Abstract
Elevated neutrophil counts in broncho-alveolar lavage (BAL) fluids of lung transplant (LTx) patients with chronic lung allograft dysfunction (CLAD) are associated with disease pathology. However, phenotypical characteristics of these cells remained largely unknown. Moreover, despite enhanced levels of the most potent human neutrophil-attracting chemokine CXCL8 in BAL fluid, no discrimination had been made between natural NH2-terminally truncated CXCL8 proteoforms, which exhibit up to 30-fold differences in biological activity. Therefore, we aimed to characterize the neutrophil maturation and activation state, as well as proteolytic activation of CXCL8, in BAL fluids and peripheral blood of LTx patients with CLAD or infection and stable LTx recipients. Flow cytometry and microscopy revealed a high diversity in neutrophil maturity in blood and BAL fluid, ranging from immature band to hypersegmented aged cells. In contrast, the activation phenotype of neutrophils in BAL fluid was remarkably homogeneous. The highly potentiated NH2-terminally truncated proteoforms CXCL8(6–77), CXCL8(8–77) and CXCL8(9–77), but also the partially inactivated CXCL8(10–77), were detected in BAL fluids of CLAD and infected LTx patients, as well as in COVID-19 and influenza patient cohorts by tandem mass spectrometry. Moreover, the most potent proteoform CXCL8(9–77) specifically correlated with the neutrophil counts in the LTx BAL fluids. Finally, rapid proteolysis of CXCL8 in BAL fluids could be inhibited by a combination of serine and metalloprotease inhibitors. In conclusion, proteolytic activation of CXCL8 promotes neutrophilic inflammation in LTx patients. Therefore, application of protease inhibitors may hold pharmacological promise for reducing excessive neutrophil-mediated inflammation and collateral tissue damage in the lungs.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05500-z.
Keywords: Proteolysis, Inflammation, Pneumonia, Transplant rejection, Chemokine
Introduction
Lung transplantation (LTx) is indicated for patients with end-stage lung diseases such as chronic obstructive pulmonary disease, cystic fibrosis and interstitial lung diseases. However, long-term patient survival is significantly hampered by infections and the development of chronic lung allograft dysfunction (CLAD). Infections represent one of the most prevalent causes of early mortality among adult lung transplant recipients, while CLAD emerges as the leading cause of death starting from the first year after transplantation [1]. CLAD is defined as a persistent decline in pulmonary function without a specific identifiable cause and irreversible by treatment. The disease is characterized by chronic graft injury and inflammation, ultimately leading to fibrosis and allograft dysfunction [2, 3].
Neutrophils are key players in multiple inflammatory lung diseases. These innate immune cells constitute the most abundant circulating leukocytes in humans and exploit a diverse array of oxidative and non-oxidative effector functions to combat pathogens and restore homeostasis [4]. Emerging research indicates that neutrophils exhibit a remarkable degree of phenotypical and functional diversity and plasticity, both during health and disease [5–7]. However, their largely non-specific antimicrobial actions and products of activation (e.g. degradative enzymes and toxic chemicals) may induce collateral damage to healthy tissue. Therefore, neutrophil recruitment needs to be tightly regulated. Chemotaxis of neutrophils to the inflammatory site is governed by multiple chemoattractants [8], including chemokines: small, structurally conserved proteins that orchestrate cell migration [9]. The activity of chemokines is regulated by chemical and enzymatic post-translational modifications. These are mediated by multiple enzymes and other protein-modifying agents, which are typically upregulated in an inflamed environment [10]. Indeed, also the biological activity of the most potent human neutrophil-attracting chemokine, i.e. interleukin (IL)-8 or CXCL8, can be increased up to 30-fold through limited natural NH2-terminal proteolysis [11]. Removal of five and potentially up to eight NH2-terminal amino acid residues of CXCL8 amplifies its potency to activate the chemokine G protein-coupled receptors CXCR1/CXCR2, resulting in enhanced neutrophil chemotactic and activating properties in vitro and in vivo [12–14]. More subtle natural proteolysis resulting in removal of only one or two NH2-terminal amino acid residues, or alternative cleavage of the signal peptide, may also occur but these modifications hardly affect the biological activity of CXCL8 [15]. However, information on proteolytic regulation of CXCL8 activity in patients is almost non-existing. Endogenous CXCL8 proteoforms have recently been identified in synovial fluids from arthritic patients using the “immunosorbent nano-scale liquid chromatography-tandem mass spectrometry proteoform analysis” (ISTAMPA) methodology [16]. This innovative method enables to discriminate between different CXCL8 proteoforms, which only slightly differ in molecular mass and peptide sequence and therefore cannot be distinguished by conventional antibody-based immunoassays (e.g. ELISA or Western blot).
Within the broncho-alveolar lavage (BAL) fluids of LTx patients with CLAD, neutrophils and their most potent chemokine attractant CXCL8 are significantly elevated compared to stable LTx recipients [17, 18] and associated with disease pathology [19, 20]. However, the pathophysiological properties of neutrophils during CLAD as well as in lung transplant recipients that are either stable or suffer from infections remain largely elusive. Furthermore, studies have been restricted to measuring total CXCL8 levels in the BAL fluids of these patients through conventional antibody-based immunoassays without discrimination between potentiated or inactivated CXCL8 proteoforms. Therefore, the objective of this study was to characterize the neutrophil phenotype and the activation state of CXCL8 by identification of post-translationally modified CXCL8 variants in the lungs and circulation of LTx patients.
Materials & methods
Study design
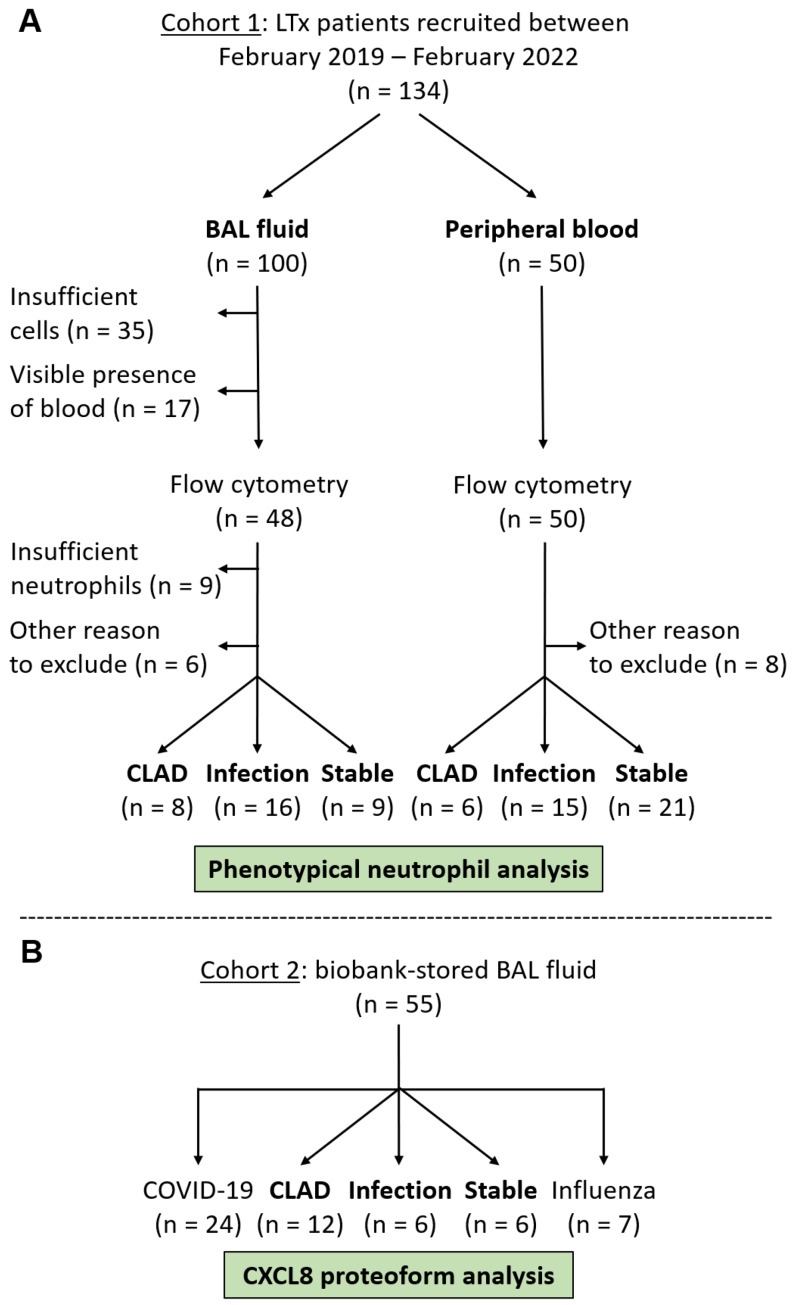
We performed an observational study relying on two patient cohorts. First, we prospectively collected fresh BAL fluid and peripheral blood samples from bilateral LTx patients for analysis of the neutrophil phenotype (Fig. 1A). These samples were collected as part of the routine follow-up consultations of the patients in the University Hospitals Leuven (UZ Leuven) on days 21, 90, 180, 360, 540 and 720 after transplantation or whenever clinically indicated (for instance upon pulmonary function decline due to infection or transplant rejection). In total, 100 BAL fluid and 50 peripheral blood samples were collected from 134 individual patients between February 2019 and February 2022. Some BAL fluid samples were excluded due to insufficient numbers of cells (n = 35) or neutrophils (n = 9) or visible presence of blood contamination (n = 17). Samples (n = 6 for BAL fluids and n = 8 for blood samples) from patients (a) on post-operative day 1, (b) upon discharge from the hospital after transplantation, (c) with other severe complications (anastomotic stricture, pulmonary embolisms) or (d) without clear diagnosis were excluded as well. Eventually, 33 BAL fluid samples and 42 peripheral blood samples were included in the neutrophil phenotype analysis part of this study. These samples were categorized retrospectively by an experienced clinician as samples derived from LTx patients with CLAD, stable LTx recipients or LTx patients with acute pulmonary infection. CLAD was defined as a persistent irreversible decline (≥ 20%) in measured forced expiratory volume in 1 s (FEV1) from the baseline value (the mean of the best two post-operative FEV1 measurements taken at least three weeks apart), without a specific other cause such as aging, infection or mechanical factors, and not reversible by treatment [2]. Infection was defined as detection of a microorganism using clinical PCR testing and/or biochemical and microbial test cultures in combination with clinical and radiological signs such as C-reactive protein increase, X-ray infiltrates or fever. Colonization was defined as detection of a microorganism without these clinical signs of infection. LTx patients were classified retrospectively as stable when no infection or rejection signs were observed, and patients remained stable (CLAD-free) for at least 6 months after the collection of their BAL fluid or peripheral blood sample. A flow chart with a detailed selection of patients for the neutrophil analysis part of the study is displayed in Fig. 1A. Clinical data for these patients are described in Table 1. Finally, peripheral blood samples from age- and sex-matched healthy controls (n = 17) were collected for neutrophil analysis.
Fig. 1.
Flow chart representing the recruitment of LTx patients for analysis of the neutrophil phenotype (cohort 1) and CXCL8 proteoforms (cohort 2). (A) To study the neutrophil phenotype, a first cohort of 100 fresh BAL fluid and 50 peripheral blood samples were collected from 134 individual patients, including 16 patients with a paired BAL fluid and peripheral blood sample. BAL fluid samples were excluded for flow cytometry analysis if cell counts were insufficient or upon severe blood contamination. Samples with insufficient neutrophils for proper flow cytometry analysis were excluded as well. Other reasons for exclusion were samples derived from patients on post-operative day 1 or upon hospital discharge immediately after transplantation, from patients with other severe complications (anastomotic stricture, pulmonary embolisms) or without clear diagnosis. Samples were categorized retrospectively to be derived from LTx patients with CLAD or pulmonary infection or stable LTx recipients according to clinical guidelines (see Materials & methods section). (B) To study CXCL8 proteoforms in LTx, COVID-19 and influenza patients, -80 °C-stored BAL fluid supernatants from a second cohort of 55 individual patients were used
Table 1.
Clinical characteristics neutrophil phenotype cohort
| BAL fluid samples | Blood samples | ||||||
|---|---|---|---|---|---|---|---|
| CLAD (n = 8) |
Infection (n = 16) |
Stable (n = 9) |
CLAD (n = 6) |
Infection (n = 15) |
Stable (n = 21) |
||
| Age (years) | 60 (52–65) | 58 (41–61) | 61 (27–65) | 56 (46–68) | 60 (56–62) | 58 (50–64) | |
| Sex |
Male Female |
3/8 (38%) 5/8 (63%) |
6/16 (38%) 10/16 (63%) |
3/9 (33%) 6/9 (67%) |
4/6 (67%) 2/6 (33%) |
7/15 (47%) 8/15 (53%) |
15/21 (71%) 6/21 (29%) |
| Underlying disease |
COPD CF ILD PPH Retransplant Other lung disease |
6/8 (75%) 0/8 (0%) 0/8 (0%) 0/8 (0%) 1/8 (13%) 1/8 (13%) |
7/16 (44%) 5/16 (31%) 4/16 (25%) 0/16 (0%) 0/16 (0%) 0/16 (0%) |
3/9 (33%) 1/9 (11%) 2/9 (22%) 0/9 (0%) 0/9 (0%) 3/9 (33%)# |
3/6 (50%) 0/6 (0%) 0/6 (0%) 0/6 (0%) 2/6 (33%) 1/6 (17%) |
9/15 (60%) 2/15 (13%) 3/15 (20%) 0/15 (0%) 0/15 (0%) 1/15 (7%) |
11/21 (52%) 4/21 (19%) 2/21 (10%) 1/21 (5%) 1/21 (5%) 2/21 (10%) |
| Time since CLAD diagnosis (days) | 277 (43–727)$ | NA | NA | 918 (452–1197) | NA | NA | |
| Future CLAD |
Yes Time to diagnosis (days) |
NA NA |
2/16 (13%) 403 (187–619) |
2/9 (22%) 313 (184–441) |
NA NA |
1/15 (7%) 619 |
3/21 (14%) 429 (184–493) |
| Mortality within 6 months | 1/8 (13%) | 0/16 (0%) | 1/9 (11%) | 1/6 (17%) | 0/15 (0%) | 0/21 (0%) | |
| POD (days) | 987 (730–2504) | 560 (201–1190)$ | 184 (92–460)** | 1560 (1383–1914) | 184 (92–366)*** | 366 (137–646)** | |
| BAL fluid |
Return (mL) Total cell count (*103/mL) Neutrophil (%) Neutrophil (*103/mL) Macrophage (%) Macrophage (*103/mL) Lymphocyte (%) Lymphocyte (*103/mL) Eosinophil (%) Eosinophil (*103/mL) |
48 (43–50) 204 (149–386) 20 (14–34) 52 (18–93) 65 (38–77) 143 (58–263) 7 (2–33) 22 (4–49) 1 (0–1) 1 (0–3) |
44 (40–55) 177 (150–846) 33 (8–79) 58 (12–698) 52 (19–88) 129 (54–158) 2 (1–8) 4 (1–23) 0 (0–1) 0 (0–3) |
56 (46–60) 169 (74–196) 3 (2–8)*, ### 3 (1–11)*, ## 94 (81–97)*, ## 148 (72–179) 3 (1–13) 6 (1–24) 0 (0–1) 0 (0–1) |
|||
| Blood |
Leukocytes (*109/L) Neutrophil (%) Neutrophil (*109/L) Monocyte (%) Monocyte (*109/L) Lymphocyte (%) Lymphocyte (*109/L) Eosinophil (%) Eosinophil (*109/L) Basophil (%) Basophil (*109/L) CRP (mg/L) |
7.6 (5.3–10.1) 74 (59–77) 5.9 (3.2–7.6) 10 (8–11) 0.7 (0.5–1.1) 11 (8–25) 1.1 (0.8–1.5) 2 (1–4) 0.1 (0.1–0.3) 1 (0–1) 0.0 (0.0-0.1) 13 (6–17) |
6.5 (5.7-8.0) 75 (65–80) 5 (3.7–5.9) 9 (8–12) 0.6 (0.4-1.0) 12 (8–24) 1.0 (0.5–1.5) 1 (0–3) 0.1 (0.0-0.2) 0 (0–1) 0.0 (0.0-0.1) 2 (1–25) |
6.3 (4.6–8.6) 70 (58–80) 4.2 (2.7–6.1) 9 (7–13) 0.5 (0.4–0.7) 18 (13–27) 1.1 (0.7–1.4) 1 (0–2) 0.1 (0.0-0.1) 0 (0–1) 0.0 (0.0-0.1) 2 (1–4) |
|||
| Biopsy |
Acute rejection LB |
1/8 (13%) 0/8 (0%) |
0/16 (0%) 1/16 (6%) |
0/9 (0%) 0/9 (0%) |
0/6 (0%) 0/6 (0%) |
0/15 (0%) 0/15 (0%) |
0/21 (0%) 0/21 (0%) |
| Infection |
General Bacterial Fungal Viral |
4/8 (50%) 3/8 (38%) 1/8 (13%) 1/8 (13%) |
16/16 (100%)** 11/16 (69%) 3/16 (19%) 4/16 (25%) |
0/9 (0%)*, #### 0/9 (0%)## 0/9 (0%) 0/9 (0%) |
1/6 (17%) 1/6 (17%) 0/6 (0%) 0/6 (0%) |
15/15 (100%)*** 12/15 (80%)* 3/15 (20%) 2/15 (13%) |
0/21 (0%)#### 0/21 (0%)#### 0/21 (0%) 0/21 (0%) |
| Colonization | 1/8 (13%) | NA | 3/9 (33%) | 0/6 (0%) | NA | 5/21 (24%) | |
| Medication use at sampling |
Corticosteroids Tacro/Cyclo/None MMF/Azathio/None Everolimus Azithromycin Montelukast Antimicrobials |
8/8 (100%) 8/0/0 6/2/0 1/8 (13%) 7/8 (88%) 7/8 (88%) 8/8 (100%) |
16/16 (100%) 11/3/2 10/4/2 5/16 (31%) 15/16 (94%) 3/16 (19%)** 16/16 (100%) |
9/9 (100%) 8/1/0 6/0/3 0/9 (0%) 9/9 (100%) 1/9 (11%)** 9/9 (100%) |
6/6 (100%) 6/0/0 4/0/2 0/6 (0%) 6/6 (100%) 6/6 (100%) 6/6 (100%) |
15/15 (100%) 13/2/0 9/5/1 3/15 (20%) 15/15 (100%) 0/15 (0%)**** 15/15 (100%) |
21/21 (100%) 18/3/0 15/3/3 1/21 (5%) 21/21 (100%) 1/21 (5%)**** 21/21 (100%) |
Characteristics of LTx patient blood and BAL fluid samples included in the flow cytometry analysis of the neutrophil phenotype (cohort 1). Continuous variables are presented as median (interquartile range). Categorical variables are presented as counts (percentage). BAL fluids or blood samples of different patient categories were statistically compared by Kruskal-Wallis tests with Dunn’s multiple comparisons tests. Mann-Whitney U tests were performed for BAL fluid-blood comparisons per patient category. Fisher’s exact tests were used to compare proportions
Abbreviations: Azathio, Azathioprine; BAL, broncho-alveolar lavage; CF, cystic fibrosis; CLAD, chronic lung allograft dysfunction; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; Cyclo, cyclosporine A; ILD, interstitial lung diseases; LB, lymphocytic bronchiolitis; MMF, mycophenolate mofetil; NA, not applicable; POD, post-operative day; PPH, primary pulmonary hypertension; Tacro, tacrolimus
* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. BAL-CLAD or Blood-CLAD. #p < 0.05; ##p < 0.01; ###p < 0.001; ####p < 0.0001 vs. BAL-Infection or Blood-Infection. $p < 0.05 vs. Blood of the same patient category
For analyses of CXCL8 proteoforms, we relied on a second cohort of biobank-stored BAL fluid supernatants obtained from 24 individual CLAD, infected or stable LTx patients (Fig. 1B). For CLAD patients, BAL fluids were obtained at CLAD diagnosis [6 bronchiolitis obliterans syndrome (BOS) and 6 restrictive allograft syndrome (RAS) patients] and free from co-infection [17]. For CLAD and infected LTx patients, neutrophil-rich BAL fluid samples (> 15% neutrophils) were selected. Clinical data for these patients are described in Table 2. Since we had no availability of BAL fluid samples from patients with chronic lung inflammation as a control for CLAD patients, we included remaining samples of a previously reported supplementary cohort of patients with acute neutrophilic inflammation due to microbial infection [21]. Therefore, BAL fluid supernatants from adult COVID-19 (n = 24) and influenza (n = 7) patients recruited at UZ Leuven were used for CXCL8 proteoform analyses (Fig. 1B). Most of these patients are, similar to the LTx patients, on active immunosuppressive treatment with corticosteroids and suffering from bacterial/fungal co-infections. Detailed clinical data and an analysis of the neutrophil phenotype of these patients are described in Supplementary Table 1.
Table 2.
Clinical characteristics CXCL8 proteoform cohort
| BAL fluid samples | ||||
|---|---|---|---|---|
| CLAD (n = 12) |
Infection (n = 6) |
Stable (n = 6) |
||
| Age (years) | 47 (29–58) | 42 (22–56) | 52 (38–55) | |
| Sex |
Male Female |
5/12 (42%) 7/12 (58%) |
1/6 (17%) 5/6 (83%) |
4/6 (67%) 2/6 (33%) |
| Underlying disease |
COPD CF ILD PPH Retransplant Other lung disease |
6/12 (50%) 3/12 (25%) 1/12 (8%) 0/12 (0%) 1/12 (8%) 1/12 (8%) |
1/6 (17%) 4/6 (67%) 1/6 (17%) 0/6 (0%) 0/6 (0%) 0/6 (0%) |
3/6 (50%) 2/6 (33%) 1/6 (17%) 0/6 (0%) 0/6 (0%) 0/6 (0%) |
| Time since CLAD diagnosis (days) | 0 | NA | NA | |
| Future CLAD |
Yes Time to diagnosis (days) |
NA NA |
2/6 (33%) 403 (187–619) |
0/6 (0%) NA |
| Mortality within 6 months | 2/12 (17%) | 0/6 (0%) | 0/6 (0%) | |
| POD (days) | 1117 (430–1700) | 1093 (149–2176) | 733 (725–746) | |
| BAL fluid |
Return (mL) Total cell count (*103/mL) Neutrophil (%) Neutrophil (*103/mL) Macrophage (%) Macrophage (*103/mL) Lymphocyte (%) Lymphocyte (*103/mL) Eosinophil (%) Eosinophil (*103/mL) |
40 (29–50) 777 (346–1086) 72 (43–79) 350 (176–829) 26 (19–47) 149 (64–228) 2 (1–5) 17 (4–31) 1 (0–3) 4 (1–34) |
42 (36–58) 831 (172–1759) 78 (49–90) 695 (75–1476) 20 (6–48) 96 (55–203) 1 (0–3) 4 (1–21) 1 (0–2) 2 (0–12) |
48 (28–67) 142 (92–311)*, # 1 (0–1)**, ## 1 (1–3)**, ## 91 (77–97)**, ## 133 (63–293) 8 (1–22) 12 (6–27) 0 (0–0)* 0 (0–0)** |
| Biopsy |
Acute rejection LB |
3/12 (25%) 3/12 (25%) |
0/6 (0%) 1/6 (17%) |
0/6 (0%) 0/6 (0%) |
| Infection |
General Bacterial Fungal Viral |
0/12 (0%) 0/12 (0%) 0/12 (0%) 0/12 (0%) |
6/6 (100%)**** 4/6 (67%)** 2/6 (33%) 2/6 (33%) |
0/6 (0%)## 0/6 (0%) 0/6 (0%) 0/6 (0%) |
| Colonization | 4/12 (33%) | NA | 1/6 (17%) | |
| Medication use at BAL |
Corticosteroids Tacro/Cyclo/None MMF/Azathio/None Azithromycin Antimicrobials |
12/12 (100%) 11/1/0 5/4/3 10/12 (83%) 12/12 (100%) |
6/6 (100%) 3/1/2 2/3/1 6/6 (100%) 6/6 (100%) |
6/6 (100%) 6/0/0 1/3/2 2/6 (33%) 6/6 (100%) |
Characteristics of LTx patient BAL fluid samples included in the analysis of CXCL8 proteoforms (cohort 2). Continuous variables are presented as median (interquartile range). Categorical variables are presented as counts (percentage). BAL fluids of different patient categories were statistically compared by Kruskal-Wallis tests with Dunn’s multiple comparisons tests. Fisher’s exact tests were used to compare proportions
Abbreviations: Azathio, Azathioprine; BAL, broncho-alveolar lavage; CF, cystic fibrosis; CLAD, chronic lung allograft dysfunction; COPD, chronic obstructive pulmonary disease; Cyclo, cyclosporine A; ILD, interstitial lung diseases; LB, lymphocytic bronchiolitis; MMF, mycophenolate mofetil; NA, not applicable; POD, post-operative day; PPH, primary pulmonary hypertension; Tacro, tacrolimus
* p < 0.05; ** p < 0.01; **** p < 0.0001 vs. BAL-CLAD. #p < 0.05; ##p < 0.01 vs. BAL-Infection
Sample collection & processing
Venous peripheral blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-coated vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA). BAL fluid samples were obtained by bronchoscopic instillation of two aliquots of 50 mL sterile saline in the right middle lobe or lingula of the lung. Returned fractions were immediately pooled and centrifuged for 8 min at 500 g. Supernatants were stored at -80 °C until use in experiments. The BAL fluid cell pellet was used for flow cytometry and microscopy analyses (vide infra).
Immunomagnetic isolation of peripheral blood neutrophils
Peripheral blood neutrophils used for phenotypical characterization were isolated from freshly collected blood by immunomagnetic negative selection according to the manufacturer’s instructions (EasySep Direct Human Neutrophil Isolation Kit; STEMCELL Technologies, Vancouver, Canada), as described in detail in [22].
Phenotypical analysis of neutrophils
The maturation and activation state of the isolated peripheral blood or BAL fluid neutrophils were analyzed by flow cytometry. Cells were incubated with 1:1000 diluted Zombie Aqua 516 (BioLegend, San Diego, CA, USA) or 1:20,000 diluted Fixable Viability Stain 620 (BD Biosciences) and 1:20 diluted Fc receptor block (Miltenyi Biotec, Bergisch Gladbach, Germany) in PBS for 15 min at room temperature. Subsequently, cells were washed with flow cytometry buffer [PBS + 2% (v/v) fetal calf serum (FCS) + 2 mM EDTA] and stained with fluorescently-labeled antibodies, which were titrated in-house and listed in Supplementary Table 2. Following incubation for 25 min at 4 °C, cells were washed with flow cytometry buffer and fixed with BD Cytofix (BD Biosciences). Results were analyzed using Diva software (BD Biosciences) on a BD LSRFortessa X-20 (BD Biosciences). FlowJo software (BD Biosciences) was used for downstream analysis. Neutrophils were gated as CD16+CD66b+ cells within the population of live, single cells (Supplementary Fig. 1) and membrane marker expression was analyzed (Supplementary Figs. 1–2).
Peripheral blood neutrophil morphology and maturation were examined by microscopy using cytospin preparations. Therefore, cell suspensions (50,000 cells in PBS) were loaded into cytospin funnels and centrifuged at 82 g for 8 min on a microscopic slide (Shandon Cytospin 2; Marshall Scientific, Hampton, NH, USA). Afterwards, these slides were immersed consecutively in Hemacolor Solutions 1, 2 and 3 (Merck, Darmstadt, Germany) to fix and stain the cells for microscopy analysis.
Quantification of total CXCL8 and neutrophil elastase by ELISA
Total CXCL8 concentrations were determined using a specific sandwich ELISA: 0.5 µg/mL polyclonal rabbit anti-human CXCL8 (#500-P28; PeproTech, Cranbury, NJ, USA) and 0.2 µg/mL biotinylated polyclonal rabbit anti-human CXCL8 antibodies (#500-P28BT; PeproTech) with HRP-conjugated streptavidin (R&D Systems, Minneapolis, MN, USA) were used for coating and detection, respectively. Both antibodies were titrated in-house. Detection was obtained through catalysis of 3,3’,5,5’-tetramethylbenzidine (TMB) substrate and quantified via spectrophotometry at 450 nm. Recombinant human CXCL8(6–77) (#200-08 M; PeproTech) was used as a standard and PBS containing 0.1% (w/v) casein and 0.05% (v/v) Tween20 was used as blocking buffer. Incubations of the coating antibody in PBS were performed overnight at 4 °C, while other incubations were performed in blocking buffer at 37 °C. Neutrophil elastase was quantified using a commercially available DuoSet ELISA (#DY9167-05; R&D Systems) according to the manufacturer’s instructions.
Immunosorbent isolation of CXCL8 from patient samples
Streptavidin-coated magnetic beads (0.25 mg Dynabeads M-280 Streptavidin; Invitrogen, Waltham, MA, USA) were coupled to 5 µg of biotinylated polyclonal rabbit anti-human CXCL8 antibodies (#500-P28BT; PeproTech) during 30 min at room temperature while rotating. Antibody-bound beads were then washed four times with PBS using a DynaMag-2 magnet (Invitrogen). Subsequently, 500 µL BAL fluid (for endogenous CXCL8 purification) or 10 µL BAL fluid spiked with 125 ng CXCL8 (vide infra) were added to the beads and incubated for 30 min at room temperature under constant rotation. During the incubation, 1% (w/v) bovine serum albumin (BSA) and 0.01% (v/v) 4-nitrophenyl β-D-glucuronide were added to prevent loss of CXCL8 due to adherence to plastic. After four washes with PBS, CXCL8 proteoforms were eluted with 0.1 M glycine (pH 2.8) in an elution volume of 20 µL.
Nano-scale liquid chromatography–top-down tandem mass spectrometry (nano-LC-MS/MS)
Elution fractions from immunosorbent isolation of CXCL8 were centrifuged at 17,000 g for 5 min, after which the supernatant was transferred to 1% (v/v) human serum albumin (HSA)-coated tubes. Five µL of sample were injected onto an UltiMate 3000 nano-scale reversed phase-ultra-high-performance liquid chromatography system (RP-UPLC; Thermo Scientific, Merelbeke, Belgium) from a cooled autosampler (5 °C). Samples were loaded on a PepMap 300 C4 capillary pre-column (5 × 0.30 mm; Thermo Scientific) in 4% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid at a flow rate of 20 µL/minute to allow for initial protein separation and desalting. Further separation and elution were obtained with a Proto 300 C4 nano UPLC column (50 × 0.15 mm; Higgins Analytical, Mountain View, CA, USA) and an increasing acetonitrile gradient in 0.1% (v/v) formic acid at a flow rate of 0.5 µL/minute. The nano UPLC column was directly connected to a captive spray ionization – ion trap AmaZon Speed ETD mass spectrometer (Bruker Daltonics, Bremen, Germany). Precursor ions for each CXCL8 proteoform were selected based on their specific mass-to-charge (m/z) values [± 2 m/z in multiple reaction monitoring (MRM) mode] and fragmented through collision-induced dissociation (CID), with the MS/MS fragmentation amplitude set at 1.0. Data were collected with the HyStar3.2 and Trap Control 8.0 software (Bruker) and analyzed with the Bruker Compass Data Analysis 5.0 software. An overview of the unique precursor ions, characteristic signature fragment ions and protein sequences of the different CXCL8 proteoforms can be found in [16] and in Supplementary Fig. 3.
Incubation of exogenous CXCL8 in BAL fluid samples & inhibition of proteolysis
To investigate proteolytic processing of CXCL8 in the presence of BAL fluids, 125 ng of recombinant human CXCL8(1–77) (#200-08; PeproTech) or CXCL8(6–77) (#200-08 M; PeproTech) was added (“spiked”) to 10 µL of BAL fluid supernatant and incubated at 37 °C for 3 h or 16 h. Tubes were pre-coated with 1% (w/v) BSA to prevent binding of CXCL8 to plastic. After incubation, CXCL8 proteoforms were purified and analyzed by nano-LC-MS/MS (vide supra). Proteolytic processing during incubation was inhibited by addition of 100 mM EDTA or 1 mg/mL Pefabloc SC - AEBSF (Merck).
Statistics
Data are displayed as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual patient sample, and statistically analyzed by Kruskal-Wallis tests with Dunn’s multiple comparisons tests or Mann-Whitney U tests. Fisher’s exact tests were used to compare proportions. Correlation analyses were performed by calculating Spearman correlation coefficients and plotted utilizing simple linear regression lines. Statistical tests for comparison were 2-sided and p < 0.05 was considered significant. Statistical analysis and visualization of the data was performed using GraphPad Prism 9 (GraphPad Software; Boston, MA, USA).
Study approval
Written informed consent was obtained from all study participants or their legal representatives according to the ethical guidelines of the Declaration of Helsinki. The Ethics Committee of the University Hospitals Leuven approved this study (S63881, S51577, S61168, S58418, S63357).
Results
Patient characteristics for the neutrophil phenotype cohort
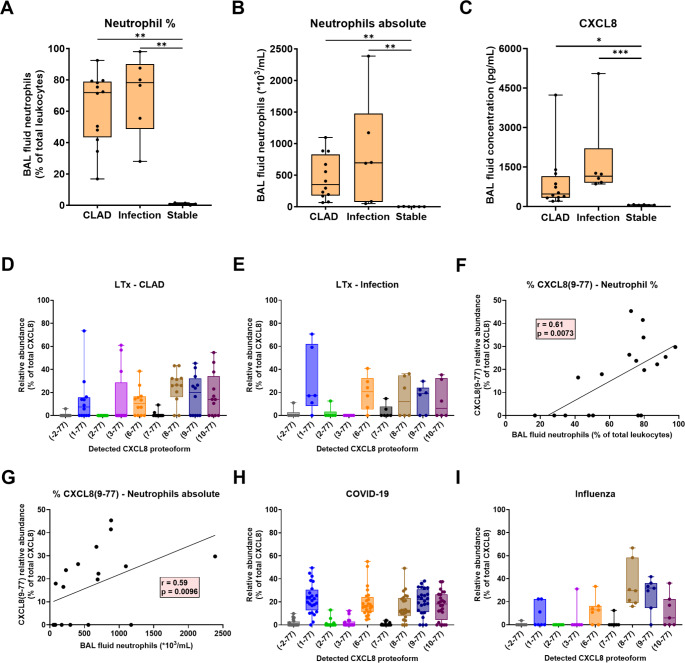
To examine the neutrophil phenotype, peripheral blood and BAL fluid samples were collected prospectively from LTx patients with CLAD, stable lung transplant recipients or LTx patients with pulmonary infection (Fig. 1A). Within the CLAD category, most patients were diagnosed with an obstructive phenotype of CLAD i.e. bronchiolitis obliterans syndrome (BOS). Only 1/8 of the BAL fluid and 1/6 of the peripheral blood samples were derived from patients with a restrictive CLAD phenotype i.e. restrictive allograft syndrome (RAS). No significant differences were observed in age, sex and underlying disease and almost no acute rejection or lymphocytic bronchiolitis was observed on biopsies taken at the time of BAL fluid or blood sampling (Table 1). Medication use was also not significantly different across the patient groups, except for treatment with montelukast for CLAD patients. Patients with CLAD were also sampled at later post-operative time points compared to infected or stable LTx patients, which is to be expected given the increased morbidity of CLAD at higher post-operative days (PODs) [1]. Total and differential blood leukocyte counts were comparable for all patient groups (Table 1; Fig. 2). In the BAL fluids, no significant differences were noted for BAL fluid return, total cells, lymphocyte and eosinophil counts. Macrophages were significantly decreased in percentage, but not in absolute numbers, in CLAD and infected relative to stable LTx patients. Finally, the percentage and absolute counts of neutrophils in the BAL fluids of CLAD and infected LTx patients were significantly increased compared to stable LTx recipients (Table 1; Fig. 2).
Fig. 2.
BAL fluid and blood neutrophil counts from LTx patients included for phenotypical neutrophil analysis. BAL fluid and peripheral blood samples were collected from CLAD (n = 8 BAL fluids & 6 blood samples), infected (n = 16 BAL fluids & 15 blood samples) and stable LTx patients (n = 9 BAL fluids & 21 blood samples). Data are shown as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual patient sample, and statistically analyzed by Kruskal-Wallis tests with Dunn’s multiple comparisons tests for comparisons between BAL fluids or blood samples of different patient categories: * p < 0.05; ** p < 0.01; *** p < 0.001
Heterogeneity in neutrophil maturity in the circulation and lungs of LTx patients
BAL fluid cells and purified peripheral blood neutrophils were subjected to multicolor flow cytometry and microscopy analysis to examine neutrophil maturity. Compared to cells from healthy donors, a (significantly) increased yet highly variable number of immature neutrophils – featured by a lack of membrane expression of CD10 [23] – was detected in both BAL fluids and blood of CLAD, infectious and stable LTx patients (Fig. 3A). No significant differences were observed between the different patient groups. Lower expression levels of the low-affinity Fcγ receptor, i.e. FcγRIII (CD16), and the adhesion molecule L-selectin (CD62L) were detected on the blood neutrophils of the LTx patients compared to healthy controls (Fig. 3B, C & Supplementary Fig. 4A). In conjunction with CD10, these two markers can be exploited to distinguish between mature (3–4 nuclear lobes) neutrophils (CD10+CD16highCD62Lhigh), hypersegmented (> 4 nuclear lobes) neutrophils (CD10+CD16highCD62Ldim) and immature neutrophils (CD10−CD16dimCD62Lhigh) [24, 25]. Confirming the data regarding CD10 expression levels, a minor but significantly increased percentage of immature neutrophils was observed in the blood of stable and infected LTx patients compared to healthy controls, showing fully mature circulating neutrophils (Fig. 3D, E). Interestingly, a significantly increased fraction of the mature neutrophils in LTx patients were characterized as hypersegmented cells, which has been associated with a hypermature, aged neutrophil phenotype (Fig. 3F) [26]. The marked variability in neutrophil maturity between different patients, regardless of the disease group, was also microscopically visible. Some LTx patient blood samples exhibited high numbers of immature neutrophils with a banded nucleus and even early neutrophil precursors, i.e. metamyelocytes. Hypermature, hypersegmented neutrophils were observed in other samples, correlating to the expression levels of CD10, CD62L and CD16 (Fig. 3G-I). Despite this considerable inter-patient variability in neutrophil maturity, no direct correlation could be established between the maturation state in the blood and the type or dosage of medication nor disease severity.
Fig. 3.
Phenotypical analysis of the maturation of BAL fluid and blood neutrophils from LTx patients. BAL fluid and peripheral blood samples were collected from CLAD (n = 8 BAL fluids & 6 blood samples), infected (n = 16 BAL fluids & 15 blood samples) and stable LTx patients (n = 9 BAL fluids & 21 blood samples), as well as healthy controls (HC, n = 17 blood samples). (A-F) Flow cytometry was used to evaluate the membrane expression of (A) CD10, (B) CD16 and (C) CD62L on BAL fluid and blood neutrophils (gated as CD16+CD66b+ cells). (D-F) Based on CD16 and CD62L expression, blood neutrophils were characterized as immature, mature or hypersegmented cells. (G-I) Representative microscopy images of blood neutrophils from a (G) healthy volunteer or from a LTx patient with (H) immature or (I) hypersegmented neutrophils, as indicated by arrows. The neutrophil expression levels of CD10, CD16 and CD62L of these individuals are indicated below the pictures. (J-L) Membrane expression of (J) CXCR4 or (K,L) CXCR4 in combination with CD10 to classify CXCR4+ BAL fluid neutrophils as immature or aged. Results represent percentages of positive neutrophils or median fluorescence intensity (MFI). Data are shown as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual patient sample, and statistically analyzed by Kruskal-Wallis tests with Dunn’s multiple comparisons tests for comparisons between BAL fluids or blood samples of different patient categories: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Mann-Whitney U tests were performed for BAL fluid-blood comparisons per patient category: $$ p < 0.01; $$$ p < 0.001; p < 0.0001 vs. blood. Some BAL fluid samples could not be included in the flow cytometry analysis for every marker, due to shortage of neutrophils
The reduced CD10 expression levels also suggested the presence of some immature neutrophils in the BAL fluids of the LTx patients (Fig. 3A). The chemokine receptor CXCR4 is typically present on immature neutrophils in the bone marrow [27], but can also be upregulated on aged neutrophils [28]. In the LTx patient BAL fluids, up to 21% of neutrophils expressed CXCR4 (Fig. 3J). Since CD62L expression is very low or absent on BAL fluid neutrophils (Fig. 3C & Supplementary Fig. 4A), CXCR4 was used in conjunction with CD10 to distinguish immature (CXCR4+CD10−) from aged (CXCR4+CD10+) neutrophils. Both subsets were found in the BAL fluids of the LTx patients (Fig. 3K, L). A significant negative correlation was found between the administered dose of tacrolimus and the percentage of CD10+ mature neutrophils in the BAL fluid (Supplementary Fig. 5A). However, this correlation was not observed with the numbers of mature peripheral blood neutrophils. Furthermore, no correlations were found with other immunosuppressive drugs or disease severity. In conclusion, our findings reveal a high level of heterogeneity in neutrophil maturation in the blood and BAL fluids of LTx patients, regardless of type or severity of disease, ranging from immature to aged, hypersegmented neutrophils.
Uniform activation phenotype of neutrophils in the lungs of stable, CLAD and infected LTx patients
Using flow cytometry, we also investigated the presence of activation markers and chemoattractant receptors on neutrophils. Expression levels of the neutrophil degranulation markers CD66b and CD11b and the integrin chain CD11c were significantly increased on BAL fluid in comparison to blood neutrophils of LTx patients with the exception of CD11b in stable LTx recipients (Fig. 4A-C). The selectin ligand CD15 (Sialyl-LewisX) only exhibited a trend to be upregulated on neutrophils in BAL fluids of LTx patients with infection (Fig. 4D). Instead, CD15 expression was significantly increased on blood neutrophils from LTx patients with infection or CLAD compared to stable LTx recipients, but no significant differences with healthy controls were observed. Neutrophil chemoattractant receptors CXCR1, CXCR2 and complement receptor C5aR were all significantly downregulated on BAL fluid compared to blood neutrophils in the different disease categories of LTx patients (Fig. 4E-G & Supplementary Fig. 4B-D). Higher administered doses of oral corticosteroids were associated with higher remaining expression levels of CXCR2 and C5aR on BAL fluid neutrophils (Supplementary Fig. 5B, C), but not on peripheral blood neutrophils. Corticosteroids or other administered drugs also did not correlate with changes in expression of degranulation markers on the neutrophils. The increased degranulation and upregulation of integrins, along with the internalization of chemoattractant receptors upon ligand-induced stimulation, suggest neutrophil activation in the lungs of all LTx patients [8, 29, 30]. However, no significant differences were observed between the different patient groups, indicating a uniform neutrophil activation phenotype in the lungs. The partial downregulation of CXCR2 on blood neutrophils of all LTx patients and the significantly increased CD66b expression on blood neutrophils of LTx patients with infection, compared to healthy controls, may indicate that patient neutrophils already become partially activated in circulation (Fig. 4A, F). However, a partial downregulation of CXCR2 may also be a sign of neutrophil aging [28], and unlike in COVID-19 patients, no internalization of the complement receptor C5aR was observed on circulating neutrophils of LTx patients [31]. Furthermore, the formyl peptide receptors FPR1 and FPR2 and, to a minor extent, CCR1 (but not CCR2) – which both are normally not expressed by neutrophils - were upregulated on BAL fluid neutrophils (Fig. 4H-J & Supplementary Fig. 4E). Minor upregulation of CCR1 on neutrophils in BAL fluid may be explained by the presence of IFN-γ which has been reported to upregulate CCR1 on neutrophils [32] and was detected in BAL fluids from lung transplant patients in a previous report [17]. Conversely, the decoy interleukin receptor IL1-R2 was significantly downregulated on BAL fluid neutrophils (Fig. 4K). Additionally, a small population of neutrophils expressed the antigen-presenting molecule HLA-DR (Fig. 4L), similar to what was observed on neutrophils from COVID-19 patients [21, 33]. No expression of the chemokine receptor CXCR3 and the active IL-1 receptor, i.e. IL1-R1, was detected on the LTx patient neutrophils. For some patients, paired BAL fluid and peripheral blood samples could be obtained. Flow cytometry analysis on these samples led to similar observations (Supplementary Fig. 6). Finally, in the BAL fluids of the LTx patients, we measured concentrations of neutrophil elastase, an important protease released during neutrophil degranulation (activation). Interestingly, neutrophil elastase levels were significantly increased in CLAD and infected compared to stable LTx patients (Fig. 4M) and correlated to the number of BAL fluid neutrophils (Fig. 4N, O). In conclusion, neutrophils in the BAL fluids of LTx patients exhibit a uniform, activated phenotype regardless of the disease state (stable, CLAD or infected) or immunosuppressive treatment of the patient. The augmented inflammation in LTx patients with CLAD or infection compared to stable LTx recipients may therefore be mostly related to increased neutrophil recruitment to the lungs, rather than an enhanced activation of individual cells.
Fig. 4.
Phenotypical analysis of activation markers and receptors on BAL fluid and blood neutrophils from LTx patients. BAL fluid and peripheral blood samples were collected from CLAD (n = 8 BAL fluids & 6 blood samples), infected (n = 16 BAL fluids & 15 blood samples) and stable LTx patients (n = 9 BAL fluids & 21 blood samples), as well as healthy controls (HC, n = 17 blood samples). Flow cytometry was used to evaluate the membrane expression of (A) CD66b, (B) CD11b, (C) CD11c, (D) CD15, (E) CXCR1, (F) CXCR2, (G) C5aR, (H) FPR1, (I) FPR2, (J) CCR1, (K) IL-1R2 and (L) HLA-DR on BAL fluid and blood neutrophils (gated as CD16+CD66b+ cells). Results represent percentages of positive neutrophils or median fluorescence intensity (MFI). (M) Neutrophil elastase levels in the BAL fluid supernatants were determined by ELISA. (N, O) Correlation between neutrophil elastase concentrations and the percentage and absolute numbers of neutrophils in the LTx BAL fluid samples (CLAD, infection and stable combined). Data are shown as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual patient sample, and statistically analyzed by Kruskal-Wallis tests with Dunn’s multiple comparisons tests for comparisons between BAL fluids or blood samples of different patient categories: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Mann-Whitney U tests were performed for BAL fluid-blood comparisons per patient category: $ p < 0.05; $$ p < 0.01; $$$ p < 0.001; p < 0.0001 vs. blood. Correlation analysis was performed calculating a Spearman correlation coefficient and plotted utilizing a simple linear regression line. Some BAL fluid samples could not be included in the flow cytometry analysis for every marker, due to shortage of neutrophils
Patient characteristics for the CXCL8 proteoform cohort
To provide insight into the putative mechanisms underlying neutrophil accumulation in the lungs of the LTx patients, we performed differential quantification of CXCL8 proteoforms known to possess up to 30-fold differences in activity. CXCL8 proteolysis was assessed in BAL fluid supernatants from a second cohort of CLAD, infected and stable LTx patients, as well as in COVID-19 and influenza patients (Fig. 1B). No significant differences were observed in age, sex, underlying disease, POD of sampling or medication use between the different patient groups (Table 2). However, total cell counts and both neutrophil percentages and absolute counts were significantly increased in the BAL fluids of LTx patients with CLAD or infection compared to stable lung transplant recipients (Fig. 5A, B). It is important to note that for CLAD and infected LTx patients, neutrophil-rich BAL fluid samples (> 15% neutrophils) were specifically selected, to be able to determine endogenous CXCL8 proteoforms. Detailed clinical data for the LTx patients can be found in Table 2. Clinical data of the COVID-19 and influenza patients are described in Supplementary Table 1.
Fig. 5.
Detection of endogenous CXCL8 proteoforms in BAL fluid. (A-C) Neutrophil counts and CXCL8 levels in BAL fluid samples from CLAD (n = 12), infected (n = 6) and stable LTx patients (n = 6) used for CXCL8 proteoform characterization. (D,E) Endogenous CXCL8 proteoforms were determined by ISTAMPA in the BAL fluid supernatants of LTx patients with CLAD or infection. Total CXCL8 levels were below the detection limit for ISTAMPA analysis in stable LTx recipients. CXCL8 proteoforms were presented according to their relative abundance in the BAL fluids, expressed as percentage of total CXCL8. (F,G) Correlation between the relative abundance of the most potent proteoform CXCL8(9–77) and the percentage and absolute numbers of neutrophils in the LTx BAL fluid samples (CLAD and infection combined). (H,I) ISTAMPA analysis of endogenous CXCL8 proteoforms in COVID-19 (n = 24) and influenza (n = 7) BAL fluid supernatants. Data are shown as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual BAL fluid sample, and statistically analyzed by Kruskal-Wallis tests with Dunn’s multiple comparisons tests: * p < 0.05; ** p < 0.01; *** p < 0.001. Correlation analysis was performed calculating a Spearman correlation coefficient and plotted utilizing a simple linear regression line
Detection of endogenous CXCL8 proteoforms in the lungs of LTx, COVID-19 and influenza patients
Similar to the increased neutrophil numbers, total CXCL8 levels were significantly elevated in the BAL fluids of LTx patients with CLAD and even more upon infection in comparison to stable LTx recipients (Fig. 5C). To characterize the specific CXCL8 proteoforms in these BAL fluid samples, we utilized the ISTAMPA method, which combines immunosorbent pre-purification of total CXCL8 (i.e. all CXCL8 proteoforms) with sensitive nano-LC-MS/MS to identify individual natural CXCL8 proteoforms [16]. In the BAL fluids of both individuals with CLAD and LTx patients with infection, CXCL8 was NH2-terminally truncated, with intact CXCL8(1–77) representing only a median of 5–20% of the total CXCL8 content (Fig. 5D, E). Apart from the full-length protein, the NH2-terminally truncated proteoforms CXCL8(6–77), CXCL8(8–77) and CXCL8(9–77) - known to exhibit 10 to 30-fold superior biological activity - were most abundant. No significant differences in CXCL8 proteoforms were found between the LTx patients with CLAD or infection. Importantly, a significant positive correlation was observed between the most active proteoform, i.e. CXCL8(9–77), and both the percentage as well as absolute counts of neutrophils in the BAL fluids of the LTx patients (Fig. 5F, G). This correlation was not observed for other CXCL8 proteoforms. Interestingly, in some BAL fluid samples, CXCL8(10–77) was detected, a proteoform never identified before in biological samples. Although presumed to be inactive due to proteolytic cleavage within the ELR motif [34], it was reported to exhibit some remaining receptor affinity and in vitro chemotactic activity, while its capacity for inducing neutrophil elastase release is strongly impaired [13]. Finally, in the BAL fluids of COVID-19 and influenza patients (Fig. 5H, I) and similar to LTx patients, CXCL8 was also mostly cleaved towards its more active proteoforms CXCL8(6–77), CXCL8(8–77) and CXCL8(9–77) and the presumably inactive proteoform CXCL8(10–77), suggesting CXCL8 proteolysis may be important during both chronic and acute neutrophilic inflammation. An overview of the top-down MS/MS spectra of detected CXCL8 proteoforms in the BAL fluids is provided in Supplementary Fig. 7. Total levels of CXCL8 are shown in Supplementary Fig. 8. In conclusion, our study demonstrates that CXCL8 consists of highly potent truncated CXCL8 proteoforms in LTx patients with CLAD and infection, as well as in patients with COVID-19 or influenza. The most potent proteoform CXCL8(9–77) thereby specifically correlated with the neutrophil counts in the LTx BAL fluids. An amplification loop, wherein (neutrophil-derived) proteases process CXCL8 into more active proteoforms to increase neutrophil chemotaxis and activation, therefore likely contributes to the excessive neutrophilic inflammation in these diseases.
Proteolytic processing of CXCL8 in BAL fluids & inhibition of proteolysis
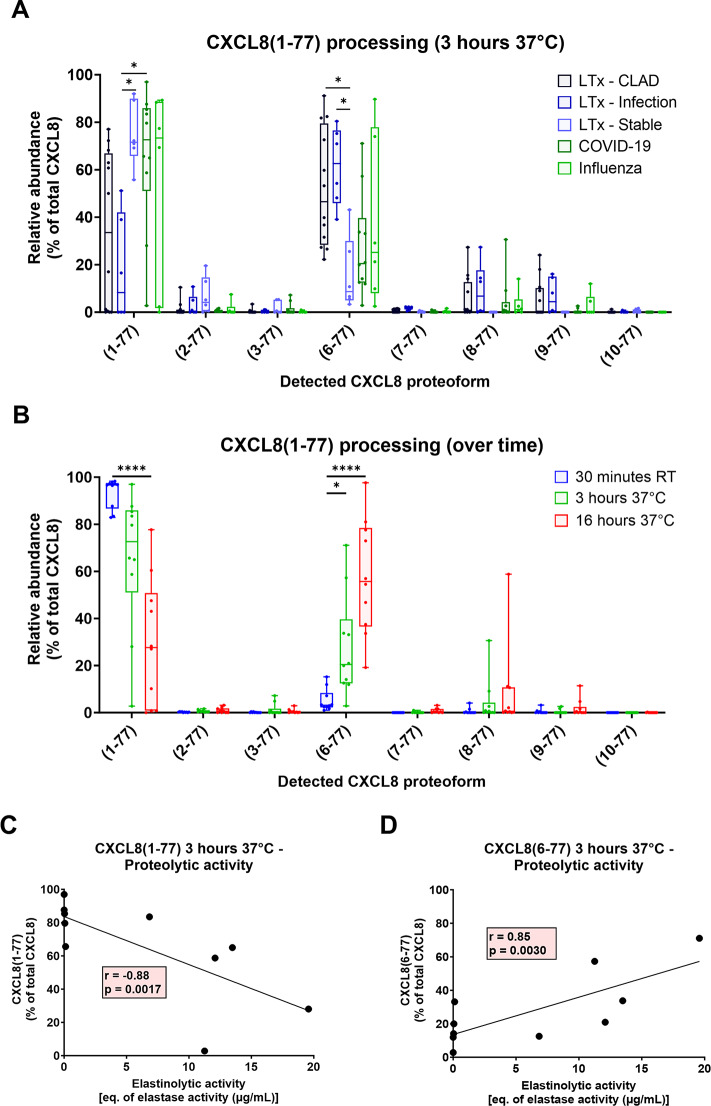
Many proteases have been reported to cleave the NH2-terminus of CXCL8 in vitro [15, 35–40]. To further understand how these CXCL8 proteoforms are generated in patients, intact CXCL8(1–77) was spiked into a small volume of LTx BAL fluid samples. After 3 h of incubation at 37 °C, allowing proteases present in the BAL fluids to process CXCL8, proteoforms were analyzed by ISTAMPA. In the LTx patients with CLAD or infection, proteolytic processing predominantly yielded CXCL8(6–77), in addition to minor amounts of the most potent proteoforms CXCL8(8–77) and CXCL8(9–77). In BAL fluids of stable LTx recipients, where endogenous CXCL8 concentrations were below the detection limit of ISTAMPA, processing of intact CXCL8(1–77) occurred significantly slower, and CXCL8(8–77) and CXCL8(9–77) were not generated (Fig. 6A). Notably, CXCL8(10–77) – which was highly abundant in the BAL fluid samples (Fig. 5) – could not be generated from intact CXCL8(1–77) within 3 h. In the COVID-19 and influenza patients, comparable proteolytic processing was observed although it tended to be slower in comparison to the CLAD and infected LTx patients (Fig. 6A). In COVID-19 BAL fluids, we noticed that shorter or longer (up to 16 h) incubation times only resulted in, respectively, decreased or increased processing towards more potent CXCL8(6–77) and the shorter CXCL8(8–77) and CXCL8(9–77) proteoforms but not to the less potent CXCL8(10–77) form (Fig. 6B). Furthermore, enhanced proteolytic (elastinolytic) activity in the COVID-19 BAL fluid samples [21] accelerated the processing speed of CXCL8(1–77) towards CXCL8(6–77) (Fig. 6C, D).
Fig. 6.
Proteolytic processing of intact CXCL8 in BAL fluid. (A) Recombinant human CXCL8(1–77) [125 ng] was spiked in 10 µL of BAL fluid supernatant from CLAD (n = 12), infected (n = 6) or stable LTx patients (n = 6) and from patients with COVID-19 (n = 10) or influenza (n = 7). After incubation for 3 h at 37 °C, CXCL8 proteolysis was analyzed by ISTAMPA. (B) CXCL8 proteoforms were also determined immediately after spiking CXCL8 [without 37 °C incubation but including the required 30 min pre-purification of the CXCL8 proteoforms at room temperature (RT) as part of the ISTAMPA procedure] and after 16 h of incubation at 37 °C in the COVID-19 BAL fluids. CXCL8 proteoforms were presented according to their relative abundance in the BAL fluids, expressed as percentage of total CXCL8. (C,D) Correlation between the relative abundance of the intact CXCL8(1–77) or the truncated CXCL8(6–77) proteoform after 3 h incubation at 37 °C and the elastinolytic activity in the COVID-19 BAL fluid samples [21]. Data are shown as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual BAL fluid sample, and statistically analyzed by Kruskal-Wallis tests with Dunn’s multiple comparisons tests: * p < 0.05; **** p < 0.0001. Correlation analysis was performed calculating a Spearman correlation coefficient and plotted utilizing a simple linear regression line
CXCL8(8–77) and CXCL8(9–77) may, at least partially, be products of further processing from the CXCL8(6–77) proteoform. To investigate this, CXCL8(6–77) was incubated in BAL fluid samples from both COVID-19 and LTx patients for 3 h at 37 °C and indeed, proteolysis towards CXCL8(7–77), CXCL8(8–77) and CXCL8(9–77) was observed. This processing was mediated by metalloproteases, as it could be inhibited by EDTA (Fig. 7A). Finally, as a proof-of-concept experiment, we aimed to block the initial processing of intact CXCL8(1–77) towards CXCL8(6–77) and the shorter proteoforms, as this has potential to reduce CXCL8 activity and consequent neutrophil chemotaxis and activation. Inhibition of serine proteases or metalloproteases with AEBSF or EDTA, respectively, could partially prevent NH2-terminal proteolysis (Fig. 7B). However, when both inhibitors were used together, near-complete inhibition of processing was achieved, suggesting that a combination of serine proteases and metalloproteases are involved in the processing of intact CXCL8 in the patient lungs. In conclusion, our findings show rapid proteolytic activation of CXCL8 in BAL fluids from LTx patients with CLAD or infection. Stable LTx recipients not only have very low total CXCL8 levels in their lungs, but also exhibit slower CXCL8 processing, which may explain their reduced airway neutrophilia. Targeting endogenous proteases in the lungs could be a promising therapeutic strategy to reduce CXCL8 processing, thus limiting excessive neutrophil infiltration, activation and inflammation, and should be further investigated in vivo.
Fig. 7.
Inhibition of proteolytic processing of CXCL8 in BAL fluid. (A) Recombinant human CXCL8(6–77) [125 ng] was spiked in 10 µL of BAL fluid supernatant (n = 3–6) from COVID-19 (indicated by dots) and CLAD patients (indicated by triangles) and incubated for 3 h at 37 °C, in the absence or presence of the metalloprotease inhibitor EDTA. (B) Recombinant human CXCL8(1–77) [125 ng] was spiked in 10 µL of BAL fluid supernatant (n = 8–9) from COVID-19 (dots) and CLAD patients (triangles) and incubated for 3 h at 37 °C, in the absence or presence of the serine protease inhibitor AEBSF or/and the metalloprotease inhibitor EDTA. After incubation, CXCL8 proteolysis was determined by ISTAMPA. All CXCL8 proteoforms were presented according to their relative abundance in the sample, expressed as percentage of total CXCL8. Data are shown as box-and-whisker plots (box: median with interquartile range, whiskers: full data distribution), with each dot representing an individual BAL fluid sample, and statistically analyzed by Mann-Whitney U tests or Kruskal-Wallis tests with Dunn’s multiple comparisons tests: * p < 0.05; *** p < 0.001; **** p < 0.0001
Discussion
Long-term survival of LTx patients is compromised by the development of CLAD, the end-stage of a disease continuum characterized by repeated lung injury, inflammation, tissue remodeling and repair, and ultimately leading to irreversible fibrosis and allograft failure [3]. Numerous studies have associated neutrophils with chronic inflammation in CLAD as part of a complex and interacting innate and adaptive immune cell response. Both neutrophil percentages, absolute numbers and CXCL8 levels have been shown to be increased in BAL fluids and around the airways of CLAD in comparison to stable LTx recipients [18, 41]. The importance of neutrophils within CLAD was demonstrated at first with the neomacrolide antibiotic azithromycin, which reduced airway neutrophilia and CXCL8 in some CLAD patients, leading to an attenuation in lung function decline [19] or preventing the development of CLAD when administered prophylactically [42]. Unfortunately, some patients do not respond to azithromycin treatment, (re-)develop high airway neutrophilia and experience worse CLAD-free and overall survival [20]. The main function of neutrophils in the lungs of LTx patients is the clearance of pathogens, though they may also participate in the alloreactive immune response. Nevertheless, it is probably the release of toxic chemicals and degradative enzymes that is the main contribution to the uncontrolled chronic graft injury and inflammation in progressing CLAD [43]. Microbial infection is therefore an important risk factor contributing to CLAD development [44]. Reducing neutrophils in the lungs to a level were pathogen-eliminating functions are maintained but harmful inflammatory collateral tissue damage is diminished may have significant pharmacological potential [45].
A detailed study focusing on the phenotypical characteristics of neutrophils in LTx patients was not performed yet. Therefore, we investigated the expression levels of established maturation and activation markers on the surface of neutrophils present in blood and BAL fluid of LTx patients with CLAD or infection and stable LTx recipients. Through flow cytometry and microscopy, we observed substantial patient variability in maturity of blood and BAL fluid neutrophils, ranging from immature to aged, hypersegmented neutrophils. The percentage of immature blood neutrophils in infected and stable LTx patients was significantly increased compared to healthy controls. However, the majority of blood neutrophils (> 90%) were still mature, in contrast to critically ill COVID-19 patients, where only 30–70% of neutrophils were mature, as we previously reported [21, 31]. These high levels of immature neutrophils in COVID-19 were indicative for emergency myelopoiesis [46, 47]. A similar, though less severe, situation may be occurring in LTx patients with infection. Immature neutrophils in the blood of some stable LTx patients could be representing a population of immunosuppressive granulocytic-myeloid-derived suppressor cells (G-MDSCs). These cells were shown to be specifically enriched in stable LTx patients, but due to the lack of specific markers we could not distinguish these cells from normal neutrophils [5, 48]. Immature progenitor neutrophils were also observed in the BAL fluid of the LTx patients, similar to what was already observed in COVID-19 patients [21, 34]. Despite their incomplete nuclear segmentation, immature neutrophils were shown to already possess the capacity to migrate, to potently release reactive oxygen species and to phagocytose bacteria [49–51]. Whether these immature neutrophils did migrate to the lungs or were formed in the lungs as part of extramedullary granulopoiesis [52] is currently unclear. Aged neutrophils, associated with re-upregulation of CXCR4 [28], may be better prepared (‘primed’) than non-aged neutrophils to defend the host against pathogens during an acute inflammatory response [53, 54]. The CD16highCD62Ldim hypersegmented neutrophils detected in the blood of LTx patients are also associated with a state of hypermaturation/aging [26]. However, the exact function of these hypersegmented cells is still a topic of ongoing research, as it was demonstrated that they are capable of suppressing T-lymphocyte proliferation [55] and may even be a specific subset only recruited to the bloodstream in response to acute inflammation [25]. Therefore, not all CD16highCD62Ldim hypersegmented neutrophils may be representing aged neutrophils [4]. In our patient cohort, no direct correlation between the maturation state and the type or dose of medication, nor type or severity of disease could be established, except for a weak positive correlation between higher tacrolimus doses and increased numbers of immature neutrophils in the BAL fluids. However, it is unlikely that tacrolimus, as a T-lymphocyte inhibitor, could directly influence neutrophil maturation in the lungs. The high variability in maturation is likely caused by a combination of physiological, disease and treatment-related factors.
Additionally, neutrophils in the lungs of LTx patients displayed an activated phenotype with upregulation of degranulation markers and integrins (CD66b, CD11b, CD11c) and downregulation of chemoattractant receptors (CXCR1, CXCR2, C5aR). A weak positive correlation was found between the administered dose of corticosteroids and the remaining expression of C5aR and CXCR2 on BAL fluid neutrophils. This may be explained by reduced internalization of these receptors due to diminished ligand stimulation [30]. However, no changes were observed in levels of degranulation markers, suggesting that corticosteroids do not directly alter the activation profile of neutrophils in the lungs of LTx patients. No correlations between the expression levels of these activation markers and disease severity, and no differences in activation phenotype between neutrophils of infected, CLAD or stable LTx patients were found. Consequently, neutrophils are activated in a uniform way upon migration to the lungs. Nevertheless, we detected enhanced levels of the degranulation product neutrophil elastase in the BAL fluids of CLAD and infected compared to stable LTx patients, correlating to the number of neutrophils in the BAL fluids. This not only proves the neutrophil activation in the lungs but also that the increased recruitment of neutrophils to the lungs, rather than phenotypical alterations in the activation state of individual cells, may be mostly related to the enhanced inflammation in LTx patients with CLAD or infection.
Neutrophils are guided towards the inflammatory site by multiple chemoattractants [8], including the most potent human neutrophil-attracting chemokine CXCL8. Discriminating between intact and processed forms of CXCL8 is a prerequisite in dismantling the importance of CXCL8 in the lungs, as truncated CXCL8 proteoforms display an enhanced potency to induce receptor signaling, chemotaxis and enzyme degranulation compared to intact CXCL8(1–77) [12–14]. Therefore, we used our recently developed ISTAMPA methodology to discriminate between different CXCL8 proteoforms [16]. To our knowledge, we demonstrate for the first time the occurrence of site-specific proteolytic modification of CXCL8 in the BAL fluids of LTx patients with CLAD or infection, as well as in COVID-19 and influenza patients, with detection of the known natural CXCL8 proteoforms. By formation of these more potent chemokine forms due to release of CXCL8-truncating proteases, neutrophil recruitment may be enhanced. Indeed, a specific positive correlation between the relative abundance of the most potent proteoform CXCL8(9–77) and the percentage and absolute numbers of neutrophils in the LTx BAL fluid samples was observed. Removal of more than eight NH2-terminal amino acids inevitably results in cleavage in or beyond the ELR motif which is important for the interaction with CXCL8 receptors. Remarkably, we now observed natural CXCL8(10–77) in the lungs of COVID-19, influenza and LTx patients. Previously, CXCL8(10–77) was only generated after incubation of CXCL8 with macrophage metalloelastase (MMP-12) in vitro [56]. Despite its cleavage in the ELR motif, this proteoform retains some capacity to bind CXCL8 receptors and to induce neutrophil chemotaxis in vitro, but has almost completely lost its ability to provoke the release of neutrophil elastase [13]. The in vivo chemotactic activity of this CXCL8 proteoform remains to be determined. Interestingly, all endogenously detected proteoforms could at least be partially obtained by incubation of intact CXCL8(1–77) in BAL fluid supernatants with the exception of CXCL8(10–77). This proteoform may therefore be a product of cell membrane-bound proteases, which were removed by centrifugation of the BAL fluids. Additionally, enhanced proteolytic activity in the BAL fluid samples promoted faster processing towards CXCL8(6–77). Finally, we showed in a proof-of-concept experiment that inhibition of serine proteases and metalloproteases may block the proteolytic activation of CXCL8 in patient samples, which may have therapeutic potential to reduce excessive neutrophil inflammation. Ideally, neutrophil recruitment should be reduced to a level where tissue destruction is minimized without immune-compromising the patient. Indeed, neutrophil-dependent clearance of pathogens and debris (resulting from tissue damage) are beneficial [57]. Therefore, it may be favorable to reduce, rather than to block, CXCL8 activity. This might be achieved by reducing CXCL8 activity through inhibition of proteolytic activation and should therefore be further investigated in vivo.
With our study we could not link the neutrophil phenotypical properties or the presence of specific CXCL8 proteoforms to patient survival or the development of CLAD. To gain further insight, it would be interesting to investigate neutrophils at the time of and just before CLAD diagnosis in a longitudinal study, examining potential changes in activation or maturation signature. Previous research suggested that numbers of BAL fluid neutrophils and CXCL8 concentrations could be predictive for development of CLAD [58, 59], although contradictory findings have been reported by others [43, 60]. Additionally, in this study, no discrimination was made between BOS and RAS for analysis of the neutrophil phenotype of CLAD patients, as only 2 RAS compared to 12 BOS patients could be included in the flow cytometry experiments. For the analysis of CXCL8 proteoforms in the BAL fluids, we did not observe significant differences between BOS and RAS patients. Moreover, precise categorization of CLAD patients is challenging, since undefined and mixed phenotypes are also observed [61]. Finally, we were not able to determine endogenous CXCL8 proteoforms in stable LTx recipients, or in CLAD and infected LTx patients with a low abundancy of neutrophils, as total CXCL8 concentrations were below the detection limit for ISTAMPA analysis. Nevertheless, we showed significantly slower processing of spiked CXCL8 in these samples, suggesting that not only total CXCL8 concentrations are significantly lower in these patients but also the proportions of the further activated proteoforms.
In conclusion, with this study we showed a significant heterogeneity in the maturation state of neutrophils within the lungs and circulation of LTx patients, ranging from immature to aged, hypersegmented cells. Regardless of disease type, severity or treatment, neutrophils exhibit a uniform activation phenotype in the lungs, characterized by upregulation of markers associated with degranulation and downregulation of chemoattractant receptors. This suggests that the number of neutrophils likely plays a more crucial role in disease pathogenesis than their phenotypical activation profile. Moreover, for the first time, we have demonstrated proteolytic activation of CXCL8 within BAL fluids of CLAD and infected LTx patients. Importantly, detection of highly potent truncated CXCL8 proteoforms positively correlated with the neutrophils counts in the lungs. Finally, rapid proteolytic processing of intact CXCL8(1–77) could be inhibited by a combination of serine and metalloprotease inhibitors in vitro. Protease inhibition may therefore be of pharmacological interest to reduce the proteolytic activation of CXCL8 and, consequently, neutrophil recruitment and activation. Further in vivo studies will be required to explore the therapeutic potential of protease inhibitors on neutrophilic inflammation in the lungs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by research grants from Katholieke Universiteit Leuven (KU Leuven; C1 grants C16/17/010 and C14/23/143) and Fonds Wetenschappelijk Onderzoek-Vlaanderen (FWO-Vlaanderen; grant G0F8822N). This work was also supported by a KU Leuven (CONTAGIOUS) and a University Hospitals Leuven (UZ Leuven; KOOR project) internal grant. SC received a PhD fellowship fundamental research (grant number 11A4220N) and FB a PhD fellowship for applied research (SB/1SHBW24N) from FWO-Vlaanderen. RV is supported by FWO-Vlaanderen as senior clinical researcher (1803521 N) and by a research grant (G060322N). The graphical abstract was created with BioRender.com.
Author contributions
SC and PP designed the experiments. SC, FB, AN, MM, NP and ACdC performed the experiments and analyzed the data. MM developed methodology. JK, EEC, HB, CH, LJC, JW, BMV and RV were involved in collection of patient samples and clinical data analysis. SC, FB and AN visualized the data. MG, SS, REM, JW, BMV, RV and PP supervised this study. SC wrote the original draft of this manuscript. All authors reviewed, edited and approved the final version of the manuscript.
Data availability
This study includes no data deposited in external repositories. Data are available from the corresponding author on request.
Declarations
Ethical approval
The Ethics Committee of the University Hospitals Leuven approved this study (S63881, S51577, S61168, S58418, S63357).
Consent to publish
The manuscript contains no individual person’s data.
Consent to participate
Written informed consent was obtained from all study participants or their legal representatives according to the ethical guidelines of the Declaration of Helsinki.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chambers DC, Cherikh WS, Harhay MO et al (2019) The international thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation Report-2019; focus theme: Donor and recipient size match. J Heart Lung Transpl 38:1042–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verleden GM, Glanville AR, Lease ED et al (2019) Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transpl 38:493–503 [DOI] [PubMed] [Google Scholar]
- 3.Bos S, Milross L, Filby AJ et al (2022) Immune processes in the pathogenesis of chronic lung allograft dysfunction: identifying the missing pieces of the puzzle. Eur Respir Rev 31:220060. 10.1183/16000617.0060-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzemaekers M, Malengier-Devlies B, Gouwy M et al (2023) Fast and furious: the neutrophil and its armamentarium in health and disease. Med Res Rev [DOI] [PubMed]
- 5.Deniset JF, Kubes P (2018) Neutrophil heterogeneity: Bona fide subsets or polarization states? J Leukoc Biol 103:829–838 [DOI] [PubMed] [Google Scholar]
- 6.Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P (2019) Neutrophil diversity in Health and Disease. Trends Immunol 40:565–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 8.Metzemaekers M, Gouwy M, Proost P (2020) Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol 17:433–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes CE, Nibbs RJB (2018) A guide to chemokines and their receptors. FEBS J 285:2944–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanheule V, Metzemaekers M, Janssens R et al (2018) How post-translational modifications influence the biological activity of chemokines. Cytokine 109:29–51 [DOI] [PubMed] [Google Scholar]
- 11.Cambier S, Gouwy M, Proost P (2023) The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol [DOI] [PMC free article] [PubMed]
- 12.Metzemaekers M, Vandendriessche S, Berghmans N et al (2020) Truncation of CXCL8 to CXCL8(9–77) enhances actin polymerization and in vivo migration of neutrophils. J Leukoc Biol 107:1167–1173 [DOI] [PubMed] [Google Scholar]
- 13.Clark-Lewis I, Schumacher C, Baggiolini M, Moser B (1991) Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem 266:23128–23134 [PubMed] [Google Scholar]
- 14.Van den Steen PE, Proost P, Wuyts A et al (2000) Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96:2673–2681 [PubMed] [Google Scholar]
- 15.Mortier A, Berghmans N, Ronsse I et al (2011) Biological activity of CXCL8 forms generated by alternative cleavage of the signal peptide or by aminopeptidase-mediated truncation. PLoS ONE 6:e23913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzemaekers M, Abouelasrar Salama S, Vandooren J et al (2021) From ELISA to Immunosorbent Tandem Mass Spectrometry Proteoform Analysis: the Example of CXCL8/Interleukin-8. Front Immunol 12:644725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verleden SE, Ruttens D, Vos R et al (2015) Differential cytokine, chemokine and growth factor expression in phenotypes of chronic lung allograft dysfunction. Transplantation 99:86–93 [DOI] [PubMed] [Google Scholar]
- 18.Bos S, Filby AJ, Vos R, Fisher AJ (2022) Effector immune cells in chronic lung allograft dysfunction: a systematic review. Immunology 166:17–37. 10.1111/imm.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verleden GM, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE (2006) Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 174:566–570. 10.1164/rccm.200601-071OC [DOI] [PubMed] [Google Scholar]
- 20.Vandermeulen E, Verleden SE, Ruttens D et al (2015) BAL neutrophilia in azithromycin-treated lung transplant recipients: clinical significance. Transpl Immunol 33:37–44. 10.1016/j.trim.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 21.Cambier S, Metzemaekers M, de Carvalho AC et al (2022) Atypical response to bacterial coinfection and persistent neutrophilic bronchoalveolar inflammation distinguish critical COVID-19 from influenza. JCI Insight 7:e155055. 10.1172/jci.insight.155055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanter M, Cambier S, De Bondt M et al (2022) Method matters: Effect of Purification Technology on Neutrophil phenotype and function. Front Immunol 13:820058. 10.3389/fimmu.2022.820058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marini O, Costa S, Bevilacqua D et al (2017) Mature CD10 + and immature CD10- neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 129:1343–1356. 10.1182/blood-2016-04-713206 [DOI] [PubMed] [Google Scholar]
- 24.Adrover JM, Del Fresno C, Crainiciuc G et al (2019) A neutrophil timer coordinates Immune Defense and Vascular Protection. Immunity 50:390–402e10. 10.1016/j.immuni.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 25.Tak T, Wijten P, Heeres M et al (2017) Human CD62Ldim neutrophils identified as a separate subset by proteome profiling and in vivo pulse-chase labeling. Blood 129:3476–3485. 10.1182/blood-2016-07-727669 [DOI] [PubMed] [Google Scholar]
- 26.Casanova-Acebes M, Pitaval C, Weiss LA et al (2013) Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153:1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eash KJ, Greenbaum AM, Gopalan PK, Link DC (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 120:2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisel KC, Bautz F, Seitz G et al (2009) Modulation of CXC chemokine receptor expression and function in human neutrophils during aging in vitro suggests a role in their clearance from circulation. Mediators Inflamm 2009:790174. 10.1155/2009/790174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengeløv H, Follin P, Kjeldsen L et al (1995) Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol 154:4157–4165 [PubMed] [Google Scholar]
- 30.Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S (2019) How do chemokines navigate neutrophils to the target site: dissecting the structural mechanisms and signaling pathways. Cell Signal 54:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzemaekers M, Cambier S, Blanter M et al (2021) Kinetics of peripheral blood neutrophils in severe coronavirus disease 2019. Clin Transl Immunol 10:e1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonecchi R, Polentarutti N, Luini W et al (1999) Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J Immunol 162(1):474–479 [PubMed] [Google Scholar]
- 33.Wauters E, Van Mol P, Garg AD et al (2021) Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res 31:272–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hébert CA, Vitangcol RV, Baker JB (1991) Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem 266:18989–18994 [PubMed] [Google Scholar]
- 35.Hébert CA, Luscinskas FW, Kiely JM et al (1990) Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils. J Immunol 145:3033–3040 [PubMed] [Google Scholar]
- 36.Nakagawa H, Hatakeyama S, Ikesue A, Miyai H (1991) Generation of interleukin-8 by plasmin from AVLPR-interleukin-8, the human fibroblast-derived neutrophil chemotactic factor. FEBS Lett 282:412–414 [DOI] [PubMed] [Google Scholar]
- 37.Padrines M, Wolf M, Walz A, Baggiolini M (1994) Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett 352:231–235 [DOI] [PubMed] [Google Scholar]
- 38.Proost P, Mortier A, Loos T et al (2007) Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood 110:37–44 [DOI] [PubMed] [Google Scholar]
- 39.Tester AM, Cox JH, Connor AR et al (2007) LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS ONE 2:e312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Repnik U, Starr AE, Overall CM, Turk B (2015) Cysteine cathepsins activate ELR chemokines and inactivate Non-ELR chemokines. J Biol Chem 290:13800–13811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandermeulen E, Lammertyn E, Verleden SE et al (2017) Immunological diversity in phenotypes of chronic lung allograft dysfunction: a comprehensive immunohistochemical analysis. Transpl Int 30:134–143. 10.1111/tri.12882 [DOI] [PubMed] [Google Scholar]
- 42.Vos R, Vanaudenaerde BM, Verleden SE et al (2011) A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 37:164–172. 10.1183/09031936.00068310 [DOI] [PubMed] [Google Scholar]
- 43.Zheng L, Whitford HM, Orsida B et al (2006) The dynamics and associations of airway neutrophilia post lung transplantation. Am J Transpl 6:599–608. 10.1111/j.1600-6143.2006.01222.x [DOI] [PubMed] [Google Scholar]
- 44.Witt CA, Meyers BF, Hachem RR (2012) Pulmonary infections following lung transplantation. Thorac Surg Clin 22:403–412. 10.1016/j.thorsurg.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boff D, Russo RC, Crijns H et al (2022) The therapeutic treatment with the GAG-Binding chemokine fragment CXCL9(74–103) attenuates neutrophilic inflammation and lung dysfunction during Klebsiella pneumoniae infection in mice. Int J Mol Sci 23:6246. 10.3390/ijms23116246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manz MG, Boettcher S (2014) Emergency granulopoiesis. Nat Rev Immunol 14:302–314. 10.1038/nri3660 [DOI] [PubMed] [Google Scholar]
- 47.Honda T, Uehara T, Matsumoto G et al (2016) Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta 457:46–53. 10.1016/j.cca.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 48.Heigl T, Singh A, Saez-Gimenez B et al (2019) Myeloid-derived suppressor cells in lung transplantation. Front Immunol 10:900. 10.3389/fimmu.2019.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Grinsven E, Textor J, Hustin LSP et al (2019) Immature neutrophils released in Acute inflammation exhibit efficient Migration despite Incomplete Segmentation of the Nucleus. J Immunol 202:207–217. 10.4049/jimmunol.1801255 [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Ai Z, Khoyratty T et al (2020) ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI Insight 5:e139163. 10.1172/jci.insight.139163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leliefeld PHC, Pillay J, Vrisekoop N et al (2018) Differential antibacterial control by neutrophil subsets. Blood Adv 2:1344–1355. 10.1182/bloodadvances.2017015578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malengier-Devlies B, Metzemaekers M, Wouters C et al (2021) Neutrophil Homeostasis and Emergency Granulopoiesis: the example of systemic juvenile idiopathic arthritis. Front Immunol 12:766620. 10.3389/fimmu.2021.766620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uhl B, Vadlau Y, Zuchtriegel G et al (2016) Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood 128:2327–2337. 10.1182/blood-2016-05-718999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang D, Chen G, Manwani D et al (2015) Neutrophil ageing is regulated by the microbiome. Nature 525:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pillay J, Kamp VM, van Hoffen E et al (2012) A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 122:327–336. 10.1172/JCI57990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dean RA, Cox JH, Bellac CL et al (2008) Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR + CXC chemokines and generates CCL2, -7, -8, and– 13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood 112:3455–3464 [DOI] [PubMed] [Google Scholar]
- 57.Reynaud-Gaubert M, Marin V, Thirion X et al (2002) Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transpl 21:721–730. 10.1016/s1053-2498(02)00392-3 [DOI] [PubMed] [Google Scholar]
- 58.Metzemaekers M et al (2023) Fast and furious: the neutrophil and its armamentarium in health and disease. Med Res Rev 43(5):1537–1606. 10.1002/med.21958 [DOI] [PubMed] [Google Scholar]
- 59.Neurohr C, Huppmann P, Samweber B et al (2009) Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transpl 28:468–474. 10.1016/j.healun.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 60.Suwara MI, Vanaudenaerde BM, Verleden SE et al (2014) Mechanistic differences between phenotypes of chronic lung allograft dysfunction after lung transplantation. Transpl Int 27:857–867. 10.1111/tri.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verleden GM, Godinas L, Vos R, Verleden SE (2022) Chronic lung allograft dysfunction and restrictive allograft syndrome: are phenotypes robust and helpful? Curr Opin Organ Transpl 27:211–216. 10.1097/MOT.0000000000000962 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study includes no data deposited in external repositories. Data are available from the corresponding author on request.