Abstract
The mechanisms underlying individual differences in core body temperature (Tc) are unexplained by genetic factors and poorly understood. Here, we investigated whether the environmental temperature during early development affects postnatal Tc. Mouse embryos were cultured from pronuclear to blastocyst stage in either standard (37 °C) or high (38 °C) temperature, and the Tc of each grown-up adult was measured. The adult 38 °C-incubated mice showed lower Tc than the 37 °C group without changes in activity levels. In the hypothalamus of the 38 °C group, insulin-like growth factor 1 (Igf1) and IGF binding protein 2 (Igfbp2) gene expression increased. The decrease in Tc in the wild-type 38 °C group was alleviated by brain neuron-specific Igfbp2 knockout. This suggests that IGFBP2 binds to IGF-1 and, inhibits its binding to the receptor, thereby interfering with the thermogenic signaling of IGF-1. These results suggest that one of the factors determining individual postnatal Tc is the ambient temperature of embryos at an early developmental stage, which could affect epigenetic changes, such as DNA methylation, leading to alterations in the Igf1 and Igfbp2 gene expressions in adulthood.
Keywords: Core body temperature, Insulin-like growth factor 1, IGF binding protein 2
Subject terms: Developmental biology, Physiology
The core body temperature (Tc) of homeotherms, including humans, fluctuates due to exercise and circadian rhythms, but it is constantly maintained around the individual’s unique “set point” by the thermoregulatory system1,2. The set point varies from person to person and decreases with age, but for both humans and mice, it generally falls within the range of 36.6 ± 0.5 °C3,4. However, the mechanisms by which individual differences in Tc occur are poorly understood and cannot be explained solely by genetic factors. One study on birds suggested a connection between heat exposure to embryos and the Tc of the grown-up adults: in a study using Japanese quail (Coturnix japonica), incubation at a temperature 0.7 °C higher than the normal incubation temperature resulted in increased mitochondrial metabolism after birth5. Considering that mitochondrial metabolism in the preoptic area (POA) of the hypothalamus, which constitutes the central mechanism for thermoregulation (Tc), is involved in Tc control6, heat exposure during the embryonic stage may influence Tc regulation in adulthood.
POA sends inhibitory signals to mediate the differential inhibitory control of the sympatho-excitatory drive, determining brown adipose tissue (BAT) thermogenesis7. Thermosensitive neurons in the POA receive thermal signals from peripheral and deep-body thermoreceptors as well as hormonal and metabolic signals for the feedback control of Tc8. Thus far, insulin-like growth factor-1 receptor (IGF-1R) is expressed in warm-sensitive neurons in the POA9. IGF-1R is a transmembrane tyrosine kinase receptor that receives insulin-like growth factor-1 (IGF-1) signaling. IGF-1 plays a crucial role in the central regulation of peripheral metabolism, and administration of IGF-1 to the POA induces dose-dependent hyperthermia9. The POA sends inhibitory signals through distinct pathways to other hypothalamic nuclei, such as the dorsomedial hypothalamus (DMH) and raphe pallidus (RPa), to mediate differential inhibitory control of the sympathoexcitatory drive that drives brown adipose tissue (BAT) thermogenesis10. IGF-1 can affect the activity of preoptic GABAergic inhibitory neurons projecting to the DMH and RPa, leading to disinhibition of the sympathoexcitatory drive and resulting in BAT activation and subsequent hyperthermia9.
Heatwave exposure during early pregnancy is likely to increase the risk of stillbirth11. Processes critical to embryonic development such as cell proliferation, migration, differentiation, and programmed cell death (apoptosis) are adversely affected by elevated maternal temperatures12. Heat exposure can cause irreversible changes in embryonic development and it cannot be ruled out that these effects may influence postnatal development and physiological functions of grown-up adults. Many studies have supported the hypothesis that there is a link between the periconceptual, fetal, and early infant phases of life and the long-term development of metabolic disorders; this hypothesis is known as the developmental origins of the health and disease (DOHaD) hypothesis13. An abnormal nutritional environment around the embryo, which may cause detrimental changes in embryonic development, can also affect the long-term physiological functions of adults14,15. Malnutrition during gestation, such as suboptimal maternal nutrition, increases the risk of late-life diseases, such as hypertension and type 2 diabetes16–18. In a rat study, pre-implantation maternal low-protein diets increased body weight and systolic blood pressure in male offspring19. We hypothesized that the thermal environment of early embryos could, like nutritional conditions, influence the long-term physiological functions of grown-up adults.
In this study, we investigated the effect of incubation temperature for early-stage embryos on the Tc of the grown-up adult mice. To this end, mouse in vitro fertilized embryos were cultured from the pronuclear stage to the blastocyst stage at either standard body temperature (37 °C group) or high temperature (38 °C group), and the Tc of each grown-up adult was measured. We could not test the low temperature condition, because most 36 °C-incubated embryos were lethal. The adult 38 °C group showed lower Tc than the 37 °C group without altering activity levels. Quantitative polymerase chain reaction (qPCR) analysis of hypothalamic tissues for candidate genes that could be responsible for the determination of Tc revealed enhanced expression of IGF-1 and insulin-like growth factor binding protein-2 (IGFBP2), which bind to and disturb the binding of IGF-1 to its receptor (IGF-1R)20,21. Brain neuron-specific Igfbp2 conditional knockout (cKO) mice showed an amelioration of the Tc reduction in the 38 °C group, suggesting that increased expression of IGFBP2 in the hypothalamus in the 38 °C group caused the reduction of Tc after growth by disturbing the Igf-1 signaling in the hypothalamus of mice.
Material and methods
Animals
The experimental protocols were approved by the Safety Committee for Recombinant DNA Experiments at Tottori University (35-071, 2024-010) and the Committee on the Ethics of Animal Experiments in Tottori University Faculty of Medicine (22-Y-6, and 23-Y-45), and were carried out following the Guidelines for Animal Experiments at Tottori University. All experiments described here conform to the ARRIVE guidelines. All mice (C57BL/6N and ICR strains) were purchased from Japan SLC (Tokyo, Japan). The CaMK2a-Cre transgenic mouse strain (B6.Cg-Tg(Camk2a-cre)20Kmik/KmikRbrc, RBRC00254) was provided by the RIKEN BRC through the National BioResource Project of MEXT/AMED, Japan22. The Igfbp2-floxed mouse line was generated as described follows in “Generation of Igfbp2-floxed mouse”. They were housed in individual plastic cages [24.5 × 17.5 × 12.5 cm; (length × width × depth)] with wood-chip bedding in a room maintained at 25 ± 1 °C. They experienced a photoperiod of 12 h light:12 h dark, with lights on at 0700 h. All mice had ad libitum access to water and standard laboratory chow.
Mouse embryo in vitro culture
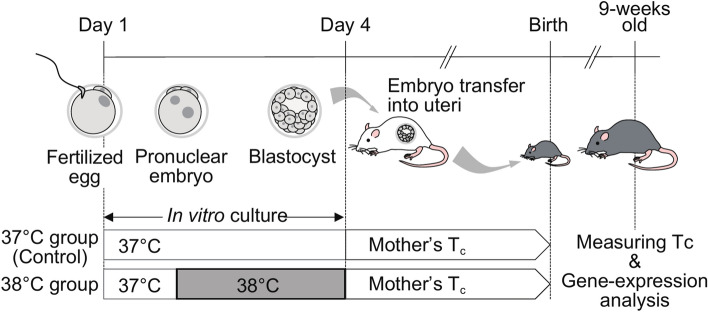
We established a mammalian animal model to validate the influence of the mother’s Tc on offspring. Here, we used mouse reproductive technologies such as in vitro fertilization, embryo culture, and embryo transfer into the uterus. We cultured in vitro mouse fertilized zygotes from the pronuclear stage to the blastocyst stage at 37 °C (37 °C group); standard culture temperature, or 38 °C (38 °C group) with CO2 incubators (Fig. 1). Briefly, C57BL/6N female mice of 3-weeks old were peritoneally injected with 5 IU/mouse pregnant mare’s serum (Serotropin, ASKA Animal Health, Japan), followed by 5 IU/mouse hCG (Gestron, Kyoritsu Seiyaku, Japan) injection for superovulation that occurred 48 h later. Eggs obtained from superovulated mice sacrificed by cervical dislocation were fertilized in vitro with C57BL/6N sperm in HTF medium (ARK Resource, Japan). The time course of embryo culture in KSOM medium (ARK Resource, Japan) is shown in Fig. 1. After fertilization was confirmed, fertilized zygotes were divided equally and randomly into the two groups, 37 °C and 38 °C groups, cultured in vitro to develop to the blastocyst stage in a CO2 incubator at either 37 or 38 °C. The embryos were transferred to the uteri of pseudo-pregnant ICR mice chosen randomly. After birth, 9-week-old male mice were subjected to body temperature and gene-expression analyses.
Fig. 1.
Experimental workflow.
Measurement of Tc and samplings of the hypothalamus and the liver
Tc was measured using a biotelemetry system (Data Science International [DSI], MN; Lange et al., 1991). In brief, mice were anesthetized by the inhalation of 5% isoflurane, and a battery-operated transmitter (TA11TA-F10, DSI) was implanted intraperitoneally (i.p.). After a 1-week-recovery period, the Tc and physical levels were measured for another two weeks. Data were obtained from the last 7 days. The area under the curve (AUC) was calculated as the integral value of Tc. After cessation of measurement, the mice were anesthetized with M/M/B consisting of medetomidine (Domitor, Nippon Zenyaku Kogyo, Japan), midazolam (Midazolam Injection, Sandoz, Japan), and butorphanol (Vetorphale, Meiji Seika Pharma, Japan) at doses of 0.3, 4, and 5 mg/kg body weight, respectively23. The whole body was perfused with 25 mL of cold saline using a microsyringe pump (CXF-101, ISIS, Japan). The hypothalamus and liver were immediately removed and frozen in liquid nitrogen. The samples were kept at – 80 °C for molecular analysis.
qPCR analysis for candidate genes, Igf1 and Igfbp2
Igf1 and Igfbp2 mRNA expression levels in the hypothalamus and liver were analyzed using qPCR. The tissue was homogenized at 4 °C with a multi-beads shocker (MB2200, Yasui Kikai, Japan), and mixed with RNase-free water (W1503, Merck, Germany) and ISOGEN II (311-07361, NIPPON GENE, Japan). After centrifugation (12,000 rpm, 15 min, 4 °C), the supernatant was collected and mixed with an equal volume of isopropanol (166-04836, FUJIFILM Wako Pure Chemical, Japan). The RNA pellet was washed twice with 75% ethanol, then dissolved in RNase-free water after a brief air drying. Total RNA was reverse-transcribed using a high-capacity cDNA reverse transcription kit with an RNase inhibitor (4374966; Thermo Fisher Scientific). The cDNA was diluted 5 times with RNase water before qPCR. qPCR was performed using the PowerUp SYBR Green Master Mix (A25742; Thermo Fisher Scientific). The qPCR primers used are listed in Supplementary Table 1.
Generation of Igfbp2-floxed mouse
Igfbp2-floxed mice were generated by the standard protocol for gene editing technology, CRISPR/Cas9 system24. Briefly, two crRNAs (crRNA1 and crRNA2) and two ssDNAs (ssDNA1 and ssDNA2) were synthesized as shown in Supplemental Table 2 (Alt-R® CRISPR-Cas9 crRNA and Ultramer® DNA Oligo, respectively; Integrated DNA Technologies [IDT], IA). Before micro-injection, 2 ng/µL of crRNAs, 8 ng/µL of Alt-R® CRISPR-Cas9 tracrRNA (1072532, IDT), 50 ng/µL of Alt-R® S.p. Cas9 Nuclease V3 (1081058, IDT), and 100 ng/µL of ssDNAs were mixed in sterilized water for the embryo (W1503, Merck), and filtrated with Ultrafree-MC (UFC30GV25, Merck). The filtered mixture was microinjected into the pronuclei and cytoplasm of the fertilized zygotes obtained from C57BL/6N female mice. The next day, the injected zygotes were transferred into the oviducts of pseudo-pregnant ICR mice. After birth, the genotypes of the pups were identified by genomic PCR and sequence analysis of the floxed region. Briefly, genomic PCR was performed using KOD FX Neo (KFX-201, TOYOBO, Japan) using a standard procedure. The genotyping primers used are listed in Supplementary Table 2. The product of genomic PCR was purified with the Wizard® SV Gel and PCR Clean-Up System (A9281, Promega, WI), and sequenced with the Value Read DNA sequence (Eurofins Genomics, Japan). Igfbp2-floxed mice were mated with CaMK2a-Cre transgenic mice to generate neuron-specific Igfbp2 cKO mice. The CaMK2a gene was also expressed in the testes, so we used Igfbp2 cKO (Igfbp2flox/-::CaMK2a-Cre) and the control mice (Igfbp2+/-::CaMK2a-Cre), which are the offspring of male F1 mice of Igfbp2-flox and CaMK2a-Cre mice, respectively. Notably, both Igfbp2 cKO and control mice harbored the CaMK2a-Cre transgene.
Igfbp2 cKO male mice were subjected to Tc and physical levels analyses, as described above. Briefly, the embryo culture was performed at 38 °C. After birth, the control and Igfbp2 cKO mice were identified by genotyping. The mice which were obviously small and weak (the body weight at 4 weeks old was under 15 g), or which had an system error at measuring the Tc were omitted. To confirm the reduction in Igfbp2 expression in Igfbp2 cKO mice, tissues from control and Igfbp2 cKO mice were collected and frozen quickly. After homogenization with a multi-beads shocker, RNA was purified using the FastGene RNA Premium Kit (FG-81050, Nippon Genetics, Japan). Total RNA was reverse-transcribed and cDNA was used for qPCR, as described above.
Statistical analysis
All results are expressed as mean ± S.E.M. Data were analyzed for statistical significance using a Student’s t-test after Shapiro–wilk and Levene tests to assess the normality and the equal variances, respectively; when the latter two tests failed to confirm those variables, data were analyzed by Mann–Whitney U tests. These tests were performed using the R software (version 2023.12.0 + 369)25. Differences were considered statistically significant at p < 0.05.
Results
High ambient temperature at an early embryonic stage causes low Tc after growth
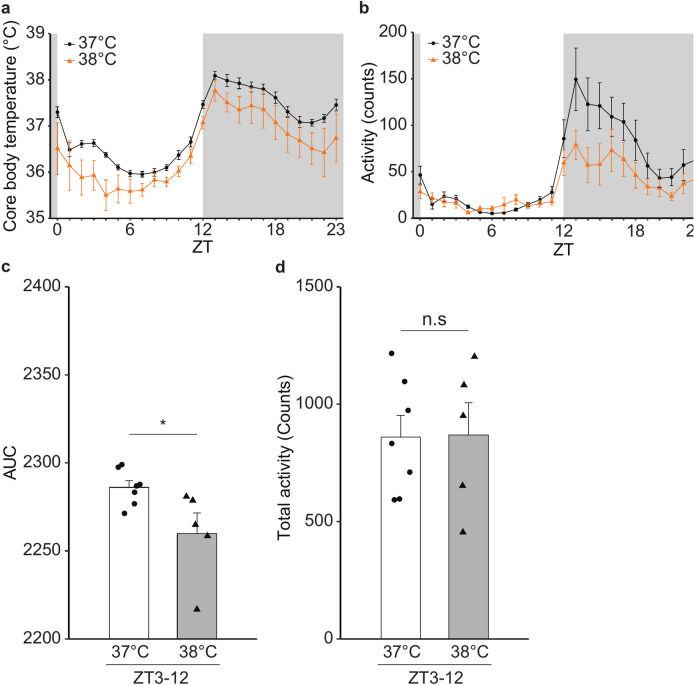
Daily changes of Tc and activity were measured on both 37 °C and 38 °C groups of mice at the same age (9-weeks old; Fig. 1). Both groups showed similar diurnal rhythm of Tc (Fig. 2a) and physical activity (Fig. 2b). However, the Tc of the 38 °C group was lower than that of the 37 °C group (Fig. 2a). AUC of Tc indicated a significant difference in Tc from Zeitgeber time (ZT) 3 to ZT12 between the two groups (Fig. 2c), while there were no significant differences in the activities during the same period (Fig. 2d).
Fig. 2.
Effect of environmental temperature near mouse embryos at the early stage on the Tc of the grown-up male mice. (a, b) Circadian rhythms of core body temperature (a) and activity (b) of the male WT mice (n = 7; 37 °C group, n = 5; 38 °C group). For a given mouse, data were obtained every 1 h for 7 days (see Material and Methods), followed by calculation of a 7-day mean of the data at each time-point. Subsequently, we obtained a mean value (namely, ● and ▲ shown in Fig. 2a and b) at each time-point for a given group. The error bar shows standard error of the mean (SEM). (c) Area under the curve (AUC) of the temperature changes from ZT3 to ZT12. Error bar shows SEM. * p-value < 0.05 (Student’s t-test). (d) Total activity from ZT3 to ZT12. Error bar shows SEM.
High ambient temperature causes upregulation of hypothalamic Igf1 and Igfbp2 expressions
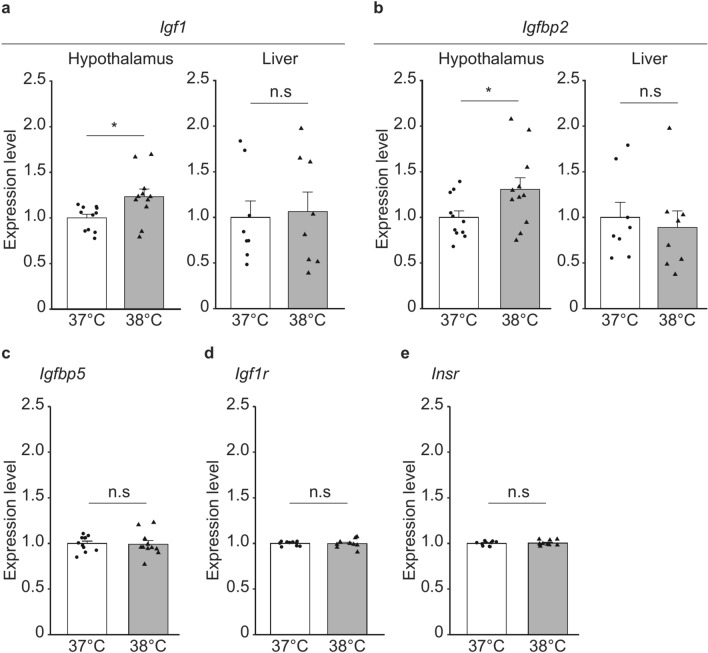
To test the possibility that thermogenic signaling through IGF-1 underlies the Tc changes in the 38 °C group, we examined the gene expression levels of IGF-1, its binding proteins (IGFBP2 and 5), and its receptors (IGF-1R, and insulin receptor [INSR]) in the grown-up adult of both groups. IGFBP2 and 5 are reported to be expressed in the brain26–28. Gene expression analysis by qPCR revealed that hypothalamic Igf1 and Igfbp2 mRNA expression was upregulated in the 38 °C group, without any changes in hepatic expression (Fig. 3a,b). The expression levels of Igfbp5 and its receptors (Igf1r and Insr) did not differ between the two groups (Fig. 3c, d,e). As IGFBP2 disturbs the binding of IGF-1 to its receptors, these data suggest that increased expression of IGFBP2 caused a reduction in the thermogenic function of IGF-1 and lowered the Tc of the 38 °C group.
Fig. 3.
Effect of the environmental temperature near mouse embryos at the early stage on the Igf1 and Igfbp2 expressions in the hypothalamus and liver and the Igfbp5, Igf1r and insr in the hypothalamus of the grown-up male mice. (a) Expression level of Igf1 mRNA in the hypothalamus (n = 10 in each of the 37 °C and 38 °C groups) and liver (n = 8 in each group). Error bar shows SEM. * p-value < 0.05 (Student’s t-test). (b) Expression level of Igfbp2 mRNA in the hypothalamus (n = 11 in each of the 37 °C and 38 °C groups) and liver (n = 8 in each group). Error bar shows SEM. * p-value < 0.05 (Student’s t-test). (c) Expression level of Igfbp5 mRNA in the hypothalamus (n = 11 in each of the 37 °C and 38 °C groups). Error bar shows SEM. (d) Expression level of Igf1r mRNA in the hypothalamus (n = 10 in each of the 37 °C and 38 °C groups). Error bar shows SEM. (e) Expression level of Insr mRNA in the hypothalamus (n = 10 in each of the 37 °C and 38 °C groups). Error bar shows SEM.
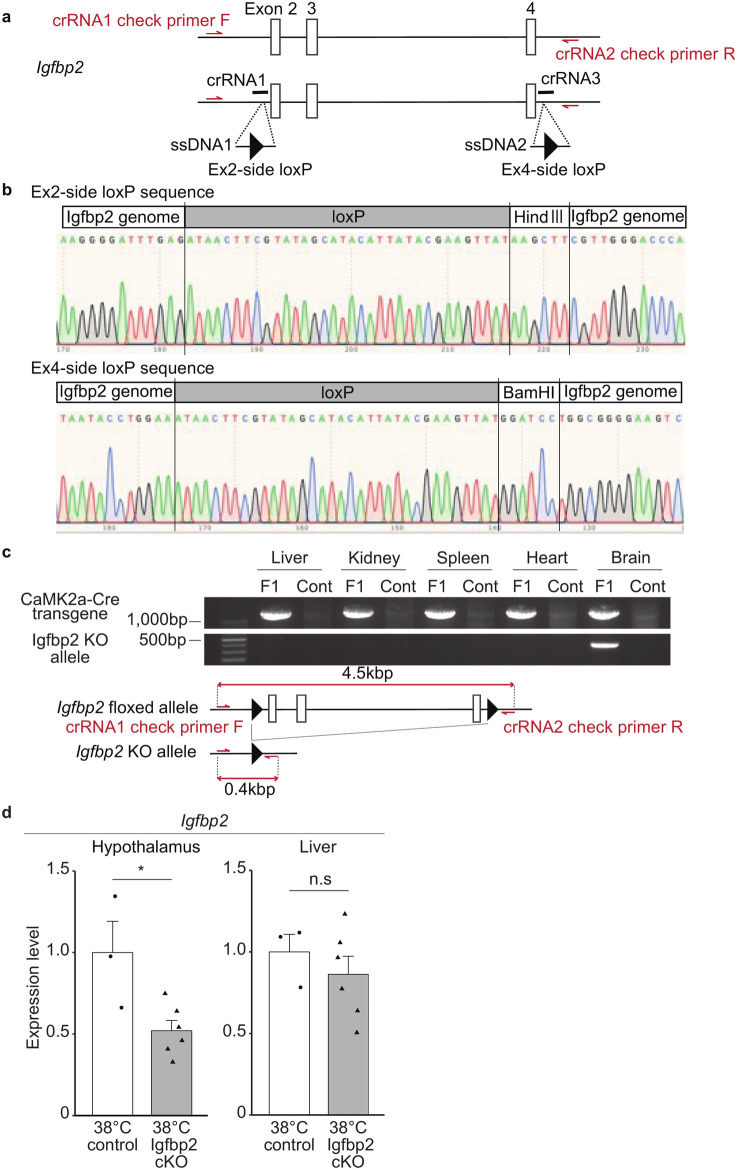
Generation of brain neuron-specific Igfbp2 cKO mouse
To test our hypothesis that Igfbp2 mediates the Tc reduction in the 38 °C group, we next generated an Igfbp2 conditional knockout mouse line. As IGFBP2 and IGF-1 are present not only in the brain, but also in the liver of vertebrates, including mice29,30, we generated an Igfbp2 floxed mouse line using the CRESPR/Cas9 system. Figure 4a shows the construction of the Igfbp2 floxed mouse, in which two loxP sites were inserted in Igfbp2 intron 1 and the downstream of the 3′ UTR, respectively. The insertion of loxP sequences into the proper target position in Igfbp2 floxed mouse was confirmed by DNA sequence analysis (Fig. 4b). To generate a brain neuron-specific Igfbp2 cKO, Igfbp2 floxed mice were mated with CaMK2a-Cre transgenic mice, in which Cre recombinase is expressed specifically in brain neurons. In the F1 mouse with the Igfbp2 floxed allele and CaMK2a-Cre transgene, the Igfbp2 KO allele was detected in the hypothalamus, but not in the liver, indicating that a brain-specific Igfbp2 cKO mouse was established (Fig. 4c and Supplemental Fig. S1).
Fig. 4.
Generation of brain-specific Igfbp2 knock-out (Igfbp2 cKO) mouse. (a) Construction of Igfbp2-floxed mouse. Red arrows show the genotyping primers, crRNA1 check primer F, and crRNA2 check primer R in Supplemental Table 2, to detect the Igfbp2 KO allele. (b) Sequence analysis to confirm the loxP knock-in. (c) PCR results to detect the Igfbp2 KO allele by Cre/loxP recombination. F1 mouse is Igfbp2+/flox::CaMK2a-Cre, and the cont is the Igfbp2+/flox mouse as a control. Bottom schema shows the construction of Igfbp2 KO allele primers. (d) Expression level of the Igfbp2 mRNA in the hypothalamus and liver of the male Igfbp2 cKO (n = 6) and the control mice (n = 3) exposed to 38 °C at their early stage of the embryo. Error bar shows SEM. * p-value < 0.05 (Student’s t-test).
In the following study, we used Igfbp2+/−::CaMK2a-Cre mice as the control, in which Igfbp2 allele is half that of the wild type, because the CaMK2a-Cre gene is also expressed in the testis, and we could not obtain Igfbp2flox/flox::CaMK2a-Cre mice. In the cKO (Igfbp2flox/−::CaMK2a-Cre) mice, IGFBP2 expression is expected to be null, specifically in neurons in the brain, and half of the wild type in other regions, including the liver. We confirmed that the Igfbp2 expression level in Igfbp2 cKO mice after growth was specifically decreased in the hypothalamus, but not in the liver (Fig. 4d).
Brain neuron-specific knockout of Igfbp2 mitigated the Tc reduction in the 38 °C group
The Igfbp2 cKO and the control mice showed indistinguishably the same circadian rhythm of Tc and activity when they were born from natural breeding (Supplemental Fig. S2), indicating that brain neuron-specific deletion of Igfbp2 does not modify the Tc. The Tc rhythm of control mice (Igfbp2+/-::CaMK2a-Cre mice) in Supplemental Fig. S2a was comparable to that of the WT 37 °C group (shown in Fig. 2a).
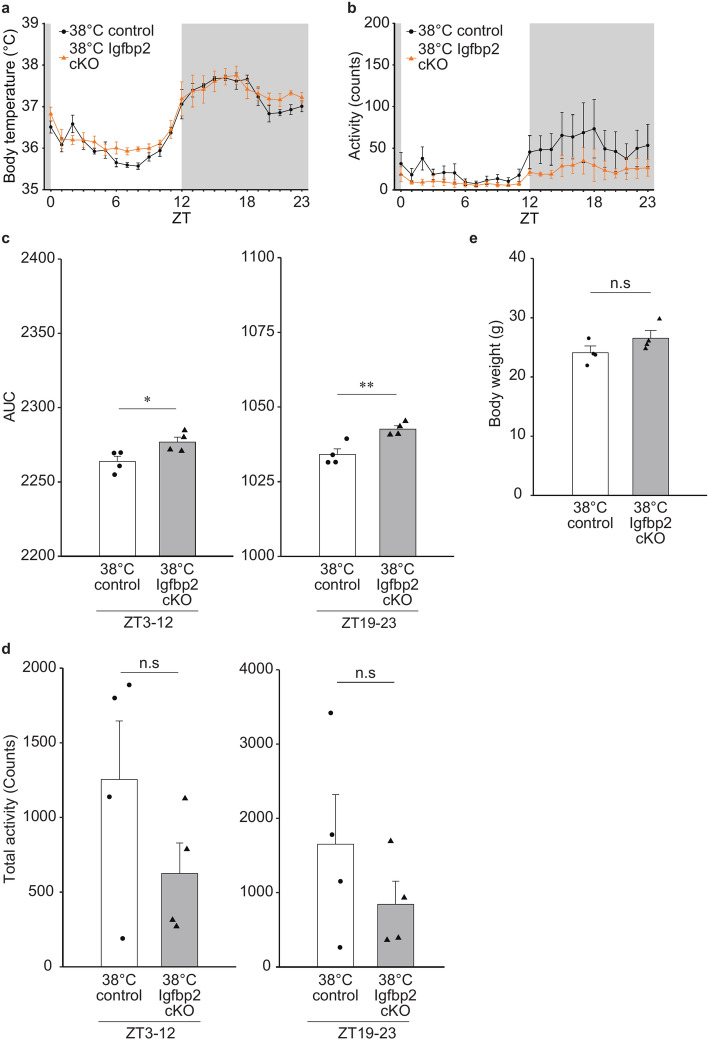
Next, we examined the mice that suffered 38 °C exposure (38 °C Igfbp2 cKO and 38 °C control). Both groups exhibited typical circadian rhythms for Tc and activity (Fig. 5a,b). The Tc rhythm of control mice in Fig. 5a was comparable to that of the WT 38 °C group (shown in Fig. 2a). Notably, both control (Igfbp2+/-::CaMK2a-Cre mice) and WT mice showed diurnal Tc changes between ~ 35.5 and ~ 37.5 °C, indicating that halving the expression of IGFBP2 throughout the body does not affect the Tc reductions by embryonic 38 °C incubation.
Fig. 5.
Effect of cKO of brain Igfbp2 on Tc of adult mice exposed to the environmental temperature of 38 °C at their early stage of embryos. (a, b) Circadian rhythms of Tc (a) and activity (b) of the male Igfbp2 cKO and the control mice (n = 4 in each group) exposed to 38 °C at their early stage of embryo. Error bar shows SEM. (c) AUC of the temperature changes from ZT3 to ZT12 and ZT19 to ZT23. Error bar shows SEM. * p-value < 0.05 (Student’s t-test). ** p-value < 0.01 (Student’s t-test). (d) Total activity (counts) from ZT3 to ZT12 and ZT19 to ZT23. Error bar shows SEM. (e) Body weight of 8-weeks old Igfbp2 cKO mice exposed to the environmental temperature of 38 °C at their early stage of the embryo. Igfbp2 cKO and the control mice (n = 4 in each group) were used. Error bar shows SEM.
In the 38 °C Igfbp2 cKO mice, however, the Tc was higher than in the 38 °C control group during the latter half of the day and night time (Fig. 5a, ZT 3–12 and 19–23, respectively). The AUCs of the 38 °C Igfbp2 cKO group were significantly higher than those of the 38 °C control mice in these time periods, suggesting that IGFBP2 in the hypothalamus neurons is critical for the Tc reduction by the heat exposure at early stages (Fig. 5c). In contrast, while the overall cKO activity appeared to be low, there were no significant differences in activity between the two groups during the same period (Fig. 5b,d). Notably, the cKO mice had no different body weight (at 8 weeks of the age) comparing to the control group with a shared genetic background, indicating that the Tc difference between cKO and control mice (at 9 weeks of the age) may not be due to differences in body mass (Fig. 5e).
Discussion
Here, a rise by 1 °C in the environmental temperature (i.e. 38 °C vs 37 °C) around embryos led to a decrease in Tc of the grown-up adult. Since there was no significant difference in the activities between the two groups, thermosensory and/or thermoeffector pathways as well as function of the thermoregulatory center might have undergone alterations by the early exposure to 38 °C. In this study, significant differences in Tc were observed during the daytime (ZT3-12), which corresponds to the sleeping period for mice (Figs. 2 and 5). This result may support our notion that the difference in body temperature is due to some changes in metabolic functions rather than behavioral activities. Expressions of Igf1 and Igfbp2 mRNAs in the hypothalamus of the 38 °C group were upregulated. IGFBP2 induced by heat exposure, inhibits IGF-1 function in the POA of the hypothalamus; this inhibitory effect exceeds the thermogenic action of IGF-1, resulting in a decrease in Tc. The hypothesis is supported by the increases in Tc in the present Igfbp2 cKO mice exposed to 38 °C at their early stage of the embryo, as compared with the 38 °C control mouse Tc. Importantly, the increase in Tc was observed only in the 38 °C-incubated Igfbp2 cKO but not in the Igfbp2 cKO born from natural breeding, confirming that this IGFBP2-mediated mechanism works only when the mice receive heat exposure at early embryonic stage.
Malnutrition during gestation to a decrease in maternal nutrition leads to low birth weight, and it is correlated with the development of metabolic diseases such as type 2 diabetes31. In such cases, the mismatch between normal and sufficient postnatal nutrition following poor intrauterine nutrition accelerates weight gain, leading to glucose intolerance, insulin resistance and lipid accumulation32,33. Our mice of the 38°C group might have undertaken such adaptive changes in the thermoregulatory system to cope with the high environmental temperature at the early stage of the embryos. However, this possibility needs to be tested in future study. Another possibility is that some studies have revealed the involvement of epigenetics in postnatal weight gain and elevated systolic blood pressure in adults, owing to maternal malnutrition before implantation19,34. It is also reported that the epigenetic modification affects the Igfbp2 expression level. Kammel et al. showed that the DNA methylation of the hepatic Igfbp2 expression level in infants has the relationship with the fatty liver35. According to McDonald’s group, IGFBP2 expression correlated the glioma with necrosis and microvascular proliferation, whose prognosis is relatively poor36. Zheng et al.37 reported that IGFBP2 expression had the association with DNA methylation in glioma. Therefore, we should investigate the role of epigenetics in the decrease in Tc of the present 38 °C group, as well.
In most mammals, fertilization and embryo growth occur in the uterus, which is located deep inside dams. Therefore, it is difficult to manipulate the intrauterine temperature (Tuterus). For example, try to increase the Tuterus could go wrong, because the thermoregulatory system of dams would take action to lower the increased Tc induced by the change in the Tuterus. Even if the Tuterus is maintained constant by experimental devises, changes in several physiological systems, such as the nervous and endocrine systems, may be induced, leading to effects other than the temperature itself exerted on the embryo. Therefore, we used mouse reproductive technologies. This allowed us to culture the mouse embryos in vitro in a CO2 incubator before conception. Embryos were transferred into the uteri of pseudo-pregnant ICR mouse after in vitro culturing. To examine the effects of environmental temperature changes on the embryo and/or fetus during the entire pregnant period, the generation of transgenic and/or knockout pregnant mice with hyperthermia would be one option. As the upregulation of uncoupling protein (UCP) in the mitochondria of brown adipose tissue results in an increase in heat production, UCP could be a target molecule38. Currently, some transgenic animals with hypothermia are available39. It would be interesting to examine the effect of maternal hypothermia on the Tc of the grown-up adults.
In this study, we generated Igfbp2 cKO mice and used them to analyze Tc controls. Considering the inhibitory effect of IGFBP2 on IGF-1, this mouse line may have other phenotypes, such as those related to the neuroendocrine system. One possible candidate is the growth hormone (GH)/IGF-1 axis40. Igf-l null mice exhibit retarded growth after birth41. At an early postnatal age (4 weeks old), Igfbp2 cKO mice gained slightly more weight than control mice (data not shown), though the difference disappeared until 8 weeks old (Fig. 5e). Since the gene deletion was performed specifically in the brain, it is possible to consider that brain IGF-1 might activate growth hormone-releasing hormone (GRH) and/or inhibit somatostatin neurons in the hypothalamus, leading to the activation of the GH/IGF-1 axis and weight gain. Igfbp2 cKO is a useful animal model for the analysis of brain development in the GH/IGF-1 axis.
The mechanisms by which individual differences in Tc arise cannot be fully explained by genetic factors alone and are poorly understood. The results of this study demonstrate for the first time that embryos exposed to a high-temperature environment during early development, such as when the mother has a high fever in early pregnancy, tend to have a lower Tc after birth. Furthermore, this mechanism is thought to involve the inhibition of IGF-1-mediated thermogenic signaling in the POA by IGFBP2. We propose that this is a possible mechanism by which individual differences in Tc arises depending on maternal Tc during early pregnancy. This mechanism may provide a clue as to why the Tc of mammals is approximately 37 °C and not some other temperature.
Supplementary Information
Acknowledgements
We thank Dr. Michio Miyoshi for the experimental support, and Dr. Satoshi Koba and Dr. Kunio Kondoh for the advice to our study. We also thank the Tottori Bio Frontier, which managed by Tottori prefecture in Japan, for use of research equipment.
Author contributions
Y.Y., T.W. and T.Y.H. conceived the study; Y.Y. and K.N. conducted the experiments; Y.Y. and T.Y.H. analyzed and interpreted the data; Y.Y. and T.Y.H. drafted and wrote the manuscript. A.F. and K.M. provided CaMK2a-Cre mice; All authors reviewed the manuscript; T.Y.H. supervised and approved the manuscript.
Funding
This study was supported by MEXT/JSPS KAKENHI (Grant nos. 15K08207, 22K06055, 21K18269, and 23H00422) and AMED (Grant no. JP23gm1510001).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuki Yoshimura, Email: yoshimura@tottori-u.ac.jp.
Takeshi Y. Hiyama, Email: hiyama@tottori-u.ac.jp
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80252-1.
References
- 1.Tan, C. L. & Knight, Z. A. Regulation of body temperature by the nervous system. Neuron98, 31–48. 10.1016/j.neuron.2018.02.022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammel, H. T. Regulation of internal body temperature. Annu. Rev. Physiol.30, 641–710. 10.1146/annurev.ph.30.030168.003233 (1968). [DOI] [PubMed] [Google Scholar]
- 3.Waalen, J. & Buxbaum, J. N. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J. Gerontol. A Biol. Sci. Med. Sci.66, 487–492. 10.1093/gerona/glr001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reitman, M. L. Of mice and men - environmental temperature, body temperature, and treatment of obesity. FEBS Lett.592, 2098–2107. 10.1002/1873-3468.13070 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Stier, A., Monaghan, P. & Metcalfe, N. B. Experimental demonstration of prenatal programming of mitochondrial aerobic metabolism lasting until adulthood. Proc. Biol. Sci.289, 20212679. 10.1098/rspb.2021.2679 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guimaraes, N. C. et al. Mitochondrial pyruvate carrier as a key regulator of fever and neuroinflammation. Brain Behav. Immun.92, 90–101. 10.1016/j.bbi.2020.11.031 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Morrison, S. F. & Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol.81, 285–308. 10.1146/annurev-physiol-020518-114546 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Tabarean, I. Central thermoreceptors. Handb. Clin. Neurol.156, 121–127. 10.1016/B978-0-444-63912-7.00007-2 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Alavez, M. et al. Insulin-like growth factor 1-mediated hyperthermia involves anterior hypothalamic insulin receptors. J. Biol. Chem.286, 14983–14990. 10.1074/jbc.M110.188540 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura, K. & Morrison, S. F. A thermosensory pathway that controls body temperature. Nat. Neurosci.11, 62–71. 10.1038/nn2027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, J., Tong, S., Williams, G. & Pan, X. Exposure to heat wave during pregnancy and adverse birth outcomes: An exploration of susceptible windows. Epidemiology30(Suppl 1), S115–S121. 10.1097/EDE.0000000000000995 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Edwards, M. J., Saunders, R. D. & Shiota, K. Effects of heat on embryos and foetuses. Int. J. Hyperthermia19, 295–324. 10.1080/0265673021000039628 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Lacagnina, S. The developmental origins of health and disease (DOHaD). Am. J. Lifestyle Med.14, 47–50. 10.1177/1559827619879694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker, D. J. In utero programming of chronic disease. Clin. Sci.95, 115–128 (1998). [PubMed] [Google Scholar]
- 15.Grilo, L. F. et al. Metabolic disease programming: From mitochondria to epigenetics, glucocorticoid signalling and beyond. Eur. J. Clin. Invest.51, e13625. 10.1111/eci.13625 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Tarry-Adkins, J. L. & Ozanne, S. E. Nutrition in early life and age-associated diseases. Ageing Res. Rev.39, 96–105. 10.1016/j.arr.2016.08.003 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Barker, D. J., Osmond, C., Golding, J., Kuh, D. & Wadsworth, M. E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ298, 564–567. 10.1136/bmj.298.6673.564 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravelli, A. C. et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet351, 173–177. 10.1016/s0140-6736(97)07244-9 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Kwong, W. Y., Wild, A. E., Roberts, P., Willis, A. C. & Fleming, T. P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development127, 4195–4202. 10.1242/dev.127.19.4195 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Binkert, C., Landwehr, J., Mary, J. L., Schwander, J. & Heinrich, G. Cloning, sequence analysis and expression of a cDNA encoding a novel insulin-like growth factor binding protein (IGFBP-2). EMBO J.8, 2497–2502. 10.1002/j.1460-2075.1989.tb08386.x (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo, V. C., Rekaris, G., Baker, N. L., Bach, L. A. & Werther, G. A. Basic fibroblast growth factor induces proteolysis of secreted and cell membrane-associated insulin-like growth factor binding protein-2 in human neuroblastoma cells. Endocrinology140, 3082–3090. 10.1210/endo.140.7.6771 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Nakayama, K. et al. RNG105/caprin1, an RNA granule protein for dendritic mRNA localization, is essential for long-term memory formation. Elife6, 29677. 10.7554/eLife.29677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai, S., Takagi, Y., Kaneko, S. & Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim.60, 481–487. 10.1538/expanim.60.481 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Horii, T. et al. Efficient generation of conditional knockout mice via sequential introduction of lox sites. Sci. Rep.7, 7891. 10.1038/s41598-017-08496-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Team, R. R: A language and environment for statistical computing, <https://www.R-project.org/>
- 26.Lee, W. H., Michels, K. M. & Bondy, C. A. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: Correlation with insulin-like growth factors I and II. Neuroscience53, 251–265. 10.1016/0306-4522(93)90303-w (1993). [DOI] [PubMed] [Google Scholar]
- 27.Bondy, C. & Lee, W. H. Correlation between insulin-like growth factor (IGF)-binding protein 5 and IGF-I gene expression during brain development. J. Neurosci.13, 5092–5104. 10.1523/JNEUROSCI.13-12-05092.1993 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan, S. IGFBP-2 signaling in the brain: From brain development to higher order brain functions. Front. Endocrinol.10, 822. 10.3389/fendo.2019.00822 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng, L. Y. et al. The fetal rat binding protein for insulin-like growth factors is expressed in the choroid plexus and cerebrospinal fluid of adult rats. Mol. Endocrinol.3, 1559–1568. 10.1210/mend-3-10-1559 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Segura, L. M., Perez, J., Pons, S., Rejas, M. T. & Torres-Aleman, I. Localization of insulin-like growth factor I (IGF-I)-like immunoreactivity in the developing and adult rat brain. Brain Res.560, 167–174. 10.1016/0006-8993(91)91228-s (1991). [DOI] [PubMed] [Google Scholar]
- 31.Hales, C. N. & Barker, D. J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia35, 595–601. 10.1007/BF00400248 (1992). [DOI] [PubMed] [Google Scholar]
- 32.Crowther, N. J., Cameron, N., Trusler, J. & Gray, I. P. Association between poor glucose tolerance and rapid post natal weight gain in seven-year-old children. Diabetologia41, 1163–1167. 10.1007/s001250051046 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Carr, S. K. et al. Maternal diet amplifies the hepatic aging trajectory of Cidea in male mice and leads to the development of fatty liver. FASEB J.28, 2191–2201. 10.1096/fj.13-242727 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Kwong, W. Y. et al. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction132, 265–277. 10.1530/rep.1.01038 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Kammel, A. et al. Early hypermethylation of hepatic Igfbp2 results in its reduced expression preceding fatty liver in mice. Hum. Mol. Genet.25, 2588–2599. 10.1093/hmg/ddw121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald, K. L. et al. IQGAP1 and IGFBP2: Valuable biomarkers for determining prognosis in glioma patients. J. Neuropathol. Exp. Neurol.66, 405–417. 10.1097/nen.0b013e31804567d7 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Zheng, S. et al. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro Oncol.13, 280–289. 10.1093/neuonc/noq190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab.29, 27–37. 10.1016/j.cmet.2018.11.002 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Hiraoka, Y. et al. Critical roles of nardilysin in the maintenance of body temperature homoeostasis. Nat. Commun.5, 3224. 10.1038/ncomms4224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Samerria, S. & Radovick, S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells10, 2664. 10.3390/cells10102664 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker, J., Liu, J. P., Robertson, E. J. & Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell75, 73–82 (1993). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.