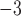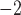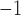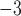Abstract
We report on the proof-of-concept of a low-mass, low-power method for collecting micron-sized sulfuric acid aerosols in bulk from the atmosphere of Venus. The collection method uses four wired meshes in a sandwich structure with a deposition area of 225 cm2. It operates in two modes: passive and electrostatic. During passive operation, aerosols are gathered on the deposition surface by aerodynamic force. During electrostatic operation, a tungsten needle discharges a high voltage of − 10 kV at the front of the grounded mesh structure. The discharge ionizes aerosols and attracts them to the mesh by Coulomb forces, resulting in improved efficiency and tentative attraction of submicron aerosols. We describe the instrument construction and testing in the laboratory under controlled conditions with aerosols composed of 25%, 50%, 70%, 80%, 90% and 98%* concentration by volume of sulfuric acid, the rest water. We demonstrated the following: (i) both modes of operation can collect the entire range of sulfuric acid solutions; (ii) the collection efficiency increases steadily (from a few percent for water to over 40% for concentrated sulfuric acid) with the increased concentration of sulfuric acid solution in water in both modes; (iii) the relative improvement in the collection of the electrostatic mode decreases as the sulfuric acid concentration increases. We also demonstrated the operation of the instrument in the field, cloud particle collection on Mt. Washington, NH, and crater-rim fumaroles’ particle collection on Kīlauea volcano, HI. The collection rate in the field is wind-speed dependent, and we observed collection rates around 0.1 ml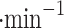 in low wind environments (1–2 m
in low wind environments (1–2 m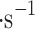 ), and around 1 ml
), and around 1 ml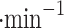 in stronger wind (7–9 m
in stronger wind (7–9 m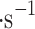 ).
).
Keywords: Venus clouds, Sulfuric acid aerosols, Cloud collection
Subject terms: Aerospace engineering, Planetary science
Introduction
Clouds of Venus
The first human-made object to fly by Venus was the Mariner 2 spacecraft nearly six decades ago1. It was followed by two decades of intense in-situ and orbital exploration with probes and balloons during the Venera, Vega, and Pioneer Venus programs; the data from these missions provided the majority of our knowledge about Venus’s clouds and haze system2. The next four decades enriched our understanding of Venus through remote observations by two fly-bys: Galileo in 1990 and Messenger in 2007, and three orbiters: Magellan in 1990–1994, Venus Express in 2006–2014, and Akatsuki. Akatsuki has been the only functional Venus probe in the last decade, and it fell silent as of May 2024. The last direct Venus cloud measurements were made in 1985 by the Vega balloons3. These missions provided significant knowledge about our current understanding of Venusian atmospheric chemistry and microphysics.
Venus’s dense atmosphere is 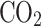 -dominated and has a global cloud coverage of approximately 20 km in thickness at an altitude of 47–68 km. This makes Venus the most extensive aerosol system among the four terrestrial planets in the Solar System2. Venusian clouds are made of photochemically-generated sulfuric acid aerosols (
-dominated and has a global cloud coverage of approximately 20 km in thickness at an altitude of 47–68 km. This makes Venus the most extensive aerosol system among the four terrestrial planets in the Solar System2. Venusian clouds are made of photochemically-generated sulfuric acid aerosols ( 75–98% concentration in water solution) of various sizes and are formed in three distinct layers. According to the Pioneer Venus Particle Size Spectrometer Experiment, clouds are composed of: (i) the upper layer, which is mainly populated by submicron (0.35
75–98% concentration in water solution) of various sizes and are formed in three distinct layers. According to the Pioneer Venus Particle Size Spectrometer Experiment, clouds are composed of: (i) the upper layer, which is mainly populated by submicron (0.35 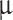 m, 200–600
m, 200–600 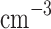 ) and micron-sized (1–2
) and micron-sized (1–2 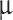 m, 10–70
m, 10–70 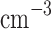 ) aerosols, called Mode 1 and Mode 2, respectively; (ii) the middle layer, which includes Mode 1 (70–140
) aerosols, called Mode 1 and Mode 2, respectively; (ii) the middle layer, which includes Mode 1 (70–140 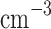 ) and Mode 2 (30–50
) and Mode 2 (30–50 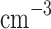 ), but also has larger Mode 3 aerosols (7–8
), but also has larger Mode 3 aerosols (7–8  m, 15–50
m, 15–50 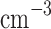 ); (iii) the lower layer, which has Mode 1 (230–560
); (iii) the lower layer, which has Mode 1 (230–560 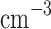 ), Mode 2 (50–70
), Mode 2 (50–70 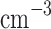 ) and the highest density of Mode 3 particles (30–170
) and the highest density of Mode 3 particles (30–170 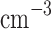 ) with the possible presence of solids4. The existence of Mode 3 particles has been questioned, and the error analysis of existing data from the last century suggests that these particles might be the continuation of Mode 25,6. However, Knollenberg reexamined the original data and argued for trimodal size distribution in the nominal
) with the possible presence of solids4. The existence of Mode 3 particles has been questioned, and the error analysis of existing data from the last century suggests that these particles might be the continuation of Mode 25,6. However, Knollenberg reexamined the original data and argued for trimodal size distribution in the nominal 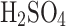 cloud deck7. The main clouds are surrounded by upper haze (up to 100 km) and lower haze (down to 33 km and possible surface haze). The lower haze layer temperature is too high for the stability of liquid sulfuric acid droplets; nevertheless, the refractive indices measured by Pioneer Venus are consistent with the main cloud, suggesting the mysterious existence of liquid
cloud deck7. The main clouds are surrounded by upper haze (up to 100 km) and lower haze (down to 33 km and possible surface haze). The lower haze layer temperature is too high for the stability of liquid sulfuric acid droplets; nevertheless, the refractive indices measured by Pioneer Venus are consistent with the main cloud, suggesting the mysterious existence of liquid 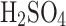 aerosols in the lower haze4. The upper haze comprises submicron particles similar to the main cloud deck, and refractive indices agree with the sulfuric acid composition. Although clouds and hazes cover the entire planet, they are not dense.
aerosols in the lower haze4. The upper haze comprises submicron particles similar to the main cloud deck, and refractive indices agree with the sulfuric acid composition. Although clouds and hazes cover the entire planet, they are not dense.
Venus clouds are comparable to light-moderate fog days on Earth with a visibility of a few kilometers. As calculated from Knollenberg and Hunten4, the density of particulate matter is approximately 0.5 µg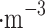 , 13 µg
, 13 µg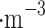 , and 37 µg
, and 37 µg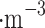 in upper, middle, and lower clouds, respectively. The cloud deck’s optical thickness is 20–40 in visible, near-ultraviolet (UV), and near-infrared (IR) wavelengths8. Low particle density and small particle sizes, in principle, make it challenging to obtain sufficient materials to meet typical analytical sensitivity requirements.
in upper, middle, and lower clouds, respectively. The cloud deck’s optical thickness is 20–40 in visible, near-ultraviolet (UV), and near-infrared (IR) wavelengths8. Low particle density and small particle sizes, in principle, make it challenging to obtain sufficient materials to meet typical analytical sensitivity requirements.
The cloud particles follow the movement of Venus’s super-rotating atmosphere. The clouds travel around Venus in over four Earth days while the planet spins in the same direction approximately 50 times slower. The atmosphere’s super-rotation starts at an altitude of 10 km and stops at 95 km. The horizontal winds in the upper clouds9 reach 100–120 m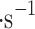 , and the upward and downward (i.e., vertical) gusts up to 3 m
, and the upward and downward (i.e., vertical) gusts up to 3 m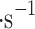 within the cloud layer10,11. An aerial sampling platform would follow horizontal winds along the clouds, resulting in a net-zero velocity of cloud particles relative to a cloud collector. However, the vertical winds are observed regardless of horizontal movement, which makes it feasible to passively sample Venusian clouds using an atmospheric probe and vertical winds.
within the cloud layer10,11. An aerial sampling platform would follow horizontal winds along the clouds, resulting in a net-zero velocity of cloud particles relative to a cloud collector. However, the vertical winds are observed regardless of horizontal movement, which makes it feasible to passively sample Venusian clouds using an atmospheric probe and vertical winds.
The National Aeronautics and Space Administration (NASA) and European Space Agency (ESA) are committed to launching space missions to Venus in the early 2030s. The planned missions include orbiters Venus Emissivity, Radio Science, InSAR, Topography, and Spectroscopy (VERITAS)12, Envision13, and atmospheric probe Deep Atmosphere Venus Investigation of Noble gases, Chemistry, and Imaging (DAVINCI)14. A Venus Flagship Mission concept15 would utilize an aerobot to study cloud and gas composition in the 52–62 km altitude range, including searching for biomolecules, and potential input from the lower atmosphere (e.g., volcanic plumes). The proposed instrument package would include an aerosol mass spectrometer and a fluorimetric microscope, which would utilize ultraviolet excitation to characterize fluorescent particles as an indication of life. The Flagship mission concept currently considers a pump-based approach for sample acquisition.
We, as part of the Morning Star Missions to Venus team (https://venuscloudlife.com/team/), are planning a series of scientific missions to ultimately search for signs of life or life itself on Venus (where aerial life has been hypothesized since the 1960s16,17), by investigating chemical signatures18–20 in the Venusian temperate cloud deck that expands from 48 km (2 bar and 80 °C) to 60 km (0.4 bar and 0 °C)21. The first in a series of planned missions is the Rocket Lab Mission to Venus22, a largely privately funded mission that aims to constrain aerosol abundance, size, shape, composition, and fluorescence by an autofluorescence nephelometer (AFN)23 during a 330-s cloud-layer descent; the probe is targeted for launch in 2026. The following missions24 focus on an aerial probe capable of in-situ analysis of liquid and solid particles by a sophisticated instrument suite25–28. The intention is to culminate the series of Morning Star Missions to Venus with a Venus Cloud Sample Return (VCSR) mission29. The VCSR concept includes a roadmap for a Venus atmospheric sample return that would be built on the groundwork of both the initial Morning Star Missions and other planetary sample return missions.
The success of the Morning Star Missions that follow the Rocket Lab Mission to Venus depends on reliable and efficient cloud liquid collection, either for direct in-situ analysis or for sample return and subsequent investigation in Earth-based laboratories. In this paper we describe an approach and the corresponding technology for bulk liquid collection from the Venusian temperate cloud deck.
An introduction to cloud liquid collection approaches
Many technologies for cloud and fog collection are designed following solutions that originate in nature. For example, numerous plants survive in highly arid environments by exclusively collecting moisture and fog for survival. In the Atacama desert, one such plant is Tillandsia landbeckii, native to Peru and Chile, which has a fluffy net structure of hydrophilic scale-like trichomes covering the leaves and branches that form a meter-scale natural fog collector30. Artificial water collection follows a similar principle. The utilization of fog harps for water harvesting is popular in dry and high mountain areas31,32; cloud collection is a common practice in environmental studies33. Aerosol collectors are categorized into (i) passive deposition or condensation of aerosols on the surface by wind, such as fog harps; and (ii) active systems with pump-driven aerosols to a collector.
Cyclone-type collectors (i.e., pump-driven with wetted walls) are widely used for aerosol collection on Earth. However, the standard ones weigh over 4–5 kg, are bulky, consume 10–20 W of power, and are not qualified for space applications34. A larger cloud collector design based on fog harps can sweep large volumes of the atmosphere and, as theoretical analysis shows, outperform cyclone-type collectors in total atmospheric swiping volume and sample collection amount29.
The cloud liquid collector has not, however, been explored as a technology that could be adapted for Solar System bodies, especially not for Venus. Although both Pioneer Venus and Vega interacted with Venus’s liquid aerosols, they unintentionally collected sulfuric acid droplets on the gas inlet (i.e., not designed for aerosols). Specifically, the droplets blocked the Pioneer Venus mass spectrometer inlet (a favorable hypothesis), producing water vapor and 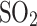 , consistent with 85%
, consistent with 85% 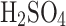 and 15%
and 15% 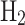 O. After falling to a lower, hotter altitude, the droplets likely evaporated, freeing the inlet35. The Vega probe pumped the atmosphere through an aerosol collecting filter from 63 to 48 km, sealed it, and heated the sample on a carbon fiber substrate to measure the vapor that mainly consisted of
O. After falling to a lower, hotter altitude, the droplets likely evaporated, freeing the inlet35. The Vega probe pumped the atmosphere through an aerosol collecting filter from 63 to 48 km, sealed it, and heated the sample on a carbon fiber substrate to measure the vapor that mainly consisted of 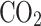 ,
, 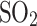 , and
, and 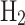 O2, supporting the view that sulfuric acid is the main component of the Venus cloud particles. The nephelometers of Pioneer Venus, Venera, and Vega derived refractive indices of cloud aerosols. Pioneer Venus measured a refractive index of 1.35–1.53,4 and Venera 9, 10 and 11 measured 1.35–1.4736. Remote observations by Venus Express found the upper cloud refractive index to vary between 1.44 and 1.5336. This is only partially consistent with an expected sulfuric acid refractive index of 1.42–1.47, and therefore gives us the uncertainty of expected sulfuric acid concentration in cloud particles for collection system design.
O2, supporting the view that sulfuric acid is the main component of the Venus cloud particles. The nephelometers of Pioneer Venus, Venera, and Vega derived refractive indices of cloud aerosols. Pioneer Venus measured a refractive index of 1.35–1.53,4 and Venera 9, 10 and 11 measured 1.35–1.4736. Remote observations by Venus Express found the upper cloud refractive index to vary between 1.44 and 1.5336. This is only partially consistent with an expected sulfuric acid refractive index of 1.42–1.47, and therefore gives us the uncertainty of expected sulfuric acid concentration in cloud particles for collection system design.
Venusian cloud collection in sufficient amounts requires prolonged exposures (i.e., large atmospheric volume sweeps) that can be achieved by a parachute, balloon, or plane. We are considering a buoyant balloon that floats in the clouds with horizontal winds. The externally mounted cloud collector would take advantage of Venusian vertical winds11 to drive particles to the collection surface.
The simplest way of collecting aerosols is by passive particle deposition by wind on fog-harp-like surfaces. The most significant limitation of the passive collection is aerodynamic force deviation, which makes aerosols follow streamlines. This deviation makes passive collection efficiently feasible only for large particles deposited on large surfaces (see discussion on Stokes number in the following section). Other factors impacting fog collectors’ efficiency are the deposition surface shape37,38 and construction materials39. To increase the water collection efficiency, many different materials, including soft and hard ones, have been tested in a variety of configurations (e.g., Raschel mesh, harp wires, grid, 3D textile, kirigami, etc.)40.
To achieve high-efficiency aerosol particle collection, Han et al. combined the charging of aerosols with a superhydrophobic surface41,42. Damak and Varanasi43 proposed electrostatic injection to overcome the aerodynamic force by electrostatic force and demonstrated improved water aerosol collection in the laboratory. Nature also learned to use electrostatic forces, where one example is spider ballooning—spiders sense electric currents in the atmosphere, align their silk, and charge it to take off while controlling their altitude with a high-voltage charge activation44; these spiders were mysteriously found flying at high altitudes and far away from the coast above the ocean. When a fog collector is operated electrostatically, the incoming flow of aerosols is ionized by a high-voltage source at a short distance from the deposition surface, which is grounded; charged aerosols are then attracted to the grounded mesh by Coulomb forces. Many studies have since employed electrostatic-based ideas with water aerosols45,46.
We expand on previous work and have constructed a multi-layer electrostatic cloud collector that we test in an environment relevant to Venus clouds. Our multi-layer electrostatic cloud collector, called the Venus Cloud Catcher (VCC), complies with the following requirements for space application: (i) Mesh design suitable for high stresses during vibration, shock, and thermal-vacuum testing; (ii) Adaptation for a compact design with low mass; (iii) Low power consumption (under 5 W) and electrically conductive mesh; and (iv) Operation in a high-temperature (80 °C) concentrated sulfuric acid environment. We evaluated the VCC performance in a custom sulfuric acid chamber. We also tested the VCC in the field by collecting clouds on Mt. Washington, USA (an extremely windy environment47 with regular cloud coverage) and fumaroles’ particles on Kīlauea volcano, USA (sulfur dioxide and sulfate aerosols emission48,49).
Methods
Venus Cloud Catcher (VCC) design
The VCC comprises four wire meshes; each is 15 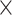 15
15 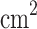 . This provides an area of 0.0225 m2 that is compact enough to fit on a small atmospheric probe and provides a minimum viable size for the collection of a sufficient amount of cloud material for analytical instrumentation. The total mass of the prototype is under 500 g, and the flight instrument is expected to be under 1 kg with the sulfuric acid-resistant frame and electronics housing; the electronics mass is approximately 200 g (see “Appendix” for details). The VCC intends to use vertical gusts10,11 to carry particles to be sampled, and we assume a vertical wind velocity of 0.72 m
. This provides an area of 0.0225 m2 that is compact enough to fit on a small atmospheric probe and provides a minimum viable size for the collection of a sufficient amount of cloud material for analytical instrumentation. The total mass of the prototype is under 500 g, and the flight instrument is expected to be under 1 kg with the sulfuric acid-resistant frame and electronics housing; the electronics mass is approximately 200 g (see “Appendix” for details). The VCC intends to use vertical gusts10,11 to carry particles to be sampled, and we assume a vertical wind velocity of 0.72 m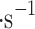 —the average of Vega 1 and Vega 2 mean values. We calculated these numbers from Vega 1 and Vega 2 raw data anemometer measurements and filtered the data to use data with the “quality flag” of two and above as annotated by the Vega team (i.e., 0—no data; 1—erroneous data with downlink problems, 2 or 3—good data, but some uncertainties in decommutation, 4—high-quality data). The Vega data is available for download in Lorenz et al.50. The mean vertical wind velocity in the middle cloud layer for Vega 1 is 0.7696 m
—the average of Vega 1 and Vega 2 mean values. We calculated these numbers from Vega 1 and Vega 2 raw data anemometer measurements and filtered the data to use data with the “quality flag” of two and above as annotated by the Vega team (i.e., 0—no data; 1—erroneous data with downlink problems, 2 or 3—good data, but some uncertainties in decommutation, 4—high-quality data). The Vega data is available for download in Lorenz et al.50. The mean vertical wind velocity in the middle cloud layer for Vega 1 is 0.7696 m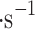 , and 0.6755 m
, and 0.6755 m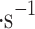 for Vega 2.
for Vega 2.
The VCC’s collection efficiency determines what fraction of the total available liquid can be gathered for the analysis. When operated on Venus, the VCC would be at a 45° angle from the gravity vector to ensure vertical winds passage through the mesh and gravity gradient for directing the collected sample to the sample delivery system. Thus, the effective area is reduced by 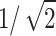 . In order to achieve a 0.0225
. In order to achieve a 0.0225 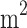 effective area on Venus, the total VCC required area is 0.0225
effective area on Venus, the total VCC required area is 0.0225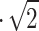 = 0.0318
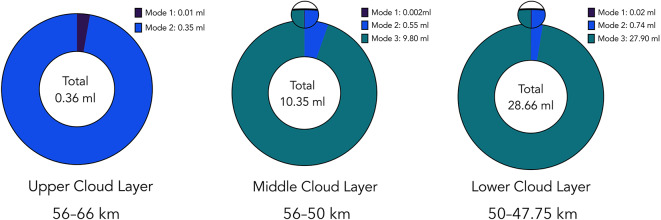
= 0.0318  . The resulting swipe volume is 1.4 million liters of atmosphere per one Earth day. Using particle number density data from Pioneer Venus4, the resulting daily bulk liquid availability is 0.36 ml, 10.35 ml, and 28.66 ml of liquid samples in the upper, middle, and lower cloud decks, respectively. Total theoretical liquid availability is visualized in Fig. 1.
. The resulting swipe volume is 1.4 million liters of atmosphere per one Earth day. Using particle number density data from Pioneer Venus4, the resulting daily bulk liquid availability is 0.36 ml, 10.35 ml, and 28.66 ml of liquid samples in the upper, middle, and lower cloud decks, respectively. Total theoretical liquid availability is visualized in Fig. 1.
Fig. 1.
Theoretical total availability of bulk liquid per one Earth day in various layers of the Venusian clouds. Calculated using 0.72 m relative wind, 0.0225-
relative wind, 0.0225- effective collector surface, and particle number density data from Knollenberg and Hunten4. Mode 1 appears as a thin fraction line in the zoomed section of the middle and lower cloud layers.
effective collector surface, and particle number density data from Knollenberg and Hunten4. Mode 1 appears as a thin fraction line in the zoomed section of the middle and lower cloud layers.
The VCC collector shown in Fig. 2 consists of a deposition surface (four meshes), with a gutter that delivers liquid samples to the vials, and control electronics for electrostatic operation (see Appendix for details on the instrument’s electronics and the underlying computer code). In the front of the deposition surface, a 2% ceriated tungsten needle (1.5 mm in diameter, 25 mm long, and 20 end) discharges − 10 kV.
end) discharges − 10 kV.
Fig. 2.
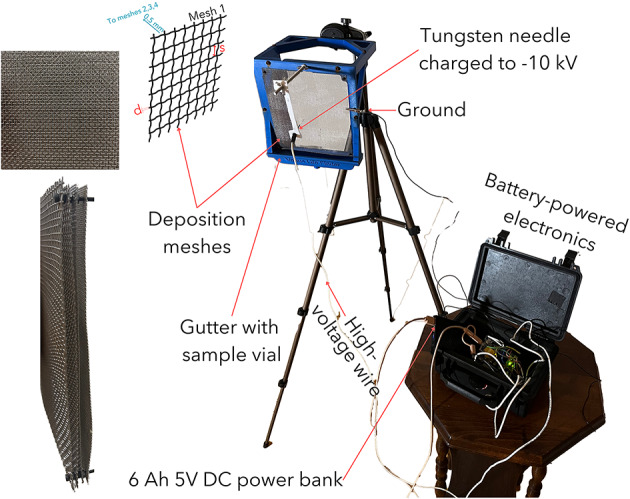
The VCC cloud collection setup. The setup is fully hand portable and designed for self-sufficient field operation. See Table 1 for further details on the mesh parameters, such as opening size and wire diameter.
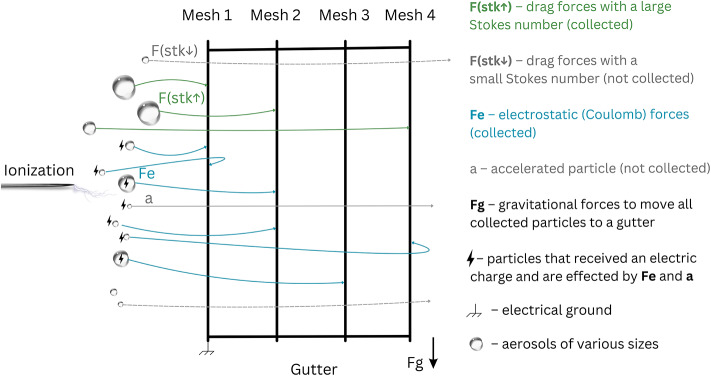
The VCC mesh design uses a multi-layer sandwich structure comprising four individual meshes that are separated by an approximately 0.5 mm gap. Each mesh has different wire thicknesses and open areas (see Table 1). We designed the multi-layer structure to increase overall collection efficiency by providing secondary probabilities of collision if an aerosol particle misses the preceding mesh. Aerosols on their trajectory first encounter the least dense Mesh 1, while some particles make it all the way to the most dense Mesh 4. The majority of particles do not collide with the mesh due to the aerodynamic deviation that makes the particles follow streamlines in the passive operation (i.e., no electrostatic discharge). When the electrostatic operation engages, a fraction of aerosols receive an electric charge and are driven to the grounded mesh by electrostatic forces without following the streamlines. Because the grid size of the individual mesh is much larger than the particle size and has a square shape, one can expect the grid to act as an aerosol accelerator on particular occasions (i.e., similar to the working principle of a gridded ion engine or electron gun). In rare cases, the accelerated aerosol would fly through all meshes without colliding with the deposition surface. However, in some instances, the electrostatic force would turn the particle around after having passed through the mesh, providing a second chance of collision. A multilayered structure partially addresses this issue by providing second, third, and fourth chances of collision when the particle misses the previous mesh. Various forces acting on individual aerosols described in this paragraph are visualized in Fig. 3.
Table 1.
Physical parameters of the four meshes that form the sandwich deposition surface with the visual identification of dimensions.
| Deposition component | Opening size (s), mm | Shade coefficient | Wire diameter (d), mm | Stokes number (Stk) | Passive collection (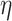 ), % ), % |
Midcloud col., 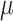 l l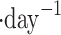
|
|---|---|---|---|---|---|---|
| Mesh 1 | 2.46 | 0.4 | 0.7 |
Mode 1: 1.12 Mode 2: 4.98 Mode 3: 6.10 |
Mode 1: 0.01 Mode 2: 0.61 Mode 3: 5.59 |
Mode 1: 0.002 Mode 2: 3.35 Mode 3: 547.82 |
| Mesh 2 | 1.9 | 0.44 | 0.63 |
Mode 1: 1.24 × 10 Mode 2: 5.53 × 10 Mode 3: 6.78 × 10 |
Mode 1: 0.02 Mode 2: 0.75 Mode 3: 6.63 |
Mode 1: 0.004 Mode 2: 4.12 Mode 3: 649.74 |
| Mesh 3 | 0.86 | 0.54 | 0.4 |
Mode 1: 1.96 × 10 Mode 2: 8.71 × 10 Mode 3: 1.07 |
Mode 1: 0.03 Mode 2: 1.42 Mode 3: 10.92 |
Mode 1: 0.006 Mode 2: 7.81 Mode 3: 1070.1 |
| Mesh 4 | 0.55 | 0.55 | 0.28 |
Mode 1: 2.80 × 10 Mode 2: 1.24 × 10 Mode 3: 1.53 |
Mode 1: 0.05 Mode 2: 2.02 Mode 3: 13.55 |
Mode 1: 0.01 Mode 2: 11.11 Mode 3: 1327.9 |
Stokes numbers are calculated for Venusian conditions at a 50 km altitude for each particle Mode’s approximate diameter: Mode 1 is 300 nm, Mode 2 is 2 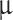 m, and Mode 3 is 7
m, and Mode 3 is 7 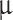 m aerosols. The collection in the middle cloud layer is calculated for each mesh individually for passive collection per one Earth day.
m aerosols. The collection in the middle cloud layer is calculated for each mesh individually for passive collection per one Earth day.
Fig. 3.
The side-view schematics of forces that are acting on aerosols during their interaction with the VCC collection surface meshes.
The parameters of the meshes are presented in Table 1 (including the square’s opening size formed by individual wires s; the shade coefficient, which is a fraction of the wire area of the collector to the total area; and each individual wire diameter) and visualized in Fig. 2. The mesh material is MONEL® nickel-copper alloy 400, and expected maximum erosion in concentrated hot (95  C) sulfuric acid is under 15
C) sulfuric acid is under 15 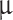 m per one Earth day51. Alternatively, stainless steel (SS) 904L can be used for components that are in direct contact with Venusian clouds. SS 316 may not be suitable for prolonged exposures to sulfuric acid but can be used for solutions up to 20% and over 90% concentration by weight (
m per one Earth day51. Alternatively, stainless steel (SS) 904L can be used for components that are in direct contact with Venusian clouds. SS 316 may not be suitable for prolonged exposures to sulfuric acid but can be used for solutions up to 20% and over 90% concentration by weight (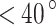 C). At certain conditions (60% concentration by weight and
C). At certain conditions (60% concentration by weight and 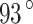 C), SS 316 erosion rate reaches over 3.4 mm per one Earth day, and SS 304 can reach 7 mm per one Earth day51.
C), SS 316 erosion rate reaches over 3.4 mm per one Earth day, and SS 304 can reach 7 mm per one Earth day51.
The primary reason for the wire mesh, as opposed to other geometries (i.e., Raschel mesh, harp wires, 3D textile, etc.), is its structural integrity for high-stress loads in high-vibration and shock environments during launch. The main disadvantage of such geometry is an aerosol passage without impact at low shade coefficients and clogging at high shade coefficients38. The shade coefficient is a fraction of the wire area of the collector to the total area. The optimal shade coefficient is 0.5–0.652. We address the clogging issue by having a non-rigid point attachment of the mesh structure, which causes it to fluctuate slightly (i.e., long-period vibration caused by stochastic aerodynamic force due to wind) and drive clogged droplets down by the gravity gradient. This approach requires launch locks for the launch and cruise, which are released during the VCC operation.
Taking into consideration total liquid availability from Fig. 1 and theoretical efficiency from the last column of Table 1, it becomes evident that it is not feasible to collect Mode 1 and Mode 2 particles in reasonable amounts by passive collection. Auxiliary force is required to drive the smallest particles in Venusian clouds to the collection surfaces. The VCC introduces electrostatic force by giving charge to an aerosol, which, in turn, is attracted to the grounded mesh by Coulomb force. In the next two paragraphs, we describe forces that act on the aerosols in a mathematical manner, and how electrostatic force helps to increase the overall collection efficiency and attract smaller particles.
Stokes number Stk characterizes particle behavior in a fluid flow and their ability to follow the streamlines. A large Stokes number increases total deposition efficiency 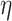 .
. 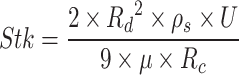 , where
, where 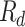 is sulfuric acid droplet radius (Mode 1 = 0.15
is sulfuric acid droplet radius (Mode 1 = 0.15 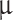 m, Mode 2 = 1
m, Mode 2 = 1 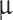 m, Mode 3 = 3.5
m, Mode 3 = 3.5 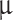 m),
m), 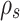 is sulfuric acid density 1830 kg
is sulfuric acid density 1830 kg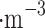 , U is the relative speed of 0.72 m
, U is the relative speed of 0.72 m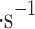 ,
, 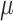 is Venus viscosity of 1.68
is Venus viscosity of 1.68 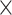 10
10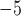 Pa
Pa s calculated at a 50 km altitude53,
s calculated at a 50 km altitude53, 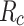 is the wire radius of each mesh that is specified in Table 1. The overall deposition efficiency is established empirically43,54
is the wire radius of each mesh that is specified in Table 1. The overall deposition efficiency is established empirically43,54
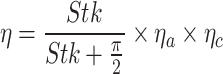 , where
, where 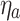 is a shade-coefficient-dependent ratio of aerosols that are moving towards the deposition area to the wire surface,
is a shade-coefficient-dependent ratio of aerosols that are moving towards the deposition area to the wire surface, 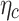 is a clogging coefficient, and 0.5 is used in the calculations. Theoretical efficiency from the last column of Table 1 is calculated using the overall deposition efficiency
is a clogging coefficient, and 0.5 is used in the calculations. Theoretical efficiency from the last column of Table 1 is calculated using the overall deposition efficiency 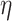 .
.
Our design introduces electrostatic force in addition to the drag force. High voltage discharge generates an electric field at the tungsten needle’s surface; air and aerosol ionization also occur here. Ion wind propagates from the needle to the mesh. Ionized particles also push neutral air molecules into motion by normal elastic collisions. The ozone smell is a good indicator of ionization occurring while running the collector in Earth’s atmosphere. The resulting Coulomb forces between charged aerosols and grounded deposition mesh is 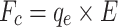 , where E is an electric field, which in case of a − 10 kV charge and 2 cm separation between the needle and mesh is E = 5
, where E is an electric field, which in case of a − 10 kV charge and 2 cm separation between the needle and mesh is E = 5 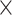 10
10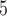 V
V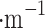 . If the aerosol is singly charged, the resulting attractive forces
. If the aerosol is singly charged, the resulting attractive forces 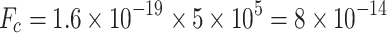 N would be responsible for attracting a considerable amount of Mode 1 and Mode 2 aerosols to the deposition surface. This is essential for the efficient capture of Mode 1 and Mode 2 particles.
N would be responsible for attracting a considerable amount of Mode 1 and Mode 2 aerosols to the deposition surface. This is essential for the efficient capture of Mode 1 and Mode 2 particles.
Laboratory framework for sulfuric acid clouds
The interest in studying sulfuric acid aerosols on Earth comes not only from the Venusian atmosphere. Earth’s atmospheric sulfur cycle is significant for climate, ecology, and health. Sulfur-containing gases are transported from Earth’s surface to the troposphere, where they react and form sulfuric acid aerosols. These aerosols play a vital role in cloud formation and act as cloud condensation nuclei55, which involves numerous laboratory studies56. Despite the large accessibility of laboratory equipment for aerosol studies, it is uncommon to purchase a large off-the-shelf chamber that generates sulfuric acid aerosols. Therefore, we have constructed a custom-built chamber for sulfuric acid aerosols specifically to test the VCC in an environment that is relevant to Venusian clouds.
For estimating the collection of sulfuric acid aerosols by the VCC, we have created a 160 l enclosed chamber with inlets for an electrical connection and an atomizer, and a 350–700 nm transparent round window, depicted in Fig. 4. The chamber is located inside a chemical fume hood for safe operations. The particles are generated by a stainless steel atomizer operated by compressed air and gravity-fed liquid. In our experiments, we used compressed air (0.4 MPa) and various concentrations of sulfuric acid by volume (25%, 50%, 70%, 80%, 90% and 98%*), the rest water. The atomizer produces a round heterogeneous aerosol pattern in an angle of 18 , optimally up to 30 cm in length, and has a pressure-dependent rate of 0.8–1 l per hour. The cloud collector is located 50 cm from the atomizer and receives particle winds (originating from the atomizer) of approximately 1.6 ± 0.4 m
, optimally up to 30 cm in length, and has a pressure-dependent rate of 0.8–1 l per hour. The cloud collector is located 50 cm from the atomizer and receives particle winds (originating from the atomizer) of approximately 1.6 ± 0.4 m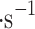 in the front of Mesh 1 and 0.8 ± 0.2 m
in the front of Mesh 1 and 0.8 ± 0.2 m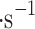 behind Mesh 4. We attempted to use ultrasonic nebulizers (i.e., peristaltically pumped liquid to vibrating fine mesh) with calibrated particle size in pure water of 8 ± 1.5
behind Mesh 4. We attempted to use ultrasonic nebulizers (i.e., peristaltically pumped liquid to vibrating fine mesh) with calibrated particle size in pure water of 8 ± 1.5 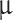 m and 4 ± 0.5
m and 4 ± 0.5 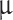 m, but operating them in concentrated sulfuric acid was not possible. The ultrasonic nebulizer operated nominally until 50%
m, but operating them in concentrated sulfuric acid was not possible. The ultrasonic nebulizer operated nominally until 50% 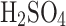 concentration. At acid concentrations higher than 50%, the viscosity of liquid and the surface tension made generating aerosols impossible. In contrast to the ultrasonic nebulizer, the atomizer performs nominally with the entire range of sulfuric acid concentrations while producing heterogeneous particles. The particle size decreases with increased
concentration. At acid concentrations higher than 50%, the viscosity of liquid and the surface tension made generating aerosols impossible. In contrast to the ultrasonic nebulizer, the atomizer performs nominally with the entire range of sulfuric acid concentrations while producing heterogeneous particles. The particle size decreases with increased 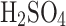 concentration due to changes in the liquid’s surface tension, viscosity, and stability.
concentration due to changes in the liquid’s surface tension, viscosity, and stability.
Fig. 4.
Safe laboratory operation by the corresponding author of our custom-built sulfuric acid aerosol chamber. (A) The full scale 160 liter chamber inside the chemical fume hood. (B) View through the glass of the VCC and discharge needle from the side surrounded by sulfuric acid aerosols. (C) The side of the chamber has an electrical interface for the collector and pressurized gas and liquid inlets for the atomizer.
Field test locations
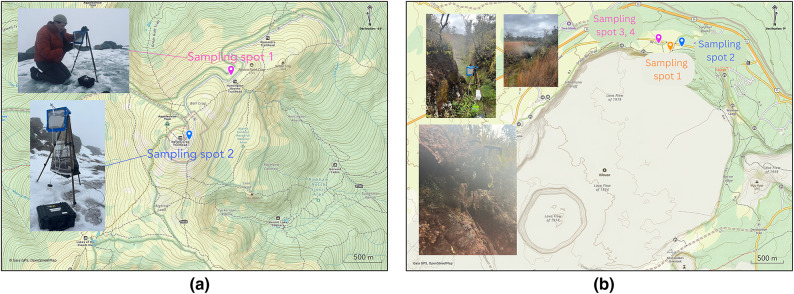
For the field evaluation of the instrument, we selected two locations—Mt. Washington, NH, USA, and Kīlauea volcano, HI, USA (Fig. 5). The field tests were conducted in October and November of 2023. These two field test locations take advantage of unique environmental conditions that are particularly suitable for VCC field testing. Mt. Washington experiences an extremely windy environment47 with regular cloud coverage, while Kīlauea volcano has high emissions of sulfur dioxide and sulfate aerosols48,49. The geographical location and elevation of each sampling spot are:
- Mt. Washington, NH, USA:
- Sampling spot 1: 44.27776 N, − 71.29454 W, elevation of 1742 m.
- Sampling spot 2: 44.27027 N, − 71.30180 W, elevation of 1891 m.
- Kīlauea crater rim:
- Sampling spot 1: 19.43021 N, − 155.26607 W, elevation of 1198 m.
- Sampling spot 2: 19.43079 N, − 155.26437 W, elevation of 1200 m.
- Sampling spot 3, 4: 19.43126 N, − 155.2678 W, elevation of 1206 m.
Fig. 5.
The sampling locations are at the creator rim of Kīlauea volcano, HI, USA, and near the summit of Mt. Washington, NH, USA. The VCC is operated by the corresponding author. The map was generated in https://www.gaiagps.com/ (accessed on July 2, 2024; no permission is needed when attributed as stated above).
Aerosol collection protocols and equipment
The concentrated sulfuric acid was purchased from Fisher Chemical  with a certified concentration range of 96–98%. Therefore, we denote it with an asterisk as 98%* to indicate the remote possibility of the solution being up to 2% less concentrated.
with a certified concentration range of 96–98%. Therefore, we denote it with an asterisk as 98%* to indicate the remote possibility of the solution being up to 2% less concentrated.
In the laboratory collection of sulfuric acid, we used the following protocol:
Prepare sulfuric acid solutions in water with concentrations by volume of 25%, 50%, 70%, 80%, 90% and 98%*. Each solution with a total volume of 30 ml is stored in a closed container. Each solution is cooled down to room temperature naturally after the highly exothermic reaction.
Locate VCC at a distance of 0.5 m from the atomizer nozzle inside the chamber. Measure the wind speed in the front of and behind VCC while running the nebulizer. Measure the weight of the empty collection container/vial. If the run is electrostatic, engage the high-voltage supply to the needle. If the run is passive, no voltage is supplied to the tungsten needle. Both passive and electrostatic operations have the same VCC geometry (i.e., the physical needle is left on the front of the collector) in order to have comparable conditions and to ensure comparable collection results.
Seal the chamber. Atomize each solution by injecting it into the atomizer interface. Let the atomizer run for 10 min after the entire liquid volume is atomized. Disengage the atomizer.
Remove the top cover of the chamber. Measure the weight of the collected sample and calculate the weight of the collected liquid solution.
Remove VCC to the cleaning area. Neutralize the sulfuric acid by sodium bicarbonate (
 ). Safely dispose of the neutralized solution in the special container and locate it in the designated waste area of hazardous materials. Clean VCC with a large amount of water. Dry VCC with compressed air.
). Safely dispose of the neutralized solution in the special container and locate it in the designated waste area of hazardous materials. Clean VCC with a large amount of water. Dry VCC with compressed air.Repeat steps 2–5 with each solution. After tests are completed, neutralize and clean the chamber after use.
During the field campaign collection of water clouds and volcanic fumaroles’ particles, we used the following protocol:
Choose a suitable location, measure wind speed and direction (with BTMETER BT-100 handheld anemometer), measure temperature, and write down sampling coordinates.
Place VCC on the tripod, connect it to clean collection vials, and orient VCC to face the wind.
Mark collection time and weigh the collected solution. Mark the vial with a unique identifier and seal it. Clean VCC with isopropyl alcohol (
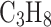 O), wash it with water and let it dry naturally.
O), wash it with water and let it dry naturally.Repeat steps 2 and 3 for each sampling location.
The UV–Vis spectra (190–840 nm) of the collected samples from the field were obtained using the Denovix DS-C Spectrophotometer with plastic cuvettes. The UV–Vis spectrometer uses a pulsed xenon flash lamp as a light source with a measurement time of two seconds and an absorbance accuracy of 1.5%. Deionized water was used as a blank for all the samples.
Results and discussion
In this section, we focus on the collection efficiency of sulfuric acid in the laboratory, the collection rates of water clouds and volcanic fumaroles’ particles in the field with the VCC, and identify cross-contamination in the field samples.
Laboratory sulfuric acid collection efficiency
Here, we report on the overall collection efficiency of various concentrations of sulfuric acid in water solution and the assessment of aerosol moisture absorption from the air. According to the protocols described in “Aerosol collection protocols and equipment” section, we atomized 30 ml of the liquid solutions and collected it inside the aerosol chamber for approximately 10 minutes, which resulted in particulate matter of 185–345 g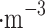 . We demonstrated that both passive and electrostatic collection modes are capable of collecting the entire range of sulfuric acid concentrations in water solution.
. We demonstrated that both passive and electrostatic collection modes are capable of collecting the entire range of sulfuric acid concentrations in water solution.
Table 2 shows the weight of each liquid before atomization and the weight of each collected solution by passive and electrostatic modes in triplicates. The relative improvement of electrostatic over passive operation is demonstrated in the last column. We observed significant improvement in collection efficiency (i.e., over 30%) for water and low-concentration solutions (i.e., under 50% 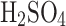 ). At concentrations over 50%, the relative efficiency improvement decreased steadily and stabilized at a few percent for concentrations over 80%.
). At concentrations over 50%, the relative efficiency improvement decreased steadily and stabilized at a few percent for concentrations over 80%.
Table 2.
Collection of aerosol particles with various sulfuric acid concentrations using the VCC collector with (i.e., electrostatic mode) and without (i.e., passive mode) high-voltage activation.
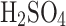 v/v v/v |
Density, kg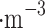
|
Weight of 30 ml, g | Collected passively, g | Collected electrostatically, g | Relative improvement, % |
|---|---|---|---|---|---|
| 0% (i.e., pure water) | 997 | 29.9 | 2.4 | 14.4 | 45.4 |
| 2.7 | 4.8 | 43.7 | |||
| 6.3 | 4.2 | 21.4 | |||
| 25% | 1178 | 35.3 | 4.6 | 9.1 | 49.4 |
| 6.1 | 9.3 | 34.4 | |||
| 6.3 | 11.7 | 46.1 | |||
| 50% | 1395 | 41.8 | 10.7 | 16.2 | 33.9 |
| 11.5 | 14.6 | 21.2 | |||
| 9.5 | 13.9 | 31.6 | |||
| 70% | 1610 | 48.3 | 17.2 | 22.5 | 23.5 |
| 16.7 | 20.3 | 17.7 | |||
| 12.4 | 19.0 | 34.7 | |||
| 80% | 1737 | 51.8 | 20.7 | 24.0 | 13.7 |
| 22.4 | 24.1 | 7.05 | |||
| 20.4 | 19.9 | − 2.5 | |||
| 90% | 1825 | 54.4 | 22.8 | 25.5 | 10.5 |
| 24.0 | 26.6 | 9.7 | |||
| 24.1 | 24.9 | 3.2 | |||
| 98%* | 1845 | 55.1 | 26.1 | 28.1 | 7.1 |
| 26.5 | 28.7 | 7.6 | |||
| 24.4 | 26.3 | 7.2 |
Each solution was run three times. 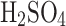 v/v is sulfuric acid concentration in water by volume. The relative improvement shows an efficiency increase of electrostatic over passive collections; if the value is negative, the passive collection was more efficient in a given run. The density of the solutions is provided at 20
v/v is sulfuric acid concentration in water by volume. The relative improvement shows an efficiency increase of electrostatic over passive collections; if the value is negative, the passive collection was more efficient in a given run. The density of the solutions is provided at 20  C.
C.
The primary findings of this experiment are the following:
It is feasible to collect aerosols composed of the entire range of sulfuric acid concentrations with the VCC collector in both modes (i.e., electrostatic and passive).
The collection efficiency increases steadily with an increased concentration of sulfuric acid solution in water in both modes.
The relative efficiency increase of electrostatic over passive collection mode is decreased with the concentration after its peak at 25%
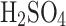 where the sulfuric acid solution has the highest conductivity (see Fig. 6). However, for the concentrations expected in Venus (over 70%
where the sulfuric acid solution has the highest conductivity (see Fig. 6). However, for the concentrations expected in Venus (over 70% 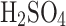 ), the complexity of electrostatic operation might not be justified for a relatively small efficiency increase, and the passive mode might be more suitable.
), the complexity of electrostatic operation might not be justified for a relatively small efficiency increase, and the passive mode might be more suitable.
As we described in “An introduction to cloud liquid collection approaches” section, we do not have general knowledge of the exact composition, acidity and concentration of 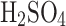 in Venus’s clouds. Our chamber generates larger particles reported by Pioneer Venus (a few microns to tens of microns), and the performance of the VCC might differ with a significant presence of submicron particles. At around 80%
in Venus’s clouds. Our chamber generates larger particles reported by Pioneer Venus (a few microns to tens of microns), and the performance of the VCC might differ with a significant presence of submicron particles. At around 80% 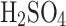 in water, the solution changes its properties from being diluted acid to becoming concentrated acid when its electrical conductivity also drops. The higher concentrations over 80%
in water, the solution changes its properties from being diluted acid to becoming concentrated acid when its electrical conductivity also drops. The higher concentrations over 80% 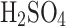 in water showed relatively small improvement between the two modes of operation in our chamber conditions. We hypothesize that the upper limit of the collection efficiency for 98%*
in water showed relatively small improvement between the two modes of operation in our chamber conditions. We hypothesize that the upper limit of the collection efficiency for 98%* 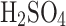 is reached due to the clogging (i.e., related to the clogging coefficient), decreased surface tension (i.e., the atomizer produces larger, smaller aerosols), increased viscosity (i.e., related to clogging), long-lived aerial aerosols (observed qualitatively in comparison with more diluted solutions), and conductivity of solution.
is reached due to the clogging (i.e., related to the clogging coefficient), decreased surface tension (i.e., the atomizer produces larger, smaller aerosols), increased viscosity (i.e., related to clogging), long-lived aerial aerosols (observed qualitatively in comparison with more diluted solutions), and conductivity of solution.
Fig. 6.
The relative efficiency of sulfuric acid aerosols collection after aerosolizing 30 ml of liquid solution by volume in the enclosed chamber for 10 min. The aerosols circulate in the chamber and pass through the deposition mesh many times. The conductivity profile from Darling57 was originally measured for water and sulfuric acid solution concentrations by weight (conductivity w/w) and then converted to the corresponding solution concentrations by volume (conductivity v/v).
Figure 6 shows the final relation of collection efficiency as a function of concentration for passive and electrostatic configurations in the closed chamber with secondary aerosol circulation for 10 min. In the auxiliary axis, we also included the conductivity of various concentrations of sulfuric acid by weight and volume at 21  C (approximate laboratory temperature) calculated from experimental data by Darling, 196457. Figure 6 visually shows how the properties of the liquid change based on the concentration.
C (approximate laboratory temperature) calculated from experimental data by Darling, 196457. Figure 6 visually shows how the properties of the liquid change based on the concentration.
It is also unclear how electrostatic discharge would impact the chemistry of aerosols and hypothetical biomolecules present in aerosol particles. While aerosols of Venus might experience natural high voltage discharges due to lightning58, the presence of lightning at Venus is controversial59. Therefore, the relatively small improvement of the electrostatic over the passive mode might not be justified in the context of our experimental conditions. However, we do not know how this system would perform with submicron particles of concentrated sulfuric acid that are extremely difficult to generate artificially but are present in the entire cloud deck of Venus. Nonetheless, the VCC is a suitable instrument for Venus cloud particle collection, but the necessity of an electrostatic mode of operation is a trade-off decision that depends on particular mission requirements.
We also considered the primary error of collection estimation by moisture absorption by 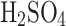 aerosols that is described in the following subsection.
aerosols that is described in the following subsection.
Collection estimation error by sulfuric acid moisture absorption
The primary error in establishing collected amounts is the absorption of atmospheric moisture by sulfuric acid aerosols. Water has a partially positive charge on the hydrogen atoms and a negative charge on the oxygen atoms. Highly electronegative oxygen atoms in sulfuric acid attract the hydrogen atoms in water by forming hydrogen bonds. The bonding process of sulfuric acid and water also occurs when sulfuric acid is exposed to humid air, which leads to a volumetric increase of sulfuric acid while reducing its concentration. When sulfuric acid bottles are opened in the laboratory (relative humidity  70%), they begin to absorb moisture from the air, and even more so when aerosolized due to increased exposed surface area. In order to qualitatively account for this error in our experiment, we measured water absorption using two open vials in the same fume hood. Two dishes of 18 mm diameter and 36.9 mm were filled with a 6 ml solution of various concentrations and monitored after 2 h and 21 h. The 25% solution of sulfuric acid loses its mass by evaporation, while the rest of the solutions (i.e., above 25% concentration) absorb the moisture. If the experiments would run longer (i.e., hours), the dilution impact on the solution concentration would need to be accounted for. However, the impact of air moisture absorption during the short test experiments was insignificant. The results are reported in Table 3.
70%), they begin to absorb moisture from the air, and even more so when aerosolized due to increased exposed surface area. In order to qualitatively account for this error in our experiment, we measured water absorption using two open vials in the same fume hood. Two dishes of 18 mm diameter and 36.9 mm were filled with a 6 ml solution of various concentrations and monitored after 2 h and 21 h. The 25% solution of sulfuric acid loses its mass by evaporation, while the rest of the solutions (i.e., above 25% concentration) absorb the moisture. If the experiments would run longer (i.e., hours), the dilution impact on the solution concentration would need to be accounted for. However, the impact of air moisture absorption during the short test experiments was insignificant. The results are reported in Table 3.
Table 3.
Absorption of moisture from the air inside the fume hood by two open surfaces with 6 ml of liquid for various concentrations of sulfuric acid shown in percentage by volume and time shown in hours.
| Open area | 25%, 2 h | 25%, 21 h | 50%, 2 h | 50%, 21 h | 75%, 2 h | 75%, 21 h | 98%, 2 h | 98%, 21 h |
|---|---|---|---|---|---|---|---|---|
255 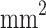
|
− 0.079 g | − 0.249 g | 0.0 g | 0.403 g | 0.066 g | 0.768 g | 0.129 g | 1.225 g |
1070 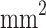
|
− 0.237 g | − 0.525 g | 0.100 g | 2.500 g | 0.800 g | 3.800 g | 0.900 g | 6.500 g |
The short-term linear regression (i.e., minutes–hours scale) for the results from Table 3 are the following for each concentration, where Abs is the absorption of moisture in grams (evaporation if negative), t is time in hours, and A is area in 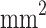 :
:
25%
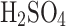 concentration by volume:
concentration by volume: 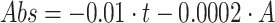 ;
;50%
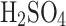 concentration by volume:
concentration by volume: 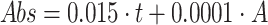 ;
;75%
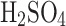 concentration by volume:
concentration by volume: 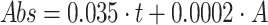 ;
;96%
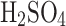 concentration by volume:
concentration by volume: 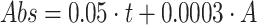 .
.
For qualitative adjustment, we calculate absorption for certain concentrations of 30 ml during 10 min into an average aerosol diameter of 10  m. The resulting correction values are: − 5.27 mg for 25%; 4.31 mg for 50%; 9.45 mg for 75%; and 13.75 mg for 96% concentrations. Error values are insignificant for such short operations and have an insignificant impact compared to collection efficiency errors (see Fig. 6). However, this effect must be considered for longer experiments and correlated with collection efficiencies.
m. The resulting correction values are: − 5.27 mg for 25%; 4.31 mg for 50%; 9.45 mg for 75%; and 13.75 mg for 96% concentrations. Error values are insignificant for such short operations and have an insignificant impact compared to collection efficiency errors (see Fig. 6). However, this effect must be considered for longer experiments and correlated with collection efficiencies.
Field sample collection rate
In this subsection, we report on collection rates with the VCC in the field campaigns. We show collection rates (amount of liquid per time), because establishing efficiency (in contrast to the laboratory tests) is impossible since we cannot determine how much liquid passes through the collector. The VCC demonstrated liquid collection both in high and low winds, despite its small surface area (as compared with conventional fog collectors). Figure 7 shows the collection rate from each sampling spot (see Tables 4, 5) and the wind profiles. The VCC is a feasible instrument for acquiring liquid samples from Earth clouds and volcanic fumaroles’ particles for analytical purposes, which further supports its applicability in collecting Venusian clouds.
Fig. 7.
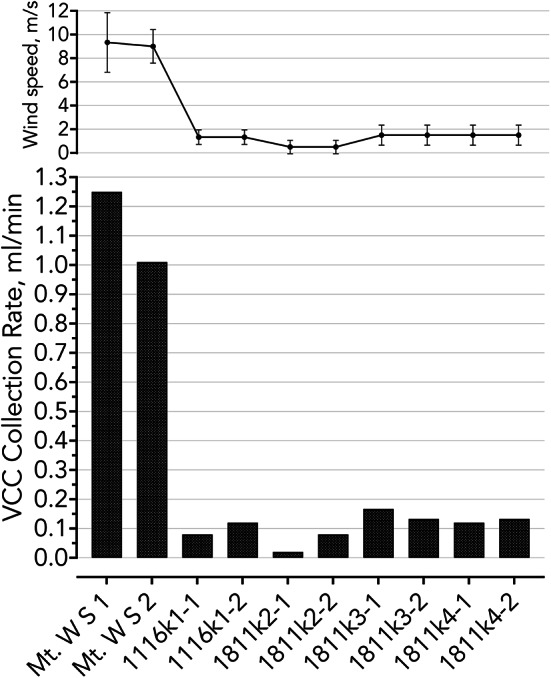
The VCC collection rate of field samples and wind profile for each sampling spot on Mt. Washington and Kīlauea crater rim. The sampling spots are described in Tables 4, 5 and Fig. 5.
Table 4.
Sample collection and conditions on Mt. Washington.
| Location | Wind, m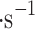
|
T,  C C |
P, mbar | Collection time, min | Collected electrostatically, g |
|---|---|---|---|---|---|
| Sample 1 | 7–9, bursts 12 | 5 | 1024.8 | 20 | 25 |
| Sample 2 | 8, bursts 10 | 13 | 1025.3 | 20 | 20.2 |
The samples were collected on October 20, 2023. T is temperature, and P is ambient pressure.
Table 5.
Sample collection and conditions at Kīlauea crater rim.
| Location | Sample no | Wind, m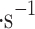
|
T,  C C |
Collecting time, min | Collected electrostatically, g |
|---|---|---|---|---|---|
| Sampling spot 1 | 1116k1-1 | 1 ± 0.2, bursts 2 | 17 | 25 | 2 |
| 1116k1-2 | 3 | ||||
| Sampling spot 2 | 1118k2-1 | 0.1–0.9 | 20.2 | 25 | 0.5 |
| 1118k2-2 | 2 | ||||
| Sampling spot 3 | 1118k3-1 | 1.5 ± 0.6 | 12 | 15 | 2.5 |
| 1118k3-2 | 2 | ||||
| Sampling spot 4 | 1118k4-1 | 1.5 ± 0.6 | 12 | 15 | 1.8 |
| 1118k4-2 | 2 |
In each sample location, the duplicate of the sample was collected by placing a tubing splitter after the gutter. The samples were collected on November 16–18, 2023. The accuracy of temperature measurements might have been impacted by hot fumaroles’ particles (50–60  C).
C).
The collection rates by electrostatic VCC operations are presented in Table 4 for Mt. Washington and in Table 5 for Kīlauea volcano. The environmental conditions on Mt. Washington drastically differed from Kīlauea, with snow coverage on the ground, fast winds, and cloud coverage with a few meters of visibility. We observed very light rain after collecting Sample 1, and for Sample 2, we moved to a higher altitude area with no rain, but we visually observed large aerosols/mist. At Kīlauea, the activity of fumaroles and weather (precipitation, temperature, wind) changed hourly; the wind was slow, and the temperature of the particles ejected from fumaroles reached 50–60  C.
C.
VCC cross-contamination in Kīlauea samples
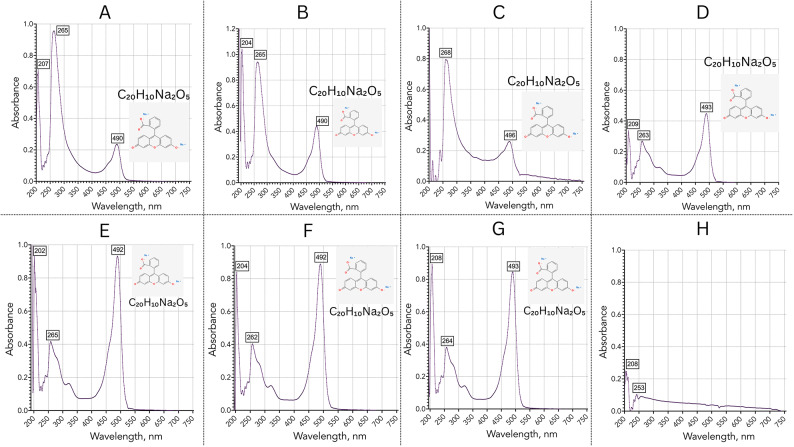
We designed the collector with the knowledge that cross-contamination for bulk samplers must be addressed in the processing of the data, as it cannot be addressed mechanically if using a single collector. Here, we demonstrate the detection of chemical compounds from laboratory studies that contaminated the field samples. Figure 8 shows UV–Vis absorbance spectra of the Kīlauea samples (A–G) collected by the VCC, and sample H acquired without the VCC directly to a vial. We observed four main peaks across the samples (190–196 nm, 202–207 nm, 262–265 nm and 490–496 nm), but our focus here is on 490–496 nm peaks.
Fig. 8.
Contamination assessment via the sample analysis using UV–Vis spectrometry on Kīlauea samples. (A) 1116k1-1 sample; (B) 1116k1-2 sample; (C) 1811k2-2 sample; (D) 1811k3-1 sample; (E) 1811k3-2 sample; (F) 1811k4-1 sample; (G) 1811k4-2 sample; (H) direct fumarole rock condensation sample. The main focus of absorbance spectra is at 490–496 nm.
To rule out contamination by the collector, we acquired samples directly from hot rock cracks where fumaroles’ particles were emerging and condensing on the rock—this is shown in the last graph “H—direct fumarole rock condensation sample”. We observed a 490–496 nm peak in UV–Vis absorbance spectra in all samples that were in contact with the VCC mesh, but not in sample H. A few months before the field tests, in the laboratory, we ran the same VCC mesh with fluorescein sodium salt (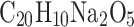 ) diluted in water, which experiences maximum absorbance in water at 494 nm. Even after a long, thorough cleaning (isopropyl alcohol and a large amount of water) and numerous operations on the collector over a long period of time, the contamination by fluorescein is strongly evident across all samples collected by the VCC but is absent in sample H, which was not in contact with the VCC mesh. The contamination assessment by fluorescein indicates that one could indeed anticipate cross-contamination from the VCC collector. The contamination assessment experiment underscores the need for careful flight instrument preparation ahead of the mission, including assessment of potential organic contamination, either biological or chemical.
) diluted in water, which experiences maximum absorbance in water at 494 nm. Even after a long, thorough cleaning (isopropyl alcohol and a large amount of water) and numerous operations on the collector over a long period of time, the contamination by fluorescein is strongly evident across all samples collected by the VCC but is absent in sample H, which was not in contact with the VCC mesh. The contamination assessment by fluorescein indicates that one could indeed anticipate cross-contamination from the VCC collector. The contamination assessment experiment underscores the need for careful flight instrument preparation ahead of the mission, including assessment of potential organic contamination, either biological or chemical.
Summary
We have demonstrated a proof-of-concept of a lightweight (below 1 kg) two-mode cloud collector (passive and electrostatic), called the Venus Cloud Catcher (VCC), operational under various sulfuric acid concentrations (25%, 50%, 70%, 80%, 90% and 98%* of sulfuric acid solution in water) in an enclosed chamber, and tested it in the field with Earth clouds and volcanic fumaroles’ particles. The four 15  15
15 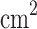 deposition surfaces are arranged in a sandwich structure of various density meshes. In electrostatic mode, the discharge of − 10 kV on the tungsten needle is achieved by custom low-power (under 2 W) electronics. The tungsten needle gives an electric charge to an individual aerosol, which, under Coulomb force, gets attracted to the ground mesh, in turn increasing the overall VCC collection efficiency and making it possible to collect smaller particles by theoretical calculations (micron–submicron level). We demonstrated the feasibility of collecting aerosols composed of the entire range of sulfuric acid concentrations with the VCC collector in both modes (i.e., electrostatic and passive). We observed a steady collection efficiency increase with the increased concentration of sulfuric acid solution in water in both modes. The relative efficiency increase of the electrostatic over the passive mode (ranging from 7% to nearly 50%) of the collection is decreased with the concentration after its peak at 25 %
deposition surfaces are arranged in a sandwich structure of various density meshes. In electrostatic mode, the discharge of − 10 kV on the tungsten needle is achieved by custom low-power (under 2 W) electronics. The tungsten needle gives an electric charge to an individual aerosol, which, under Coulomb force, gets attracted to the ground mesh, in turn increasing the overall VCC collection efficiency and making it possible to collect smaller particles by theoretical calculations (micron–submicron level). We demonstrated the feasibility of collecting aerosols composed of the entire range of sulfuric acid concentrations with the VCC collector in both modes (i.e., electrostatic and passive). We observed a steady collection efficiency increase with the increased concentration of sulfuric acid solution in water in both modes. The relative efficiency increase of the electrostatic over the passive mode (ranging from 7% to nearly 50%) of the collection is decreased with the concentration after its peak at 25 % 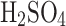 concentration, where the sulfuric acid solution has the highest conductivity (see Fig. 6). However, for the acid concentrations expected in Venus clouds (over 70%
concentration, where the sulfuric acid solution has the highest conductivity (see Fig. 6). However, for the acid concentrations expected in Venus clouds (over 70% 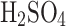 ), the complexity of electrostatic operation might not be justified for a relatively small efficiency increase, and the passive mode might be more suitable for such operations. We think a small efficiency increase is related to the longer lifetime of sulfuric acid aerosols (i.e., their stability), change in electrical conductivity, lower surface tension of the liquid, higher viscosity as compared to water, and lower electrical conductivity. The efficiency of collections is represented in an enclosed environment, where aerosols (that range between a few and tens of microns in size) circulate through the deposition surface multiple times with fixed atomized volume.
), the complexity of electrostatic operation might not be justified for a relatively small efficiency increase, and the passive mode might be more suitable for such operations. We think a small efficiency increase is related to the longer lifetime of sulfuric acid aerosols (i.e., their stability), change in electrical conductivity, lower surface tension of the liquid, higher viscosity as compared to water, and lower electrical conductivity. The efficiency of collections is represented in an enclosed environment, where aerosols (that range between a few and tens of microns in size) circulate through the deposition surface multiple times with fixed atomized volume.
We demonstrated the VCC performance in the field by collecting Earth clouds on Mt. Washington, NH, USA, and volcanic fumaroles’ particles from Kīlauea crater rim, HI, USA. The collection rate is wind-speed dependent, and as expected, the rate is higher with stronger wind. We observed collection rates around 0.1 ml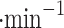 in low wind environments (1–2 m
in low wind environments (1–2 m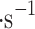 ), and around 1 ml
), and around 1 ml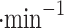 in stronger wind (7–9 m
in stronger wind (7–9 m
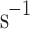 ). Even after thorough cleaning, the UV–Vis analysis of the Earth fumaroles’ particles points to cross-contamination challenges. We detected fluorescein sodium salt across all samples in contact with the VCC, a contaminant from previous laboratory experiments. The contamination assessment assay provides insight into the requirements for the flight instrument regarding its cleaning, contamination, materials used for fabrication, and identification of possible false-positive results from contaminants (especially organic).
). Even after thorough cleaning, the UV–Vis analysis of the Earth fumaroles’ particles points to cross-contamination challenges. We detected fluorescein sodium salt across all samples in contact with the VCC, a contaminant from previous laboratory experiments. The contamination assessment assay provides insight into the requirements for the flight instrument regarding its cleaning, contamination, materials used for fabrication, and identification of possible false-positive results from contaminants (especially organic).
Our team’s next step is VCC integration into an end-to-end sample handling system for the collection, storage, preparation, and transportation of samples to analytical instruments. Future efforts will involve mission-specific mesh sizing and the design of a flight-deployable version of the VCC.
The VCC makes it feasible to collect milliliter-scale samples during a multi-day aerial mission (e.g., balloon), enabling detailed in-situ analysis and, eventually, a sample return to Earth. The VCC was primarily developed for Morning Star Missions to Venus. It could potentially provide an enhanced collection of liquid samples in comparison to the pump-based approach considered by other mission concepts (e.g., Venus Flagship Mission).
Acknowledgements
Iakubivskyi’s postdoctoral efforts are supported by the Estonian Research Council Grant PUTJD1125 and MIT. This study was partially supported by NASA Innovative Advanced Concepts Phase I “Venus Atmosphere and Cloud Particle Sample Return for Astrobiology 80NSSC22KO759” and MIT. The initial concept for this work is the direct outcome of the former “Venus Life Finder Study” sponsored by Breakthrough Initiatives. We thank the members of the Morning Star Missions to Venus team (https://venuscloudlife.com/team/) for the meaningful discussions and contributions. The corresponding author thanks Shira Wolfe for proofreading the paper and Pekka Janhunen for discussing forces acting on aerosols.
Appendix
Electrostatic operation
The electronics prototype used for this work is based on the Arduino UNO R4 WiFi and DC-DC converter from HVM Technology SMHV05100N. The total power consumption is under 2 W. The breadboard is shown in Fig. 9 and the flight electronics configuration in the housing is shown in Fig. 10.
Fig. 9.
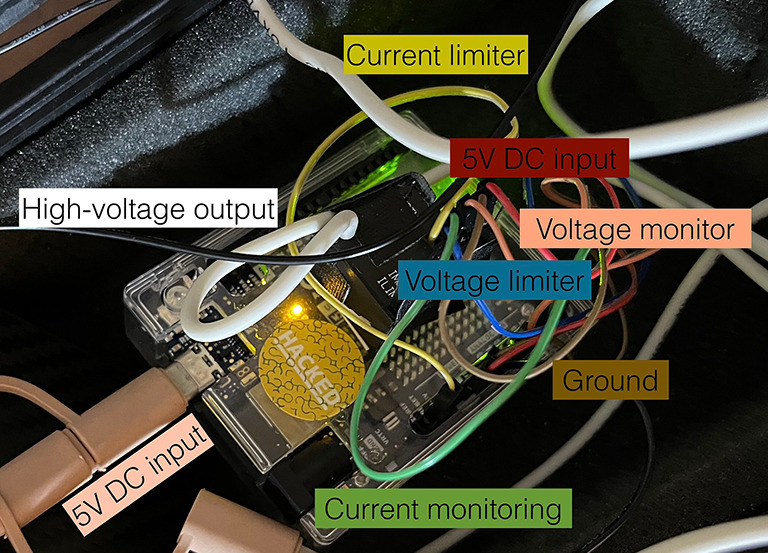
Photo of control electronics prototype inside the small Pelikan® waterproof case. Input 5V DC voltage is supplied by a 6 Ah power bank weighing 150 g.
Fig. 10.
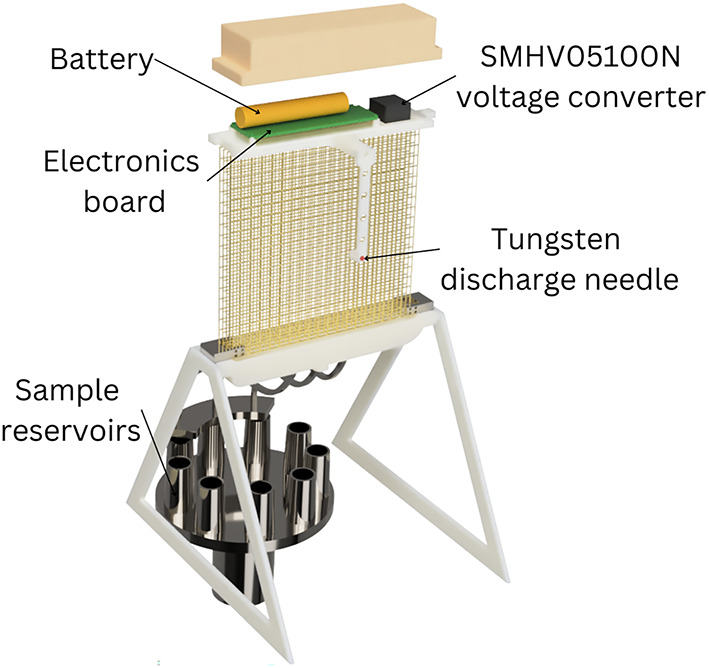
Electronics configuration on the VCC.
Author contributions
I.I. conceived and conducted the experiment and field testing, analyzed the data, built VCC, built sulfuric acid chamber, and wrote the manuscript; S.S., J.J.P., and C.E.C. mentored and supervised the experiments and development; C.E.C. provided the vertical winds model; R.A. assisted in the laboratory and contributed to engineering; R.A. and M.R.A.M. assisted in the field campaign; S.N contributed to the design of the experiments. All authors reviewed the manuscript.
Data availability
The data is provided in the tables within the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barath, F., Barrett, A., Copeland, J., Jones, D. & Lilley, A. Mariner II: Preliminary reports on measurements of Venus: Microwave radiometers. Science139, 908–909. 10.1126/science.139.3558.908 (1963). [DOI] [PubMed] [Google Scholar]
- 2.Titov, D. V., Ignatiev, N. I., McGouldrick, K., Wilquet, V. & Wilson, C. F. Clouds and hazes of Venus. Space Sci. Rev.214, 1–61. 10.1007/s11214-018-0552-z (2018). [Google Scholar]
- 3.Ragent, B. et al. Particulate matter in the Venus atmosphere. Adv. Space Res.5, 85–115. 10.1016/0273-1177(85)90199-1 (1985). [Google Scholar]
- 4.Knollenberg, R. G. & Hunten, D. M. The microphysics of the clouds of Venus: Results of the Pioneer Venus particle size spectrometer experiment. J. Geophys. Res. Space Phys.85, 8039–8058. 10.1029/JA085iA13p08039 (1980). [Google Scholar]
- 5.Toon, O. B., Ragent, B., Colburn, D., Blamont, J. & Cot, C. Large, solid particles in the clouds of Venus: Do they exist? Icarus57, 143–160. 10.1016/0019-1035(84)90063-0 (1984). [Google Scholar]
- 6.Zasova, L., Moroz, V. & Linkin, V. Venera-15, 16 and vega mission results as sources for improvements of the venus reference atmosphere. Adv. Space Res.17, 171–180. 10.1016/0273-1177(95)00747-3 (1996). [Google Scholar]
- 7.Knollenberg, R. G. A reexamination of the evidence for large, solid particles in the clouds of Venus. Icarus57, 161–183. 10.1016/0019-1035(84)90064-2 (1984). [Google Scholar]
- 8.Moroz, V. Estimates of visibility of the surface of Venus from descent probes and balloons. Planet. Space Sci.50, 287–297. 10.1016/S0032-0633(01)00128-3 (2002). [Google Scholar]
- 9.Sánchez-Lavega, A., Lebonnois, S., Imamura, T., Read, P. & Luz, D. The atmospheric dynamics of Venus. Space Sci. Rev.212, 1541–1616 (2017). [Google Scholar]
- 10.Linkin, V. et al. VEGA balloon dynamics and vertical winds in the Venus middle cloud region. Science231, 1417–1419. 10.1126/science.231.4744.1417 (1986). [DOI] [PubMed] [Google Scholar]
- 11.Sagdeev, R. et al. Overview of VEGA Venus balloon in situ meteorological measurements. Science231, 1411–1414. 10.1126/science.231.4744.1411 (1986). [DOI] [PubMed] [Google Scholar]
- 12.Smrekar, S. et al. VERITAS (Venus emissivity, radio science, InSAR, topography, and spectroscopy): A discovery mission. In 2022 IEEE Aerospace Conference (AERO) 1–20. 10.1109/AERO53065.2022.9843269 (2022).
- 13.Straume-Lindner, A.-G. et al. EnVision: An ESA medium-class mission to Venus in collaboration with NASA. In European Planetary Science Congress, EPSC2022–196. 10.5194/epsc2022-196 (2022).
- 14.Garvin, J. B. et al. Revealing the mysteries of Venus: The DAVINCI mission. Planet. Sci. J.3, 117. 10.3847/PSJ/ac63c2 (2022). [Google Scholar]
- 15.Beauchamp, P. et al. Venus flagship mission concept: A decadal survey study. In 2021 IEEE Aerospace Conference 1–18. 10.1109/AERO50100.2021.9438335 (2021). [DOI] [PMC free article] [PubMed]
- 16.Morowitz, H. & Sagan, C. Life in the clouds of Venus? Nature215, 1259–1260. 10.1038/2151259a0 (1967). [Google Scholar]
- 17.Cockell, C. S. Life on Venus. Planet. Space Sci.47, 1487–1501. 10.1016/S0032-0633(99)00036-7 (1999). [Google Scholar]
- 18.Seager, S. et al. The Venusian lower atmosphere haze as a depot for desiccated microbial life: A proposed life cycle for persistence of the Venusian aerial biosphere. Astrobiology21, 1206–1223. 10.1089/ast.2020.2244 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Seager, S. et al. Stability of nucleic acid bases in concentrated sulfuric acid: Implications for the habitability of Venus’ clouds. Proc. Natl. Acad. Sci.120, e2220007120. 10.1073/pnas.2220007120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seager, M. D., Seager, S., Bains, W. & Petkowski, J. J. Stability of 20 biogenic amino acids in concentrated sulfuric acid: Implications for the habitability of Venus’ clouds. Astrobiology24, 386–396. 10.1089/ast.2023.0082 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seager, S. et al. Venus life finder mission study. Preprint at https://arxiv.org/abs/2112.05153 (2021).
- 22.French, R. et al. Rocket Lab mission to Venus. Aerospace9, 445. 10.3390/aerospace9080445 (2022). [Google Scholar]
- 23.Baumgardner, D. et al. Deducing the composition of Venus cloud particles with the autofluorescence nephelometer (AFN). Aerospace9, 492. 10.3390/aerospace9090492 (2022). [Google Scholar]
- 24.Seager, S. et al. Venus life finder habitability mission: Motivation, science objectives, and instrumentation. Aerospace9, 733. 10.3390/aerospace9110733 (2022). [Google Scholar]
- 25.Ligterink, N. F. W. et al. The ORIGIN space instrument for detecting biosignatures and habitability indicators on a Venus life finder mission. Aerospace9, 312. 10.3390/aerospace9060312 (2022). [Google Scholar]
- 26.Kaasik, L. et al. Sensor for determining single droplet acidities in the Venusian atmosphere. Aerospace9, 560. 10.3390/aerospace9100560 (2022). [Google Scholar]
- 27.Isaac, C. & Jones, N. Leading-edge vortex lift (LEVL) sample probe for Venusian atmosphere. Aerospace9, 471. 10.3390/aerospace9090471 (2022). [Google Scholar]
- 28.Tiensuu, K. et al. Mini fluorescence microscope: Prototype results and further development. In 74th International Astronautical Congress, IAC, Proceedings of the International Astronautical Congress, IAC (International Astronautical Federation, IAF, 2023).
- 29.Agrawal, R. et al. Venus cloud sample return concept for astrobiology. Adv. Space Res.74, 490–504. 10.1016/j.asr.2024.03.065 (2024). [Google Scholar]
- 30.Azeem, M. Scientific Design of Multilayer Fog Collectors. Thesis dissertation at the Technical University of Liberec. https://dspace.tul.cz/bitstream/handle/15240/164921/Azeem_Thesis_final_0610.pdf (2021).
- 31.Goodman, J. The collection of fog drip. Water Resour. Res.21, 392–394. 10.1029/WR021i003p00392 (1985). [Google Scholar]
- 32.Schemenauer, R. S. & Joe, P. I. The collection efficiency of a massive fog collector. Atmos. Res.24, 53–69. 10.1016/0169-8095(89)90036-7 (1989). [Google Scholar]
- 33.Walters, P., Moore, M. & Webb, A. A separator for obtaining samples of cloud water in aircraft. Atmos. Environ.1967(17), 1083–1091. 10.1016/0004-6981(83)90331-1 (1983). [Google Scholar]
- 34.Marius Dybwad, G. S. & Blatny, J. M. Comparative testing and evaluation of nine different air samplers: End-to-end sampling efficiencies as specific performance measurements for bioaerosol applications. Aerosol Sci. Technol.48, 282–295. 10.1080/02786826.2013.871501 (2014). [Google Scholar]
- 35.Hoffman, J. H., Oyama, V. I. & von Zahn, U. Measurements of the Venus lower atmosphere composition: A comparison of results. J. Geophys. Res. Space Phys.85, 7871–7881. 10.1029/JA085iA13p07871 (1980). [Google Scholar]
- 36.Marov, M., Lystsev, V., Lebedev, V., Lukashevich, N. & Shari, V. The structure and microphysical properties of the Venus clouds: Venera 9, 10, and 11 data. Icarus44, 608–639. 10.1016/0019-1035(80)90131-1 (1980). [Google Scholar]
- 37.Schunk, C. et al. Testing water yield, efficiency of different meshes and water quality with a novel fog collector for high wind speeds. Aerosol Air Qual. Res.18, 240–253. 10.4209/aaqr.2016.12.0528 (2018). [Google Scholar]
- 38.Kennedy, B. S. & Boreyko, J. B. Bio-inspired fog harvesting meshes: A review. Adv. Funct. Mater.1, 2306162. 10.1002/adfm.202306162 (2023). [Google Scholar]
- 39.Kowalski, N. G., Shi, W., Kennedy, B. S. & Boreyko, J. B. Optimizing fog harps. ACS Appl. Mater. Interfaces13, 38826–38834. 10.1021/acsami.1c08995 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Bintein, P.-B. et al. Kirigami fog nets: How strips improve water collection. NPJ Clean Water6, 54. 10.1038/s41545-023-00266-6 (2023). [Google Scholar]
- 41.Han, T. et al. Design and evaluation of the field-deployable electrostatic precipitator with superhydrophobic surface (FDEPSS) with high concentration rate. Aerosol Air Qual. Res.15, 2397–2408. 10.4209/aaqr.2015.04.0206 (2015). [Google Scholar]
- 42.TaewonHan, D. F. & Mainelis, G. Development and optimization of the electrostatic precipitator with superhydrophobic surface (EPSS) Mark II for collection of bioaerosols. Aerosol Sci. Technol.49, 210–219. 10.1080/02786826.2015.1017040 (2015). [Google Scholar]
- 43.Damak, M. & Varanasi, K. K. Electrostatically driven fog collection using space charge injection. Sci. Adv.4, 5323. 10.1126/sciadv.aao5323 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morley, E. L. & Robert, D. Electric fields elicit ballooning in spiders. Curr. Biol.28, 2324–2330. 10.1016/j.cub.2018.05.057 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruzat, D. & Jerez-Hanckes, C. Electrostatic fog water collection. J. Electrostat.96, 128–133. 10.1016/j.elstat.2018.10.009 (2018). [Google Scholar]
- 46.Jiang, Y., Xu, R., Liu, S., Liu, G. & Yan, X. Electrostatic fog collection mechanism and design of an electrostatic fog collector with nearly perfect fog collection efficiency. Chem. Eng. Sci.247, 117034. 10.1016/j.ces.2021.117034 (2022). [Google Scholar]
- 47.Pagliuca, S. Measurement of winds of super-Hurricane force on Mt. Washington, NH. Bull. Am. Meteor. Soc.15, 172–174. 10.1175/1520-0477-15.6-7.172 (1934). [Google Scholar]
- 48.Darzi, M. Fumarolic aerosols from Kilauea volcano, Hawaii. Nucl. Instrum. Methods181, 359–365. 10.1016/0029-554X(81)90636-4 (1981). [Google Scholar]
- 49.Pattantyus, A. K., Businger, S. & Howell, S. G. Review of sulfur dioxide to sulfate aerosol chemistry at Kīlauea Volcano, Hawaií. Atmos. Environ.185, 262–271. 10.1016/j.atmosenv.2018.04.055 (2018). [Google Scholar]
- 50.Lorenz, R., Crisp, D. & Huber, L. Vega 1 and Vega 2 Balloon and Lander Archive. VEGA1/VEGA2-V-2/3-VENUS-1.0, NASA Planetary Data System. https://atmos.nmsu.edu/data_and_services/atmospheres_data/VENUS/vega.html (2020).
- 51.Forest, I.-S. The corrosion resistance of nickel-containing alloys in sulfuric acid and related compounds. INCO Int. Nickel Comp.1, 51–82 (1983). [Google Scholar]
- 52.de Dios Rivera, J. Aerodynamic collection efficiency of fog water collectors. Atmos. Res.102, 335–342. 10.1016/j.atmosres.2011.08.005 (2011). [Google Scholar]
- 53.Petropoulos, B. Physical parameters of the atmosphere of Venus. Earth Moon Planet.42, 29–40. 10.1007/BF00118037 (1988). [Google Scholar]
- 54.Park, K.-C., Chhatre, S. S., Srinivasan, S., Cohen, R. E. & McKinley, G. H. Optimal design of permeable fiber network structures for fog harvesting. Langmuir29, 13269–13277. 10.1021/la402409f (2013). [DOI] [PubMed] [Google Scholar]
- 55.Pirjola, L. et al. Formation of sulphuric acid aerosols and cloud condensation nuclei: An expression for significant nucleation and model comprarison. J. Aerosol Sci.30, 1079–1094. 10.1016/S0021-8502(98)00776-9 (1999). [Google Scholar]
- 56.Middlebrook, A. M., Thomson, D. S. & Murphy, D. M. On the purity of laboratory-generated sulfuric acid droplets and ambient particles studied by laser mass spectrometry. Aerosol Sci. Technol.27, 293–307. 10.1080/02786829708965475 (1997). [Google Scholar]
- 57.Darling, H. E. Conductivity of sulfuric acid solutions. J. Chem. Eng. Data9, 421–426. 10.1021/je60022a041 (1964). [Google Scholar]
- 58.Lorenz, R. D. Lightning detection on Venus: A critical review. Prog. Earth Planet Sci.5, 1–25. 10.1186/s40645-018-0181-x (2018). [Google Scholar]
- 59.Blaske, C. H., O’Rourke, J. G., Desch, S. J. & Borrelli, M. E. Meteors may masquerade as lightning in the atmosphere of Venus. J. Geophys. Res. Planets128, e2023JE007914. 10.1029/2023JE007914 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is provided in the tables within the manuscript.