl.5, 566–572 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Leen, A. M. et al. T-cell immunotherapy for adenoviral infections of stem-cell transplant recipients. Ann. N. Y Acad. Sci.1062, 104–115 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Pakakasama, S. et al. Treatment of Epstein-Barr virus lymphoproliferative disease after hematopoietic stem-cell transplantation with hydroxyurea and cytotoxic T-cell lymphocytes. Transplantation78, 755–757 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Haque, T. et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood110, 1123–1131 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Haque, T. et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet360, 436–442 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Mahadeo, K. M. et al. Tabelecleucel for allogeneic haematopoietic stem-cell or solid organ transplant recipients with Epstein-Barr virus-positive post-transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE): a phase 3, multicentre, open-label trial. Lancet Oncol.25, 376–387 (2024). [DOI] [PubMed] [Google Scholar]
- 43.Prockop, S. E. et al. Third-party cytomegalovirus-specific T cells improved survival in refractory cytomegalovirus viremia after hematopoietic transplant. J. Clin. Invest.13310.1172/JCI165476 (2023). [DOI] [PMC free article] [PubMed]
- 44.Withers, B. et al. Establishment and operation of a third-party virus-specific t cell bank within an allogeneic stem cell transplant program. Biol. Blood Marrow Transpl.24, 2433–2442 (2018). [DOI] [PubMed] [Google Scholar]
- 45.O’Reilly, R. J., Prockop, S. & Oved, J. H. Virus-specific T-cells from third party or transplant donors for treatment of EBV lymphoproliferative diseases arising post hematopoietic cell or solid organ transplantation. Front Immunol.14, 1290059 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma, C. et al. Adoptive transfer of CMV-specific TCR-T cells for the treatment of CMV infection after haploidentical hematopoietic stem cell transplantation. J. Immunother. Cancer1210.1136/jitc-2023-007735 (2024). [DOI] [PMC free article] [PubMed]
- 47.Leen, A. M. et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat. Med12, 1160–1166 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Blyth, E. et al. BK virus-specific T cells for use in cellular therapy show specificity to multiple antigens and polyfunctional cytokine responses. Transplantation92, 1077–1084 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Di Ciaccio, P. R. et al. Successful treatment of CMV, EBV, and adenovirus tissue infection following HLA-mismatched allogeneic stem cell transplant using infusion of third-party T cells from multiple donors in addition to antivirals, rituximab, and surgery. Transpl. Infect. Dis.23, e13528 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Jiang, W. et al. Third-party CMV- and EBV-specific T-cells for first viral reactivation after allogeneic stem cell transplant. Blood Adv.6, 4949–4966 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castellano-Gonzalez, G., Clancy, L. E. & Gottlieb, D. Prospects for adoptive T-cell therapy for invasive fungal disease. Curr. Opin. Infect. Dis.30, 518–527 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Smith, C. & Khanna, R. Generation of cytotoxic T lymphocytes for immunotherapy of EBV-associated malignancies. Methods Mol. Biol. (Clifton, NJ)651, 49–59 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Sinha, D. et al. ‘Off-the-shelf’ allogeneic antigen-specific adoptive T-cell therapy for the treatment of multiple EBV-associated malignancies. J. Immunother. Cancer910.1136/jitc-2020-001608 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
We have provided a detailed list of the peptides used for manufacturing T cell therapies in Supplementary Data 1 and clinical history of patients in Supplementary Data 2. Any additional data that support the findings of this study are included in the supplementary information or available from the authors, as are the unique reagents used in this manuscript. The raw numbers for charts and graphs are available in the source data file wherever possible. Source data are provided with this paper.